Abstract
We studied the association between bisphosphonate use and risk of atrial fibrillation or flutter and the effect of confounders such as heart and lung disease in a nationwide retrospective cohort from Denmark. All users of bisphosphonates and other drugs against osteoporosis between 1996 and 2006 (n = 103,562) were the exposed group and three age- and gender-matched controls from the general population (n = 310,683) were the nonexposed group. The main outcome measure was atrial fibrillation or atrial flutter. Before initiation of treatment against osteoporosis, no excess of atrial fibrillation or flutter was present for any drug except for etidronate (OR = 1.22, 95% CI 1.15–1.29). After initiation of treatment, raloxifene was not associated with any excess risk of atrial fibrillation (OR = 0.98, 95% CI 0.72–1.33). Etidronate (HR = 1.08, 95% CI 1.02–1.14) and alendronate (HR = 1.09, 95% CI 1.00–1.20) were associated with an excess risk of atrial fibrillation after treatment start if statistical adjustments were made for cardiovascular disease. However, this association disappeared upon statistical adjustment for chronic obstructive pulmonary disease (COPD) (etidronate HR = 1.04, 95% CI 0.98–1.10; alendronate HR = 1.05, 95% CI 0.96–1.15). In patients using etidronate (12.5% vs. 3.8%) and alendronate (11.4% vs. 4.6%) major differences were present in prevalence of COPD at start of treatment compared to matched controls. In conclusion, oral bisphosphonates do not seem to be associated with an excess risk of atrial fibrillation. Any excess risk seen in prior studies may be due to confounding from COPD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Intravenous treatment with zoledronic acid has been associated with an excess risk of atrial fibrillation compared to placebo in randomized controlled trials [1]. In the study by Black et al. [1] any event of atrial fibrillation occurred in 2.4% of the zoledronic acid–treated and 1.9% of the placebo-treated groups after 3 years (P = 0.12), while a significant difference was observed if the events were classified as serious adverse events (1.3% vs. 0.5%, P < 0.01). However, in the study by Lyles et al. [2] no excess risk of atrial fibrillation was observed (2.8% vs. 2.6% after 3 years for any event of atrial fibrillation, 1.1% vs. 1.3% for serious events). With oral alendronate an excess risk of atrial fibrillation has been reported in an observational study [3]. The hazard ratio (HR) in this study was 1.18 (95% confidence interval [CI] 1.08–1.29) [3] after statistical adjustment for cardiovascular medications, concomitant medications, and Charlson index [4]. One further observational study reported that the odds ratio (OR) of atrial fibrillation with bisphosphonates was 1.86 (95% CI 1.09–3.15) after statistical adjustment for osteoporosis and a number of cardiovascular confounders [5].
However, a case–control study on bisphosphonate use could not confirm this observation of an excess risk of atrial fibrillation (OR = 0.95, 95% CI 0.84–1.07) after statistical adjustment for a number of confounders including hyperthyroidism, cardiovascular disease, and pulmonary disease [6]. A further claims-based study [7] examined patients undergoing angiography and also found no relationship between atrial fibrillation and use of bisphosphonates in the crude analysis (0.82, 95% CI 0.66–1.01), but the number of cases of atrial fibrillation was low (10 cases among 98 users).
A meta-analysis [8] repeated the findings in the studies above, with a significant excess risk of atrial fibrillation as a serious adverse event in the randomized controlled trials but no excess risk in observational trials and if all events of atrial fibrillation were considered in the randomized controlled trials.
The studies presented above differ in terms of statistical adjustments performed. The study by Abrahamsen et al. [3] adjusted for cardiovascular drugs, while the study by Sorensen et al. [6] adjusted for hyperthyroidism, pulmonary disease, cardiovascular disease, and cardiovascular drugs. The studies by Heckbert et al. [5] and Bunch et al. [7] mainly used cardiovascular variables as confounders. However, many risk factors for atrial fibrillation exist, and the more potent of these include heart valve disease, congestive heart disease, and hyperthyroidism [9]. Weaker risk factors include alcoholism [10] and overweight [11]. Another important risk factor is chronic obstructive pulmonary disease (COPD), which may give rise to pulmonary hypertension, with dilation of the right atrium leading to atrial fibrillation or flutter. Many cardiovascular drugs may actually be used to treat atrial fibrillation, such as oral anticoagulants, beta-blockers, digoxin, and amiodarone; hence, statistical adjustments including these may actually overadjust because the drugs are used to treat atrial fibrillation and are not a cause of this condition.
Furthermore, some of the diseases associated with atrial fibrillation may also be associated with osteoporosis and thus prescription of bisphosphonates. These diseases associated with both osteoporosis and atrial fibrillation are primarily hyperthyroidism [12] and to some degree cardiovascular disease [13, 14]. For COPD, which is a risk factor for atrial fibrillation, use of prednisolone may further contribute to the relationship with osteoporosis. The randomized controlled trials did not report on premorbid occurrence of cardiovascular diseases or COPD [1, 2]. Any unbalance in these risk factors may thus explain some of the difference in occurrence of atrial fibrillation. Furthermore, none of the studies has reported on occurrence of atrial fibrillation before initiation of bisphosphonates or after initiation of other drugs against osteoporosis besides bisphosphonates. We therefore undertook a large-scale cohort study of use of any drug against osteoporosis and risk of atrial fibrillation before and after initiation of these drugs, adjusted for confounders. In our analysis cardiovascular drugs used to treat atrial fibrillation (digoxin and amiodarone) were excluded, to avoid confounding by indication. Beta-blockers were included in the analyses as they may be used to treat atrial fibrillation but mainly are used for hypertension and to some degree heart failure.
The objectives were to assess if use of bisphosphonates or other drugs against osteoporosis was associated with an excess risk of atrial fibrillation and the role of potential confounders for any association observed.
Materials and Methods
Study Design
The study was designed as a cohort study, with patients exposed to drugs against osteoporosis being compared to an age- and gender-matched control group of nonexposed subjects.
Patients Exposed to Drugs against Osteoporosis (Exposed Group)
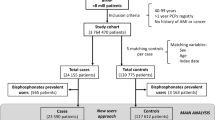
All patients who had filed a prescription for a drug against osteoporosis between January 1, 1996, and December 31, 2006, were included. These included bisphosphonates (ATC codes M05BA01 [etidronate], M05BA02 [clodronate], M05BA03 [pamidronate], M05BA04 [alendronate], M05BA06 [ibandronate], M05BA07 [risedronate], M05BA08 [zoledronate], M05BB01 [etidronate plus calcium], M05BB03 [alendronate plus vitamin D]), raloxifene (ATC code G03XC01), strontium ranelate (ATC code M05BX03), and parathyroid hormone or analogues (ATC codes H05AA02, H05AA03). All drugs were administered orally except zoledronate and pamidronate, which were administered intravenously, and parathyroid hormone, which was administered subcutaneously.
Controls (Nonexposed Group)
For each exposed subject three nonexposed control subjects matched on age (same birth year) and gender were randomly selected from the background population from the same period as the exposed patients. Nonexposed controls had not used any of the drugs against osteoporosis mentioned above for the case patients during the study period (January 1, 1996, to December 31, 2006). The day that defined start of follow-up “after use” among the nonexposed was defined as a dummy date that was the same as the date where the matched exposed subject had used a drug against osteoporosis for the first time.
Variables
The main outcome variable was a diagnosis of atrial fibrillation or flutter during the observation period (atrial fibrillation and flutter are combined into the term “atrial fibrillation” used throughout the text as atrial flutter was rare and often degenerated into atrial fibrillation). The definition of atrial fibrillation or flutter was a recorded incident episode of such leading to either hospitalization or an outpatient contact.
Registers Used
The information on fracture occurrence and occurrence of other diseases or alcoholism came from the National Hospital Discharge Register [15]. The National Hospital Discharge Register was founded in 1977 [15]. It covers all inpatient contacts from 1977 to 1994 and from 1995 also all outpatient visits to hospitals, outpatient clinics, and emergency rooms [15]. Upon discharge, the physician codes the reason for the contact using the ICD system. The code used is at the discretion of the individual physician. The register has a nationwide coverage and an almost 100% capture of contacts [15]. In general, the validity of registrations is high [16]. The validity of the diagnosis of atrial fibrillation is high (99%) [17], and all cases are managed in the hospital system (i.e., all patients are registered).
The Danish Medicines Agency keeps a nationwide register of all drugs sold at pharmacies throughout the country from 1996 and onward (National Pharmacological Database run by the Danish Medicines Agency, http://www.dkma.dk). Any drugs bought are registered with an ATC code, dosage sold, form of medication (tablets, injections, etc.), and date of sale. As all sales are registered to the individual who redeemed the prescription, the capture and validity are high. To allow comparisons between different drugs, the exposure was expressed as defined daily dose (DDD), which is a standardized variable for drug use, with 1 DDD being equivelant to the average dose of the drug an individual would normally use in 1 day.
Information on vital status and migrations came from the National Person Register. All patients were followed up until December 31, 2006, in all registers used.
It is possible to link these sources of information through the Central Person Register Number, which is a unique registration code given to every inhabitant. This code is similar to the U.S. social security number.
The project was approved and controlled by the National Board of Health and the Danish Data Protection Agency.
Statistics
Mean and standard deviation were used as descriptive statistics. Quantitative variables were defined by either use or nonuse of a drug or presence or absence of a given disease or condition during the observation period. Fractional values of exposure (say, DDD) were determined to allow for a meaningful exposure and a high number of exposed in the category.
Analyses were performed for incident cases of atrial fibrillation before the start date of the drug in question or the matched date among the nonexposed.
Crude and adjusted ORs and 95% CIs were calculated for exposure vs. outcome before and after the start of the drug in question or the dummy date among the nonexposed.
A conditional logistic regression analysis was used to assess the association between atrial fibrillation and the exposure variable before the start date of any drug against osteoporosis. The final regression model for the time before start of the drug (or the matched dummy date among the nonexposed) was created using this conditional logistic regression model, with all variables entered or entered stepwise as detailed in the tables.
A Cox proportional hazard analysis was applied for risk of the outcome after initiation of any drug against osteoporosis. The final regression model for the time after start of the drug in question (or the matched dummy date among the nonexposed) was created using this Cox proportional hazard model with all variables either entered or entered stepwise as detailed in the tables. The proportional hazard assumption was tested by examination of Kaplan–Meier plots of atrial fibrillation–free survival.
Analyses were performed using STATA 8.2 (StataCorp, College Station, TX) and SPSS 15.0 (SPSS, Inc., Chicago IL), both in the UNIX version.
The following confounders were included in the statistical analyses: congestive heart failure, use of diuretics, use of antihypertensives (angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel antagonists, and beta-blockers), heart valve disease, hyperthyroidism, COPD, use of bronchodilator drugs and inhaled corticosteroids, and alcoholism (defined as a hospital diagnosis of alcoholism or use of disulfiram).
For the time period before start of the drug in question or the corresponding dummy date among the controls, adjustments were made only for confounders that actually occurred before the onset of atrial fibrillation (i.e., COPD diagnosed later than atrial fibrillation was not considered as no causal association could be considered to be present for COPD being diagnosed after atrial fibrillation). For the time period after start of the drug in question or the dummy date among the controls, only confounder variables that occurred before the day of atrial fibrillation or flutter were included as, e.g., occurrence of COPD after atrial fibrillation or flutter was diagnosed may not signal a causal relationship.
Results
Table 1 shows the baseline characteristics of the patients who had used drugs against osteoporosis and of the controls. Patients and controls were well matched concerning age and gender. In general, patients had more comorbid factors and more often used drugs for various conditions than controls. In particular, a large absolute and relative difference was present concerning COPD and use of bronchodilator drugs.
Table 2 shows differences in comorbid conditions and other variables stratified by major drugs against osteoporosis (alendronate, etidronate, and raloxifene). Patients prescribed etidronate and alendronate in general were older than patients on raloxifene. Although significant differences existed for several conditions, the major absolute difference was present for COPD (2.7% for raloxifene, 6.8% for alendronate, and 8.7% for etidronate). For etidronate and alendronate the relative difference in COPD was larger than that for raloxifene (P < 0.01).
Table 3 shows the crude OR for atrial fibrillation before and after start of a drug against osteoporosis. For raloxifene no excess risk was present either before or after initiation of treatment. For etidronate and alendronate an increased risk of atrial fibrillation was present after initiation of treatment.
Figure 1 shows the risk of atrial fibrillation stratified by two of the major confounders, i.e., heart failure and COPD. The absolute risk of atrial fibrillation with alendronate was higher at 7 years with heart failure (40% in cases and 37% in controls) than with COPD (18% vs. 14%). However, the relative excess risk was higher with COPD than with heart failure (RR = 1.2 vs. 1.1). The risk of atrial fibrillation was not different in patients and controls with heart failure or COPD.
Table 4 shows the HR of atrial fibrillation after initiation of various drugs against osteoporosis after statistical adjustment for multiple confounders in a stepwise fashion. With the introduction of diuretics, the significance seen for alendronate and etidronate started to disappear; but it reappeared with the introduction of other cardiovascular drugs (angiotensin-converting enzyme inhibitors, calcium channel inhibitors, and beta-blockers). Upon introduction of COPD the association between atrial fibrillation and etidronate or alendronate lost its significance and did not reappear. It did not change the association to introduce systemic corticosteroids before or after COPD was introduced (data not shown). There was no effect modification of age or gender (data not shown).
Discussion
In this large-scale cohort study with extended follow-up, the oral bisphosphonates etidronate and alendronate did not seem to be associated with an excess risk of atrial fibrillation after statistical adjustment for cardiovascular confounders and COPD. This study thus adds important data on the causes of the seeming association between use of bisphosphonates and atrial fibrillation seen in prior epidemiological and randomized controlled trials.
This effect of COPD may explain the difference between the prior study of Abrahamsen et al. [3], where an association was reported, and the study by Sorensen et al. [6], where no statistically significant association was present between bisphosphonate use and risk of atrial fibrillation. The study by Abrahamsen et al. [3] adjusted for heart disease but not for pulmonary disease, in contrast to the study by Sorensen et al. [6], which adjusted for cardiovascular disease, pulmonary disease, and a number of other confounders.
In our study no association between use of raloxifene and risk of atrial fibrillation was present in the crude analysis, while an association was present for etidronate and alendronate; but this association disappeared after statistical adjustment for COPD. For raloxifene the difference in the prevalence of COPD was much smaller between users and controls than it was for etidronate and alendronate, and this may explain the absence of any excess risk of atrial fibrillation in the crude analysis.
COPD is associated with osteoporosis in a number of ways, among them corticosteroid use [18], smoking [19], physical inactivity due to reduced lung capacity, CO2 excess [20], malnutrition, weight loss [21, 22], and thus potential vitamin D deficiency [21]. However, COPD is also associated with an excess risk of atrial fibrillation both from increased pressure in the right atrium and from use of beta agonists, which may increase heart rate and thus increase the risk of atrial fibrillation or flutter [23]. COPD patients may thus be more exposed to drugs against osteoporosis because of a higher risk of osteoporosis linked to, e.g., prednisolone use. As COPD per se is associated with atrial fibrillation, this may explain the association between use of drugs against osteoporosis and atrial fibrillation if no statistical adjustments are made.
The younger the patients were, the fewer cases of COPD were present, in accordance with the epidemiology of COPD in the general population. This may also explain why raloxifene users, who were generally younger, had no excess of atrial fibrillation in the crude analysis.
The reason that statistical adjustment for use of systemic corticosteroids did not change the risk of atrial fibrillation may be an effect of confounding by indication. Statistical adjustment for COPD already had taken into account the effects of the underlying disease. Statistical adjustment for systemic corticosteroids thus did not add any information and did not change the risk estimates.
The prior randomized controlled trials [1, 2] did not specifically report on the baseline prevalence of heart and lung disease; and although randomization is supposed to counter selection bias, small differences in baseline risk of these important confounders may explain the excess in patients receiving i.v. zoledronate. In our study the number of zoledronate users was too low to study the effects of zoledronate. More studies are thus needed in this field.
The excess risk of atrial fibrillation with COPD and heart failure did not occur early after initiation of the drugs against osteoporosis but appeared first at least 2–3 years after the start. This indicates that there is no causal effect of the drugs but, rather, an effect of the underlying disease.
The main advantages of this study are the large sample size, the uniform nature of the data, the long-term follow-up, and the completeness of follow-up. The main drawbacks are the lack of individual data on smoking and body weight. However, weight is a weak risk factor for atrial fibrillation [11]; and whereas overweight predisposes to atrial fibrillation, underweight predisposes to osteoporosis [22] and thus treatment with bisphosphonates. However, the fact that no difference in risk of atrial fibrillation could be shown for raloxifene argues against any major effect of body weight.
Bias may include information bias and selection bias. Bias from selection was low as the entire population was covered by the data set and as the capture of the data set was high. Information bias may arise from misclassification of exposure and outcome. The precision of the outcome variable (atrial fibrillation and flutter) was high, as judged from validation studies. The validity of the exposure was high as it referred to drugs actually bought at the pharmacies and not just prescribed drugs. However, whether the drugs were actually ingested cannot be determined from the registers. As the drugs were purchased, the intake can be regarded as potentially high, but noncompliance cannot be excluded.
The external validity may be good as the study was nationwide. However, extrapolation of results to non-Caucasians should be done with caution as the majority of the population studied was Caucasian.
The main strengths compared to other studies are the detailed analysis of which confounders changed the apparent association between atrial fibrillation and use of oral bisphosphonates. The main limitation is the lack of data on intravenous bisphosphonates. Regarding the diagnosis of atrial fibrillation or flutter, it may take some time before the patient comes into contact with the health-care system; the date of hospitalization or outpatient contact may thus not reflect the true date of onset. However, as this may not have biased the allocation to bisphosphonates, it may not have biased the results significantly.
The main difference compared to prior studies is the fact that COPD may be responsible for the association between atrial fibrillation and bisphosphonates in the crude analysis. Furthermore, the study provides absolute risk estimates over time. The possible explanation for the association with COPD may be an increased pressure in the right atrium predisposing to atrial fibrillation and, on the other hand, a strong association between COPD and osteoporosis, and thus bisphosphonate use, due to, among other things, use of prednisolone. In the clinic the study results mean that any association between atrial fibrillation and oral bisphosphonates may not be causal but due to confounding. Oral bisphosphonates may thus safely be prescribed to patients at risk of atrial fibrillation.
Further research is needed on intravenous bisphosphonates, risk of atrial fibrillation, and potential confounding from COPD.
In conclusion, oral bisphosphonates do not seem to be associated with an excess risk of atrial fibrillation. Any excess risk seen in prior studies may be due to confounding from COPD.
References
Black D, Delmas P, Eastell R, Reid I, Boonen S, Cauley J et al (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356:1809–1822
Lyles K, Colon-Emeric C, Magaziner J, Adachi J, Pieper C, Mautalen C et al (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357:1799–1809
Abrahamsen B, Eiken P, Brixen K (2009) Atrial fibrillation in fracture patients treated with oral bisphosphonates. J Intern Med 265:581–592
Charlson M, Pompei P, Ales K, MacKenzie C (1987) A method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 40:373–383
Heckbert SR, Li G, Cummings SR, Smith NL, Psaty BM (2008) Use of alendronate and risk of incident atrial fibrillation in women. Arch Intern Med 168:826–831
Sorensen H, Christensen S, Mehnert F, Pedersen L, Chapurlat R, Cummings S et al (2008) Use of bisphosphonates among women and risk of atrial fibrillation and flutter: population based case-control study. BMJ 336:813–816
Bunch TJ, Anderson JL, May HT, Muhlestein JB, Horne BD, Crandall BG et al (2009) Relation of bisphosphonate therapies and risk of developing atrial fibrillation. Am J Cardiol 103:824–828
Loke Y, Jeevanantham V, Singh S (2009) Bisphosphonates and atrial fibrillation: systematic review and meta-analysis. Drug Saf 32:219–228
Frost L, Vestergaard P, Mosekilde L (2004) Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Arch Intern Med 164:1675–1678
Frost L, Vestergaard P (2004) Alcohol and risk of atrial fibrillation or flutter: a cohort study. Arch Intern Med 164:1993–1998
Frost L, Hune L, Vestergaard P (2005) Overweight and obesity as risk factors for atrial fibrillation or flutter: the Danish Diet, Cancer, and Health Study. Am J Med 118:489–495
Vestergaard P, Mosekilde L (2003) Hyperthyroidism, bone mineral, and fracture risk—a meta-analysis. Thyroid 13:585–593
Tanko LB, Bagger YZ, Christiansen C (2003) Low bone mineral density in the hip as a marker of advanced atherosclerosis in elderly women. Calcif Tissue Int 73:15–20
Vestergaard P, Rejnmark L, Mosekilde L (2009) Hypertension is a risk factor for fractures. Calcif Tissue Int 84:103–111
Andersen T, Madsen M, Jørgensen J, Mellemkjær L, Olsen J (1999) The Danish National Hospital Register. Dan Med Bull 46:263–268
Mosbech J, Jørgensen J, Madsen M, Rostgaard K, Thornberg K, Poulsen T (1995) The Danish National Patient Register: evaluation of data quality [in Danish]. Ugeskr Laeger 157:3741–3745
Frost L, Vestergaard P, Mosekilde L, Mortensen L (2005) Trends in incidence and mortality in the hospital diagnosis of atrial fibrillation or flutter in Denmark, 1980–1999. Int J Cardiol 103:78–84
Vestergaard P, Rejnmark L, Mosekilde L (2005) Fracture risk associated with systemic and topical corticosteroids. J Intern Med 257:374–384
Vestergaard P, Mosekilde L (2003) Fracture risk associated with smoking—a meta-analysis. J Intern Med 254:572–583
Dimal H, Domej W, Leb G, Lau K (2001) Bone loss in patients with untreated chronic obstructive pulmonary disease is mediated by an increase in bone resorption associated with hypercapnia. J Bone Miner Res 16:2132–2141
Forli L, Halse J, Haug E, Bjortuft O, Vatn M, Kofstad J et al (2004) Vitamin D deficiency, bone mineral density and weight in patients with advanced pulmonary disease. J Intern Med 256:56–62
De Laet C, Kanis J, Oden A, Johanson H, Johnell O, Delmas P et al (2005) Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int 16:1330–1338
Hanrahan JP, Grogan DR, Baumgartner RA, Wilson A, Cheng H, Zimetbaum PJ et al (2008) Arrhythmias in patients with chronic obstructive pulmonary disease (COPD): occurrence frequency and the effect of treatment with the inhaled long-acting beta2-agonists arformoterol and salmeterol. Medicine (Baltimore) 87:319–328
Acknowledgement
This study was funded by an unrestricted grant from Servier Denmark and the Dandy Foundation. None of the sponsors had any role in obtaining data, analyzing data, or writing the report.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Vestergaard, P., Schwartz, K., Pinholt, E.M. et al. Risk of Atrial Fibrillation Associated with Use of Bisphosphonates and Other Drugs Against Osteoporosis: A Cohort Study. Calcif Tissue Int 86, 335–342 (2010). https://doi.org/10.1007/s00223-010-9349-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-010-9349-0