Abstract
Both physical activity and body mass affect bone properties. In this study we examined how diet-induced obesity combined with voluntary physical activity affects bone properties. Forty 7-week-old male C57BL/6J mice were assigned to four groups evenly: control diet (C), control diet + running (CR), high-fat diet (HF, 60% energy from fat), and high-fat diet + running (HFR). After 21-week intervention, all mice were killed and the left femur was dissected for pQCT and mechanical measurements. Body mass increased 80% in HF and 62% in HFR, with increased epididymal fat pad weight and impaired insulin sensitivity. Except for total and trabecular volumetric bone mineral density (BMD), bone traits correlated positively with body mass, fat pad, leptin, and osteoprotegerin. Obesity induced by a high-fat diet resulted in increased femoral bone cross-sectional area, mineral content (BMC), polar moment of inertia, and mechanical parameters. Of the mice accessing the running wheel, those fed the control diet had thinner cortex and less total metaphyseal BMC and BMD, with enlarged metaphyseal marrow cavity, whereas mice fed the high-fat diet had significantly higher trabecular BMD and smaller marrow cavity. However, the runners had a weaker femoral neck as indicated by decreased maximum flexure load. These results suggest that voluntary running exercise affects bone properties in a site-specific manner and that there is a complex interaction between physical activity and obesity. Thus, both diet and exercise should be considered when optimizing the effects on body composition and bone, even though the underlying mechanisms remain partly unknown.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis and obesity, two disorders of body composition, have grown in prevalence during recent years [1]. Epidemiological data and animal studies indicate that both of these diseases involve genetic predisposition [2], share a common progenitor cell [3], and are influenced by nutrition and sedentary lifestyles [4, 5]. Numerous data have shown that dietary fat intake is closely associated with increased body mass in both humans and other animals [6, 7]. Both body mass and exercise evidently act on bone through altered mechanical loading. Mechanical loading modifies bone structure or density by stimulating signaling pathways that regulate bone formation and resorption. Furthermore, abundant evidence suggests that adipose tissue as an endocrinal organ affects bone metabolism through secreted adipokines [8–11].
Physical activity has been shown to maintain and possibly improve bone properties in humans and other animals. In fact, in humans, physical activity has a positive effect on bone mass and microstructure in adolescents [12], adults [13, 14], and the elderly [15, 16]. In animal models, forced exercise on a treadmill or jumping has also shown a positive effect on bone strength [17, 18]. However, since forced-exercise models may be problematic due to stress, voluntary wheel running may be an alternative model. A few studies have indicated that voluntary exercise can improve bone properties through bone modeling or remodeling [19, 20]. Overall, though, data on the effect of voluntary exercise on bone in animal models remain limited.
In order to gain insight into the effects of obesity and exercise on bone properties, we used an obesity model in which mice were fed a high-fat diet and allowed to freely run on a running wheel. Finally, we evaluated the geometry and density of femoral mid-shaft and distal region by pQCT and the mechanical properties of the femoral neck by mechanical testing.
Materials and Methods
Animals and Diets
This study was approved by the National Animal Experiment Board, Finland. Forty male C57BL/6J mice were obtained from Taconic (Ejby, Denmark) at the age of 6 weeks. Mice were housed, one per cage, in a humidity- and temperature-controlled room on a 12:12 h dark–light cycle (08.00–20.00) and allowed to adapt to their new environment for 1 week before being allocated into one of the four intervention groups (10 in each): control diet (C), control diet + voluntary running (CR), high-fat diet (HF), and high-fat diet + voluntary running (HFR).
For studying the effect of voluntary wheel-running exercise, animals in the CR and HFR groups were housed in custom-made cages with a running wheel (12 cm diameter, 8 cm width) with free access to the wheel 24 h/day for 21 weeks. Total wheel revolutions were recorded daily by a magnetic switch, with total exercise performed per day determined by multiplying the number of wheel rotations by the circumference of the wheel. C and HF animals were housed in similar cages without the running wheel. Mice had continuous access to the control or high-fat diet and regular tap water. The control diet was a standard rodent diet (R36: 4% fat, 55.7% carbohydrate, 18.5% protein, 3 kcal/g; Labfor, Stockholm, Sweden). The high-fat diet was a lard-based purified diet (D12492: 60% fat, 20% carbohydrate, 20% protein, 5.24 kcal/g; Research Diets, New Brunswick, NJ).
Body Mass, Food Consumption, and Feeding Efficiency
Body mass and food consumption were measured at 2-week intervals. Feeding efficiency over the intervention period was calculated as body mass gain per unit energy intake (mg/kcal).
Endurance Running Capacity Assessment
At week 16 of the intervention, all mice were exposed to a forced maximum running test on a motor-driven treadmill. The test was started at the speed of 10 m/minute for 5 min against an angle of 0.8º. The speed was increased in 5-minute intervals by 1 m/minute until exhaustion. The total distance covered was recorded.
Glucose Homeostasis
The glucose and insulin tolerance tests (GTT and ITT, respectively) were performed at 1-week interval for all mice after 10- and 18-week intervention. Animals were fasted for 5 h before GTT and for 2 h before ITT. A glucose (2 g/kg body mass) or insulin (0.75 U/kg body mass; Humuline® Regular; Eli Lilly, Indianapolis, IN) solution was injected into the intraperitoneal cavity, followed by blood sampling at time points 0, 15 (ITT only), 30, 60, 90, and 120 min.
Blood Biochemistry
After 5-hour fasting, a blood sample was collected at 10 and 18 weeks of intervention. The blood glucose level was determined by the B-Glucose photometer (HemoCue, Ängelholm, Sweden). After 21 weeks of intervention, the mice were killed by cervical dislocation. Blood samples were collected and serum samples were separated after 1-hour clotting and stored at −70°C for further analysis. Levels of leptin, osteoprotegerin (OPG), and osteocalcin in serum were measured using the Milliplex mouse bone metabolism panel (Millipore, Bedford, MA) according to the manufacturer’s instruction.
Tissue Sample Collection
After the mice were killed, the left femur was separated and trimmed of attached soft and connective tissues, wrapped within phosphate-buffered saline-soaked gauze, and stored frozen at −20°C. Liver and epididymal fat pads were surgically removed, weighed, snap-frozen in liquid nitrogen, and stored at −70°C.
pQCT Densitometry
A pQCT apparatus (Stratec XCT Research; Stratec Medizintechnik, Pforzheim, Germany), calibrated using a hydroxyapatite standard cone phantom, was used for bone densitometry. A voxel size of 0.07 × 0.07 × 0.5 mm was used for all measurements. The femur was thawed overnight at 4°C and inserted into a specially constructed plastic syringe with the shaft in the axial direction. The scout view was obtained for the whole femur. Four slices were scanned at the metaphyseal region at 0.5-mm intervals starting from a 12.5% landmark and one slice from a 50% landmark for the diaphyseal area. For the best separation, the third slice from the metaphysis and the slice from the diaphysis were used for further analysis. All scanned slices were analyzed by bone analysis software (Geanie 2.1; Commit, Espoo, Finland). A contour-detecting algorithm (k-mode) was used, with a threshold value of 500 mg/cm3 for the separation of trabecular and cortical bone and 100 mg/cm3 for marrow and bone separation. Parameters reported for the diaphyseal region included total bone cross-sectional area (tCSA), total bone mineral density (tBMD), total bone mineral content (tBMC), cortical cross-sectional area (cCSA), cortical bone mineral density (cBMD), cortical bone mineral content (cBMC), cortical thickness (ThC, using a ring model), marrow cross sectional area (mCSA), and density weighted polar moment of inertia (Ipolar). Parameters reported for the metaphyseal region included tCSA, tBMD, tBMC, trabecular cross-sectional area (traCSA), trabecular bone mineral density (traBMD), trabecular bone mineral content (traBMC), mCSA, and Ipolar.
Biomechanical Testing
After pQCT measurement, a cantilever bending test of the femoral neck was performed (Instron 3343; Instron, Norwood, MA) [21, 22]. Briefly, the proximal half of the femur was fixed axially in a methylmethacrylate plate stage and compressed in a direction parallel to the femoral shaft axis at a constant speed of 4.5 mm/minute. The following parameters were obtained from the recorded load curve: maximum flexure load (N), energy at break point (kJ), and elastic modulus of the femur.
Statistics
Results are expressed as mean ± SD. The Shapiro–Wilk test was used to investigate within-group normality for a given parameter of interest. Levene’s test was conducted to assess the homogeneity of the variance assumption. When normality or equality of variance assumptions was not met, log transformations were investigated. Two-way ANOVA was used to determine the effect of diet (with two levels: control and high-fat diet), exercise (with two levels: with and without voluntary running), and their interaction on the measured bone traits. Differences among the means of the intervention groups were evaluated, and the significance of differences was determined by post hoc testing using Tukey’s HSD test. A Pearson correlation coefficient was used to determine the relationship between body mass, fat pad, serum factors, and the measured bone variables. All statistical analyses were performed with SPSS 15.0 (SPSS, Inc., Chicago, IL). Differences of P < 0.05 were considered significant.
Results
Body Mass, Food Consumption, and Efficiency
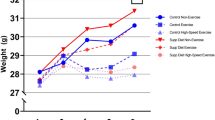
The development of body mass in the four groups is shown in Fig. 1c. After 4 weeks of intervention, significantly higher body mass was observed in both HF and HFR compared to C and CR. From this time point on, the body mass of mice fed the high-fat diet increased continuously. The body mass of mice fed the control diet reached a peak after 12 weeks of intervention. Finally, the HF mice gained 18.0 ± 4.0 g compared with 5.3 ± 1.8 g for the C mice. The running groups gained slightly less body mass (15.4 ± 3.8 g for HFR, 5.2 ± 1.4 g for CR), but the differences compared to nonrunners were statistically nonsignificant. Consistently with their body mass, mice fed the high-fat diet had a heavier liver and fat pad than their counterparts on a low-fat diet (Table 1). Significant differences in total energy consumption and feeding efficiency were observed in mice fed the high-fat diet compared with control mice, regardless of access to a running wheel (Fig. 1a, b).
Total energy intake (a) (black, fat; gray protein; white carbohydrate) and feeding efficiency (b) as body mass gained (mg) per unit energy consumed (kcal) during the 21-week intervention in mice. Mean body mass of the mice in each group during the diet intervention c. ** P < 0.01, * P < 0.05; n = 10 mice/group
Voluntary Running
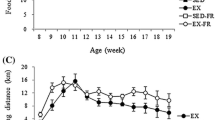
Figure 2a shows the voluntary running distances covered by the CR and HFR groups. There were no significant differences between the CR and HFR groups in either daily average or cumulative distances. Over 4 weeks, both CR and HFR reached their maximum running distance and then decreased gradually.
Figure 2b shows the results of the exhaustive running tests. Voluntary exercise training improved the running capacity of mice, especially in group HFR.
Glucose Homeostasis
The fasting glucose level (Fig. 3a) was significantly higher in HF and HFR compared to C and CR. CR had lower fasting glucose than C, but no differences were observed between HF and HFR. GTT and ITT curves were constructed and the area under the curves (AUC) was calculated using the trapezoidal rule (Figs. 3b, c). Two-way ANOVA showed a significant diet effect on AUC-GTT, with a reduced response of obese mice to glucose (Fig. 3b). Similarly, a significant dietary effect was found on AUC-ITT whereby obese mice had a larger AUC-ITT, suggesting insulin resistance (Fig. 3c). CR had smaller AUC-GTT and -ITT than C, but no significant differences were found between HF and HFR.
Blood glucose concentrations during glucose (GTT) and insulin (ITT) tolerance tests after 18-week intervention in mice. Fasting glucose concentration (a), area under curve (AUC-GTT) during glucose tolerance test (b), area under curve (AUC-ITT) during insulin tolerance test (c). ** P < 0.01, * P < 0.05; n = 10 mice/group
Blood Sample Biochemistry
Table 1 shows plasma leptin levels, which were over sevenfold higher in HF and HFR than in C and CR, while no significant differences were found either between C and CR or between HF and HFR. The high-fat diet also increased the OPG level, but no significant differences in osteocalcin were observed between the four groups.
Effects of High-Fat Diet and Voluntary Running on Bone
Most of the measured bone traits, except for tBMD, correlated positively with body mass and fat mass, as well as leptin and OPG in both the diaphyseal and metaphyseal regions. No correlation between osteocalcin and the measured bone traits were found (Table 2).
The diet- and running-induced effects on bone traits measured by pQCT are shown in Table 3. Two-way ANOVA showed a significant overall interaction of diet by running on tBMD and ThC in the diaphysis and on tBMD, tBMC, traBMD, and mCSA in the metaphysis. In both the diaphysis and metaphysis, voluntary wheel running decreased tBMD in mice on the control diet but increased tBMD for those on the high-fat diet. Of the mice with access to the running wheel, those fed the high-fat diet had a thicker diaphyseal cortex and higher metaphyseal tBMC compared to those fed the control diet, the interaction thus overriding the main effect of running. Actually, CR mice had a thinner cortex and lower metaphyseal tBMC. Voluntary wheel running increased traBMD only in mice fed the high-fat diet. For mCSA in the metaphysis, there was a “crossover” effect; for those on the control diet running increased mCSA but for those on the high-fat diet it decreased mCSA.
Apart from the above interactions, the high-fat diet had a significant main effect by improving all the other bone traits at both bone sites, while the main effect of running was seen only in the increased metaphyseal traBMC.
Table 4 shows the biomechanical properties of the femoral neck. No significant interactions of diet by running were found. The high-fat diet had a significant main effect by increasing maximum flexure load and elastic modulus, whereas voluntary running decreased the maximum flexure load.
Discussion
In this study, by feeding C57BL/6J mice a high-fat diet for 21 weeks, we obtained obese mice with over 60% increased body mass and insulin resistance. These mice also showed a sevenfold increase in leptin level. Voluntary wheel running increased running capacity under the control and high-fat diets, but under the high-fat diet it had no significant effect on insulin sensitivity. The effects of diet or running on bone properties were complex in a site-specific manner.
Our main findings were as follows: (1) Heavier body mass induced by the high-fat diet accompanied increased total bone cross-sectional area, mineral content, and polar moment of inertia in both the diaphyseal and metaphyseal regions of the femur. Diaphyseal cortical cross-sectional area, marrow cavity, mineral content and density, metaphyseal trabecular cross-sectional area, and mineral content as well as femoral neck maximum flexure load and elastic modulus were also increased. (2) Voluntary wheel running under the control diet decreased diaphyseal cortical thickness and total metaphyseal bone mineral content and density and increased metaphyseal marrow cavity. For those on the high-fat diet, voluntary wheel running increased metaphyseal trabecular density, decreased marrow cavity, and decreased maximum flexure load in the femoral neck.
Epidemiological evidence has established a close link between body mass (fat mass and/or fat free mass) and bone mass in humans [11]. It is also known that obesity is inversely correlated with fracture risk [8]. Cobayashi et al. [23] have shown that obese and overweight adolescents in the final stages of sexual maturity had higher BMD of the lumbar spine compared to their normal-weight counterparts, suggesting a positive role of obesity on growing bone. More recently, Aubertin-Leheudre et al. [24] suggested that even in sarcopenic postmenopausal women obesity might offer some positive effects against osteoporosis. Similarly, low body mass index increasing the risk of osteoporotic fracture due to low bone mass independent of age and sex was reported by Galusca et al. [25]. In animal studies, similar to our results, Brahmabhatt et al. [26] reported that the weight gain produced by diet-induced obesity may lead to bone adaptation and improved biomechanics of the femur in mature male rats. In contrast, Cao et al. [27] showed that obesity induced by a high-fat diet had negative effects on cancellous bone mass but no effects on cortical bone mass in the tibia of mice. This discrepancy may be due to differences in the analytical procedures and weight-bearing skeleton sites.
Our results add to a growing body of evidence that bone mass and density are highly correlated with body mass. Obese mice had larger and stronger bone compared to lean mice. This positive effect of obesity on both bone mass and strength has been explained by several mechanisms. In obesity, the increase of bone mass and strength may be firstly due to higher mechanical loading stimulated by increased body mass. In our study, the body mass of mice fed the high-fat diet was increased more than 60%, suggesting that body mass is a major influencing factor for bone traits. Another potential explanation is the increased secretion of bone-protecting factors from adipose tissue. During recent years, adipose tissue has been considered not just as a passive tissue for the storage of excess energy as triglycerides but also as an active endocrine organ secreting a variety of biologically active molecules, e.g., leptin [28] and adiponectin [8]. It has been shown that leptin inhibits bone formation through the central nervous system [29]. Moreover, it is proposed that peripheral leptin is associated positively with bone formation and negatively with bone resorption, leading to increased bone mass [30]. In accordance with earlier reports (on humans and other animals), our obese mice showed high serum leptin levels. We also noticed that there was a positive correlation between serum leptin level and the measured bone traits except tBMD in both the diaphyseal and metaphyseal regions. However, the correlation was diminished when adjusted for body mass; thus, further studies are needed to address the specific role of leptin in explaining the association between obesity and bone geometry and strength.
Voluntary running improved the endurance running capacity of mice especially under the high-fat diet and was associated with a concomitant statistically nonsignificant improvement in insulin sensitivity. However, body mass was not significantly reduced. Runners gained somewhat more calories from their diets, but due to increased energy expenditure, their body mass was not increased. These effects of reduced body mass on bone under the control diet were negative, but under the high-fat diet they were positive. Similar to our results, Hamrick et al. [31] reported that reduced body mass due to energy restriction resulted in decreased cortical bone mass and spared trabecular bone in mice.
Epidemiological data from randomized control trials or intervention studies in humans after partially controlling factors (intrinsic: age, genetic background, sex, hormones; extrinsic: nutrition, exercise, and other environmental factors) that influence bone properties assessed by pQCT, DEXA, or ultrasound [32] have proved that physical activity is associated with bone mass and strength and has positive effects on bone properties. Especially the data from people who participated in special exercise training programs showed more clearly beneficial effects of exercise on bone mass or bone strength [15, 16]. More interestingly, voluntary leisure-time physical activity also showed positive effects on both cortical thickness and trabecular bone after controlling for genetic background [33, 34]. In accordance with our results in obese mice, previous animal studies have also shown protective effects of exercise on trabecular bone density [18, 35]. Somewhat unexpectedly, in the present study voluntary running under the control diet had detrimental effects on bone mass and strength in both the femoral diaphysis and distal metaphysis in terms of enlarged marrow cavity. This was consistent with bone mechanical properties showing that runners had lower maximum flexure load but somewhat higher elastic modulus, meaning higher possibility of breaking. Similar findings have been reported for the rat proximal tibia after intensive treadmill running [36] and for the mouse tibia after 3-week weight-bearing running during growth [37]. A recent study showed reduced mid-tibial volumetric BMD and strength in 23-week-old female C57BL/6J mice after 1 month of voluntary wheel running [38]. This discrepancy might be explained by activated modeling or remodeling of bone during growth under continuous mechanical stimulation [39, 40]. Histomorphometric analysis [36] has proven that in young rats reduced longitudinal bone growth and bone loss under strenuous training were caused by decreased osteoblastic activity rather than a global adaptation of bone remodeling. Later on, Mori et al. [19] showed more complicated results in vivo and in vitro in 8-week-old C57BL/6J mice after voluntary climbing exercise. They showed that intermittent climbing initially increased trabecular bone volume and reduced bone resorption, partially due to downregulated marrow osteoclastogenesis and upregulated osteogenesis, while further exercise desensitized both pathways.
In general, in C57BL/6J mice from the age of 6 weeks [41, 42] trabecular volume and trabecular number decrease continuously. However, trabecular spacing, trabecular thickness, and cortical area increase up to 24 weeks of age. In our study, the voluntary running exercise lasted for 20 weeks during the growth period of the mice. Arrays of data have indicated that during the growth period physical activity imposes its effect on bone more efficiently [43].
It has been documented that BMD and BMC are powerful predictors of fracture risk [44, 45]. Interestingly, our results show positive effects of voluntary running on bone with the high-fat diet since the runners had higher trabecular BMC and BMD and smaller marrow cavity, similar to our previous twin study [34]. Consequently, both diet and exercise should be considered when optimizing the effects on body composition and bone, even though the underlying mechanisms remain partly unknown.
In conclusion, mice with obesity induced by a high-fat diet had larger and stronger femur, thicker diaphyseal cortex, but lower distal metaphyseal BMD. Voluntary running had protective effects on distal metaphyseal BMC and BMD, particularly in the high-fat diet group. These results suggest that voluntary running exercise affects bone properties in a site-specific manner and that there is a complex interaction between physical activity and obesity.
References
Rosen CJ, Bouxsein ML (2006) Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2:35–43
Gimble JM, Zvonic S, Floyd ZE, Kassem M, Nuttall ME (2006) Playing with bone and fat. J Cell Biochem 98:251–266
Hausman GJ, Hausman DB (2006) Search for the preadipocyte progenitor cell. J Clin Investig 116:3103–3106
Jebb SA, Moore MS (1999) Contribution of a sedentary lifestyle and inactivity to the etiology of overweight and obesity: current evidence and research issues. Med Sci Sports Exerc 31:S534–S541
Resnick HE, Carter EA, Aloia M, Phillips B (2006) Cross-sectional relationship of reported fatigue to obesity, diet, and physical activity: results from the Third National Health and Nutrition Examination Survey. J Clin Sleep Med 2:163–169
Williams TD, Chambers JB, Roberts LM, Henderson RP, Overton JM (2003) Diet-induced obesity and cardiovascular regulation in C57BL/6J mice. Clin Exp Pharmacol Physiol 30:769–778
Shuldiner AR (2008) Obesity genes and gene–environment–behavior interactions: recommendations for a way forward. Obesity (Silver Spring) 16:S79–S81
Gomez-Ambrosi J, Rodriguez A, Catalan V, Fruhbeck G (2008) The bone–adipose axis in obesity and weight loss. Obes Surg 18:1134–1143
Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G (2002) Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305–317
Pollock NK, Laing EM, Baile CA, Hamrick MW, Hall DB, Lewis RD (2007) Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am J Clin Nutr 86:1530–1538
Reid IR (2008) Relationships between fat and bone. Osteoporos Int 19:595–606
Janz KF, Gilmore JM, Levy SM, Letuchy EM, Burns TL, Beck TJ (2007) Physical activity and femoral neck bone strength during childhood: the Iowa Bone Development Study. Bone 41:216–222
Sone T, Imai Y, Joo YI, Onodera S, Tomomitsu T, Fukunaga M (2006) Side-to-side differences in cortical bone mineral density of tibiae in young male athletes. Bone 38:708–713
Falk B, Galili Y, Zigel L, Constantini N, Eliakim A (2007) A cumulative effect of physical training on bone strength in males. Int J Sports Med 28:449–455
Daly RM, Bass SL (2006) Lifetime sport and leisure activity participation is associated with greater bone size, quality and strength in older men. Osteoporos Int 17:1258–1267
Nurzenski MK, Briffa NK, Price RI, Khoo BC, Devine A, Beck TJ, Prince RL (2007) Geometric indices of bone strength are associated with physical activity and dietary calcium intake in healthy older women. J Bone Miner Res 22:416–424
Umemura Y, Baylink DJ, Wergedal JE, Mohan S, Srivastava AK (2002) A time course of bone response to jump exercise in C57BL/6J mice. J Bone Miner Metab 20:209–215
Hamrick MW, Skedros JG, Pennington C, McNeil PL (2006) Increased osteogenic response to exercise in metaphyseal versus diaphyseal cortical bone. J Musculoskelet Neuronal Interact 6:258–263
Mori T, Okimoto N, Sakai A, Okazaki Y, Nakura N, Notomi T, Nakamura T (2003) Climbing exercise increases bone mass and trabecular bone turnover through transient regulation of marrow osteogenic and osteoclastogenic potentials in mice. J Bone Miner Res 18:2002–2009
Banu J, Bhattacharya A, Rahman M, O’Shea M, Fernandes G (2006) Effects of conjugated linoleic acid and exercise on bone mass in young male Balb/C mice. Lipids Health Dis 5:7
Peng ZQ, Vaananen HK, Tuukkanen J (1997) Ovariectomy-induced bone loss can be affected by different intensities of treadmill running exercise in rats. Calcif Tissue Int 60:441–448
Tuukkanen J, Koivukangas A, Jamsa T, Sundquist K, Mackay CA, Marks SC Jr (2000) Mineral density and bone strength are dissociated in long bones of rat osteopetrotic mutations. J Bone Miner Res 15:1905–1911
Cobayashi F, Lopes LA, Taddei JA (2005) Bone mineral density in overweight and obese adolescents. J Pediatr (Rio J) 81:337–342
Aubertin-Leheudre M, Lord C, Labonte M, Khalil A, Dionne IJ (2008) Relationship between sarcopenia and fracture risks in obese postmenopausal women. J Women Aging 20:297–308
Galusca B, Zouch M, Germain N, Bossu C, Frere D, Lang F, Lafage-Proust MH, Thomas T, Vico L, Estour B (2008) Constitutional thinness: unusual human phenotype of low bone quality. J Clin Endocrinol Metab 93:110–117
Brahmabhatt V, Rho J, Bernardis L, Gillespie R, Ziv I (1998) The effects of dietary-induced obesity on the biomechanical properties of femora in male rats. Int J Obes Relat Metab Disord 22:813–818
Cao JJ, Gregoire BR, Gao H (2009) High-fat diet decreases cancellous bone mass but has no effect on cortical bone mass in the tibia in mice. Bone 44:1097–1104
Hamrick MW, Ferrari SL (2008) Leptin and the sympathetic connection of fat to bone. Osteoporos Int 19:905–912
Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G (2000) Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207
Hamrick MW, Della-Fera MA, Choi YH, Pennington C, Hartzell D, Baile CA (2005) Leptin treatment induces loss of bone marrow adipocytes and increases bone formation in leptin-deficient ob/ob mice. J Bone Miner Res 20:994–1001
Hamrick MW, Ding KH, Ponnala S, Ferrari SL, Isales CM (2008) Caloric restriction decreases cortical bone mass but spares trabecular bone in the mouse skeleton: implications for the regulation of bone mass by body weight. J Bone Miner Res 23:870–878
Lorentzon M, Mellstrom D, Ohlsson C (2005) Association of amount of physical activity with cortical bone size and trabecular volumetric BMD in young adult men: the GOOD study. J Bone Miner Res 20:1936–1943
Iuliano-Burns S, Stone J, Hopper JL, Seeman E (2005) Diet and exercise during growth have site-specific skeletal effects: a co-twin control study. Osteoporos Int 16:1225–1232
Ma H, Leskinen T, Alen M, Cheng S, Sipila S, Heinonen A, Kaprio J, Suominen H, Kujala UM (2009) Long-term leisure time physical activity and properties of bone: a twin study. J Bone Miner Res 24:1427–1433
Plochocki JH, Rivera JP, Zhang C, Ebba SA (2008) Bone modeling response to voluntary exercise in the hindlimb of mice. J Morphol 269:313–318
Bourrin S, Genty C, Palle S, Gharib C, Alexandre C (1994) Adverse effects of strenuous exercise: a densitometric and histomorphometric study in the rat. J Appl Physiol 76:1999–2005
Wallace JM, Rajachar RM, Allen MR, Bloomfield SA, Robey PG, Young MF, Kohn DH (2007) Exercise-induced changes in the cortical bone of growing mice are bone- and gender-specific. Bone 40:1120–1127
Warren GL, Moran AL, Hogan HA, Lin AS, Guldberg RE, Lowe DA (2007) Voluntary run training but not estradiol deficiency alters the tibial bone–soleus muscle functional relationship in mice. Am J Physiol Regul Integr Comp Physiol 293:R2015–R2026
Luu YK, Capilla E, Rosen CJ, Gilsanz V, Pessin JE, Judex S, Rubin CT (2009) Mechanical stimulation of mesenchymal stem cell proliferation and differentiation promotes osteogenesis while preventing dietary-induced obesity. J Bone Miner Res 24:50–61
Kyung TW, Lee JE, Van Phan T, Yu R, Choi HS (2009) Osteoclastogenesis by bone marrow-derived macrophages is enhanced in obese mice. J Nutr 139:502–506
Halloran BP, Ferguson VL, Simske SJ, Burghardt A, Venton LL, Majumdar S (2002) Changes in bone structure and mass with advancing age in the male C57BL/6J mouse. J Bone Miner Res 17:1044–1050
Somerville JM, Aspden RM, Armour KE, Armour KJ, Reid DM (2004) Growth of C57BL/6 mice and the material and mechanical properties of cortical bone from the tibia. Calcif Tissue Int 74:469–475
Daly RM (2007) The effect of exercise on bone mass and structural geometry during growth. Med Sport Sci 51:33–49
Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, Genant HK, Palermo L, Scott J, Vogt TM (1993) Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet 341:72–75
Kanis JA (2002) Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359:1929–1936
Acknowledgements
This study was funded by the Academy of Finland (grant 124 037). H. M. was supported by the National Graduate School of Musculoskeletal Disorders and Biomaterials, Finland. We thank Leena-Kaisa Tulla and Erkki Helkala for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors declare no conflict of interests.
Rights and permissions
About this article
Cite this article
Ma, H., Torvinen, S., Silvennoinen, M. et al. Effects of Diet-Induced Obesity and Voluntary Wheel Running on Bone Properties in Young Male C57BL/6J Mice. Calcif Tissue Int 86, 411–419 (2010). https://doi.org/10.1007/s00223-010-9346-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-010-9346-3