Abstract
Observational studies have indicated a high but heterogeneous prevalence of low bone mineral density for adult patients with cystic fibrosis. Fracture complications were also described. The objective of this study was to determine the prevalence of osteoporosis, osteopenia, and fractures among adult patients with cystic fibrosis. A systematic literature review was conducted using electronic databases. The keywords used were “cystic fibrosis [MeSH] AND bone density.” Original studies were eligible if they reported the prevalence of osteoporosis and/or osteopenia and/or fractures in adult patients with cystic fibrosis. A meta-analysis of pooled proportions was performed. Heterogeneity was tested with the Cochran Q statistic, and in the case of heterogeneity a random effect model was used. Of 117 studies, 12 were selected, i.e., that represented a total of 1055 patients. Mean age ranged from 18.5 to 32 years (median: 28.2 years). Mean body mass index ranged from 19.9 to 22.4 (median: 20.7); 53.8% were men. The pooled prevalence of osteoporosis in adults with cystic fibrosis was 23.5% (95% CI, 16.6–31.0). The pooled prevalence of osteopenia was 38% (95% CI, 28.2–48.3). The pooled prevalences of radiological vertebral fractures and nonvertebral fractures were 14% (95% CI, 7.8–21.7) and 19.7% (95% CI, 6.0–38.8), respectively. In conclusion, this systematic literature review with meta-analysis emphasized the high prevalence of osteopenia and osteoporosis in young adults with cystic fibrosis. The prevalence of fracture was also high.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Cystic fibrosis (CF) is the most common autosomal recessive disease with fatal outcome in Caucasians [1]. The incidence is about 1 of 2,000 newborns in Europe. Due to improved therapy, life expectancy has increased to more than 30 years [2]. As a consequence, patients with CF develop a number of new comorbidities including CF-associated osteoporosis. Osteoporosis is defined as a systemic skeletal disorder characterized by low bone mass, alterations of bone quality including micro architectural deterioration of bone tissue, with a consequent increase in bone fragility and fractures. Several studies have indicated a high but varying prevalence of low bone mineral density (BMD) in these patients [3–6]. Dual-energy X-ray absorptiometry (DXA) represents the gold standard for BMD measurement. The World Health Organization (WHO) has pooled reduced BMD into categories. Osteoporosis is defined as a bone density <2.5 SD of the mean BMD of a gender-matched, young healthy population. Osteopenia is an intermediate category of reduced bone density defined as a T-score between −1 and −2.5. Although these categories were created to classify postmenopausal women, they are often applied to other adult populations.
An increased fracture rate compared with healthy age-matched controls has also been described [7, 8], and rib and vertebral fractures in particular may lead to deterioration in lung function. Moreover, vertebral fractures represent a contraindication for lung transplantation.
However, these studies are of small magnitude and have shown heterogeneous results. With these issues in mind, we undertook a systematic review with meta-analysis of the published literature with the following objectives: to assess the prevalence of osteopenia, osteoporosis, and fractures among adults with CF.
Materials and Methods
Search Strategy
A systematic review of the literature was conducted in April 2008 using electronic databases (Medline, Pubmed, Cochrane) supplemented by a hand-search of reference lists, review articles, and conference abstracts in 2006, 2007, and 2008. The keywords used were “cystic fibrosis [MeSH] AND bone density,” without restriction of languages or publication dates. Titles and abstracts were scanned by one reviewer.
Inclusion and Exclusion Criteria
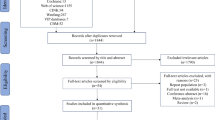
Articles were eligible (Fig. 1) if they reported the results of original or longitudinal studies, either cross-sectional studies or case–control, on the prevalence of osteoporosis and/or osteopenia and/or fractures. Only studies of adults and older adolescents with CF (diagnosed symptomatically and genetically) were included. Osteopenia and osteoporosis were collected if defined according to the WHO criteria. They were used to define osteopenia (T-score between −1 and −2.5) and osteoporosis (T-score ≤ −2.5) at any of the following sites: lumbar spine, total hip, or femoral neck. Vertebral fractures were assessed by systematic spine radiographs. Nonvertebral fractures were collected based on self-report. Articles were excluded if all the outcomes of interest were not reported or if they reported data on children. Articles were also excluded if they only contained data on CF patients on a lung transplant list or who had received a prior organ transplant.
Data Extraction
Data were extracted by one reviewer on study design and participants (number, mean age, country, and date of recruitment). Demographic data from the different studies were recorded on a standardized form. The following parameters were also recorded: body mass index (BMI), rate of δF508 mutation, corticosteroid use, age at puberty, and forced expiratory volume in 1 s (FEV1). FEV1 was measured by spirometry and is expressed as a percentage of predicted value. Mean data were collected with measures of dispersion; if median values were reported and if the number of participants was >30, these were analyzed similarly to mean values. The outcomes collected were reduced BMD (osteopenia and/or osteoporosis) and fractures (vertebral and/or nonvertebral) as absolute numbers, as available in the articles.
Quality of Publication
To assess the quality of publication, we examined patient inclusion criteria as well as the quality of the reporting. Articles were excluded if definitions of outcomes were unclear.
Statistical Analysis
Descriptive statistics are expressed as mean ± standard deviation (SD). Pooled proportion was assessed by using the arcsin-transformed proportions [9] and the method of the inverse of the variance [10]. Heterogeneity was tested with the Cochran Q statistic, and in the case of significant heterogeneity a random effect model was used. Ninety-five percent confidence intervals (CI) were calculated. Potential sources of heterogeneity (publication date of the study, mean age, corticosteroid use, country, gender ratio, BMI, rate of δF508 mutation, and mean FEV1) were investigated by subgroup analyses or meta-regression.
A sensitivity analysis was performed: the pooled prevalence was assessed n − 1 times, studies being taken out one by one. We assumed that publication bias may result in an overestimate of the prevalence in small studies (in other words, a small study had more chance to be published if it showed a high prevalence). Consequently, we explored the publication by assessing the correlation between the prevalence and the sample size (Kendall’s τ). For practical reasons, the figures exploring heterogeneity are not shown.
Results
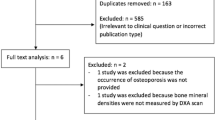
The review process is summarized in Fig. 1. Using our search strategy, 117 citations were retrieved, of which 32 articles were reviewed in their full text format. Twelve of these met the inclusion and exclusion criteria and had data available for at least one of the outcomes [3, 6, 11–20]. All of them were cross-sectional studies (Table 1); there were no longitudinal studies. A total of 1,055 patients were analyzed. The largest sample size was 191 patients (18.1% of the total) [14]. Mean age ranged from 18.5 to 32 years (median: 28.2 years). When it was provided (in nine studies), the SD of the age was about 8 years (except in Ref. 15), indicating a high heterogeneity in the samples. Mean body mass index was provided in 11 studies and ranged from 19.9 to 22.4 (median: 20.7). The SD was about 3 years (except in Ref. 15), indicating heterogeneity in the samples. Fifty-three and eight-tenths percent were men. The percentage of women ranged from 38.0 to 61.7% (median: 50.0%). Fifty-six percent of patients were homozygous for the δF508 mutation and 31% were heterozygous (data were provided in seven studies). Mean FEV1 was 54.4% (data were provided in nine studies). Data concerning corticosteroid use were available in six studies. The percentage of patients with corticosteroids ranged from 4 to 84% (median: 30.5%). Intravenous antibiotic use and age at puberty were not available in the studies.
Prevalence of Osteoporosis
For osteoporosis, 10 studies [3, 11–16, 18–20] included data relevant for this analysis, i.e., a total of 888 patients were analyzed (Table 1). The prevalence of osteoporosis ranged from 9.0 [12] to 59.1% [16]. The pooled prevalence of osteoporosis in adult CF was 23.5% (95% CI, 16.6–31.0). The forest plot is shown in Fig. 2. Significant heterogeneity was found (p < 0.001) for the prevalence of osteoporosis. The year when the studies began was suspected to be a heterogeneity factor. The meta-regression indicated that the prevalence decreased when the year of commencement increased: the regression parameter was −0.045 (SD: 0.022), with a p-value of 0.07 (Fig. 3, top left). The goodness of fit of the model was acceptable (R 2 = 0.35). From the plot representing the prevalence of osteoporosis versus the percentage of patients using corticosteroids, use of corticosteroids cannot be suspected as a heterogeneity factor. A meta-regression confirmed this (p = 0.65 and R 2 = 0.06).
Plots of heterogeneity factors. Top left: prevalence of osteoporosis according to the year studies began. Top right: prevalence of vertebral fractures according to mean age. Bottom left: prevalence of vertebral fractures according to mean body mass index. Regression lines are represented by the straight line
Prevalence of Osteopenia
For osteopenia, nine studies [3, 11–13, 15, 16, 18–20] included data relevant for this analysis, i.e., a total of 697 patients were analyzed (Table 1). The prevalence of osteopenia ranged from 12.1 [18] to 70.0% [19]. The pooled prevalence of osteopenia was 38.0% (95% CI, 28.2–48.3). The forest plot is shown in Fig. 2. Significant heterogeneity was found (p < 0.001) for the prevalence of osteopenia. As for osteoporosis, the use of corticosteroids cannot be suspected to be a heterogeneity factor (meta-regression: p = 0.91, with R 2 = 0.01).
Prevalence of Vertebral Fractures
For vertebral fractures, six studies [3, 12–14, 17, 18] included data relevant for this analysis, i.e., a total of 683 patients were analyzed (Table 1). The prevalence of vertebral fractures assessed by systematic spine radiographs ranged from 5.7 [13] to 31.3% [17]. The pooled prevalence of vertebral fractures was 14.0% (95% CI, 7.8–21.7). The forest plot is shown in Fig. 2. Significant heterogeneity was found (p < 0.001) for the prevalence of vertebral fractures. Mean age in the study was found to be a source of heterogeneity. The meta-regression indicated that the prevalence decreased when the mean age increased: the regression parameter was −0.035 (SD: 0.011), with a p-value of 0.04 (Fig. 3, top right). The goodness of fit of the model was strong (R 2 = 0.71). Mean body mass index was also suspected to be a source of heterogeneity. The meta-regression indicated that the prevalence decreased when the mean BMI increased: the regression parameter was −0.090 (SD: 0.037), with a p-value of 0.07 (Fig. 3, bottom left). The goodness of fit of the model was acceptable (R 2 = 0.59). The use of corticosteroid was not suspected to be a heterogeneity factor (meta-regression: p = 0.65, with R 2 = 0.12).
Prevalence of Nonvertebral Fractures
For nonvertebral fractures, four studies [6, 13, 15, 18] included data relevant for this analysis, i.e., a total of 553 patients were analyzed (Table 1). The prevalence of non-vertebral fractures ranged from 20.0 [15] to 38.6% [13]. The pooled prevalence of nonvertebral fractures was 19.7% (95% CI, 6.0–38.8). The forest plot is shown in Fig. 2. Significant heterogeneity was found (p < 0.001) for the prevalence of nonvertebral fractures. Potential sources of heterogeneity were not investigated due to the low number of studies on this issue.
Sensitivity Analysis
Osteoporosis
The pooled prevalence ranged from 21.1%, when the study by Ionescu et al. [16] was removed, to 25.6%, when the study by Stephenson et al. [12] was removed. Heterogeneity was always significant.
Osteopenia
The pooled prevalence ranged from 34.2% when the study by Aris et al. [19] was removed, to 42.2%, when the study by Elkin et al. [18] was removed. Heterogeneity was always significant.
Vertebral Fractures
The pooled prevalence ranged from 10.9% when the study by Rossini et al. [14] was removed, to 16.1%, when the study by King et al. [13] was removed. Heterogeneity was always significant.
Nonvertebral Fractures
The pooled prevalence ranged from 30.6%, when the study by King et al. [13] was removed, to 35.7%, when the study by Flohr et al. [15] was removed. Without the study by King et al. [13], heterogeneity was not significant (p = 0.11), as well as without the study by Flohr et al. [15] (p = 0.63).
Publication Bias
A publications bias may be suspected for osteoporosis. The prevalence of osteoporosis was negatively correlated with the sample size: studies with a small sample size had a higher prevalence. Kendall’s test led to an estimate of the correlation equal to −0.38 and a p-value of 0.13. For osteopenia and vertebral and nonvertebral fractures, prevalences were not correlated with sample size (Kendall’s test p-values were 0.40, 0.85, and 1, respectively).
Discussion
In this systematic review with meta-analysis of the published literature, the prevalence of osteoporosis was 23.5% (95% CI, 16.6–31.0) and the prevalence of osteopenia was 38.0% (95% CI, 28.2–48.3) in young adults with CF. A high prevalence of vertebral (14.0%; 95% CI, 7.8–,21.7) and nonvertebral fractures (19.7%; 95% CI 6.0–38.8) was also observed.
The designation of osteoporosis (T-score ≤ −2.5) and osteopenia (−2.5 > T-score > −1) is more meaningful than reduced BMD. Although this classification was created for postmenopausal women, it is often applied to other adult populations, although its use in men and premenopausal women is controversial. BMD measurements are usually performed at the following sites: lumbar spine (L2–L4), femoral neck, and total hip. One limitation of this systematic literature review concerned the number of articles excluded because they only expressed the results of the BMD for total body or as the mean T-score, without information on patients with osteopenia or osteoporosis. It is important to note that the prevalence of osteoporosis seemed to decrease when the year of commencement of the studies increased, but the meta-regression indicated that it was not significant. This could be due to a better management of bone disease with vitamin D adequacy, calcium supplementation, and maximization of nutritional status.
Prevalence of vertebral fractures assessed by systematic spine radiographs in the literature varied between 5.0 and 31.0% [13, 17]. Stephenson et al. [12] found that 7% of CF patients had vertebral fractures. This is comparable to that reported in untreated, postmenopausal, osteoporotic women [21, 22], despite the fact that the CF population is much younger. The method used to assess vertebral fractures varied between studies. Some of them [12, 14, 18] used the semiquantitative method described by Genant et al. [23] on lateral thoracic and lumbar spine radiographs. One of them [13] used another method, described by Eastell et al. [24], to assess vertebral fractures also on lateral thoracic and lumbar spine radiographs. Unfortunately, some of them [3, 17] used only a chest X-ray exam to assess the prevalence of vertebral deformities. These different methods used to define vertebral fracture were a limitation in this meta-analysis but cannot totally explain the controversial findings. Indeed, Grey et al. [17] and Conway et al. [3] only used a chest X-ray exam but found 31 and 26% vertebral fractures, respectively. The key differences are probably the BMI, patient age, and %FEV1. Severity of lung disease has been consistently associated with reduced BMD [3, 6, 18], which may also impact on bone quality and fracture risk. But the potential sources of heterogeneity (sample size, publication date of the study, mean age, gender ratio, BMI, rate of δF508 mutation, and mean FEV1) were investigated and it was not significant for these parameters. Use of corticosteroids was not a heterogeneity factor, but only six studies provided the percentage of patients using corticosteroids. The studies included in the present meta-analysis are observational and the use of corticosteroids surely may not be random. It may be the reason why corticosteroids do not explain heterogeneity, even though it is a known risk factor.
The purpose of the meta-regressions was to identify heterogeneity factors, but a lack of power resulted from the low number of studies. Moreover, there is a risk of false-positive results and the associations between factors and prevalences need to be confirmed in patient-level data studies.
Publication bias was explored and correlations between prevalences and sample sizes were tested. This exploration was limited by the low number of studies: the test has a poor power in this situation. A negative correlation was found for osteoporosis, and even though it was not significant, we concluded that a publication bias may be suspected.
Furthermore, it is important to note that vertebral fractures occurred particularly at the thoracic level. Rossini et al. [14] found that 95% of patients with fractures had at least one thoracic vertebral fracture. Stephenson et al. [12] found that 18 of 19 fractures occurred in the lower thoracic spine, with 74% occurring between T9 and T12, and Elkin [18] found that 21 of 23 fractures occurred in the thoracic spine. Another interesting point is the fact that prevalence of vertebral fractures decreases with increasing age. The meta-regression indicated that the prevalence decreased when the mean age increased. Indeed, we would imagine that as the CF patient ages, the bone disease and fracture rate would increase rather than decrease. Certainly the fact that the prevalence of vertebral fractures decreased with a higher BMI makes clinical sense.
Published studies included in this systematic review of the literature have documented the prevalence of nonvertebral fractures to be as high as 20% [15] to 40% [13]. The method used to assess nonvertebral fractures varied among studies. In most of them [13, 15, 18], patients were asked about previous fractures. In one study [6], patients were asked about previous fractures confirmed radiographically. It is not possible to know whether these fractures were really osteoporotic. King et al. [13] reported that all fractures were associated with significant trauma. Fractures occurred at various sites not further differentiated. For Haworth [6] the forearm was the most common site, and for Elkin et al. [18] the ribs were the most common site. This could explain the overestimation of nonvertebral fractures as a result of inclusion of rib fractures, which are often not verified radiologically.
The pathogenesis of low BMD in these patients is not fully understood. A variety of potential risk factors may contribute to the development of osteoporosis, such as calcium and vitamin D malabsorption, malnutrition, low BMI, delayed puberty, hypogonadism, glucocorticoid therapy and increased levels of osteoclast-activating cytokines such as interleukin-1, interleukin-6, and tumor necrosis factor α. As a consequence, young patients with CF may fail to reach an optimal peak bone mass [25], and some studies have shown that adult patients have accelerated bone loss, leading to early manifestation of osteoporosis [26], but the literature in this area is conflicting.
Osteopenia and osteoporosis are quite common in the adult CF population, particularly as the median age is low. The fracture prevalence is also high but the relationship between BMD fracture risk in this age group is uncertain. Vertebral fractures in patients with CF may contribute to an accelerated decline in lung function and can be a contraindication to lung transplantation. That is why it is particularly important to promote the screening of osteoporosis in these patients. It is important to focus on vitamin D adequacy, calcium supplementation, and activity, maximization of nutritional status and lung health to manage and prevent bone loss, and initiation of antifracture therapy in patients at high risk [27, 28]. Longitudinal studies in adults with CF are necessary to appreciate the prevalence and incidence of low BMD and fractures in these patients.
References
Stern RC (1997) The diagnosis of cystic fibrosis. N Engl J Med 336:487–491
Frederiksen B, Lang S, Koch C, Hoiby N (1996) Improved survival in the Danish center-treated cystic fibrosis patients: results of aggressive treatment. Pediatr Pulmonol 21:153–158
Conway SP, Morton AM, Oldroyd B et al (2000) Osteoporosis and osteopenia in adults and adolescents with cystic fibrosis: prevalence and associated factors. Thorax 55:798–804
Laursen EM, Molgaard C, Michaelsen KF, Koch C, Muller J (1999) Bone mineral status in 134 patients with cystic fibrosis. Arch Dis Child 81:235–240
Donovan DS Jr, Papadopoulos A, Staron RB et al (1998) Bone mass and vitamin D deficiency in adults with advanced cystic fibrosis lung disease. Am J Respir Crit Care Med 157:1892–1899
Haworth CS, Selby PL, Webb AK et al (1999) Low bone mineral density in adults with cystic fibrosis. Thorax 54:961–967
Aris RM, Renner JB, Winders AD et al (1998) Increased rate of fractures and severe kyphosis: sequelae of living into adulthood with cystic fibrosis. Ann Intern Med 128:186–193
Henderson RC, Specter BB (1994) Kyphosis and fractures in children and young adults with cystic fibrosis. J Pediatr 125:208–212
Stuart A, Ord JK (1994) Kendall’s advanced theory of statistics, 6th edn. Edward Arnold, London
Egger M, Davey Smith G, Altman DG (eds) (2001) Systematic reviews in health care: meta-analysis in context, 2nd edn. BMJ Books, London
Brenckmann C, Papaioannou A, Freitag A et al (2003) Osteoporosis in Canadian adult cystic fibrosis patients: a descriptive study. BMC Musculoskel Disord 4–13
Stephenson A, Jamal S, Dowdell T et al (2006) Prevalence of vertebral fractures in adults with cystic fibrosis and their relationship to bone mineral density. Chest 130:539–544
King SJ, Topliss DJ, Kotsimbos T, Nyulasi IB, Bailey M, Ebeling PR, Wilson JW (2005) Reduced bone density in cystic fibrosis:DeltaF508 mutation is an independent risk factor. Eur Respir J 25:54–61
Rossini M, Del Marco A, Dal Santo F et al (2004) Prevalence and correlates of vertebral fractures in adults with cystic fibrosis. Bone 35:771–776
Flohr F, Lutz A, App EM, Matthys H, Reincke M (2002) Bone mineral density and quantitative ultrasound in adults with cystic fibrosis. Eur J Endocrinol 146:531–536
Ionescu AA, Nixon LS, Evans WD et al (2000) Bone density, body composition, and inflammatory status in cystic fibrosis. Am J Respir Crit Care Med 162:789–794
Grey AB, Ames RW, Matthews RD, Reid IR (1993) Bone mineral density and body composition in adult patients with cystic fibrosis. Thorax 48:589–593
Elkin SL, Fairney A, Burnett S, Kemp M, Kyd P, Burgess J, Compston JE, Hodson ME (2001) Vertebral deformities and low bone mineral density in adults with cystic fibrosis: a cross-sectional study. Osteoporos Int 12:366–372
Aris RM, Ontjes DA, Buell HE, Blackwood AD, Lark RK, Caminiti M, Brown SA, Renner JB, Chalermskulrat W, Lester G (2002) Abnormal bone turnover in cystic fibrosis adults. Osteoporos Int 13:151–157
Lang SM, Fischer R, Stratakis DF, Huber RM (2004) High prevalence of osteoporosis in adult cystic fibrosis patients. Dtsch Med Wochenschr 129:1551–1555
Liberman UA, Weiss SR, Broll J et al (1995) Effect of oral alendronate on BMD and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med 333:1437–1443
Black DM, Cummings SR, Karpf DB et al (1996) Effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet 348:1535–1541
Genant HK, Jergas M, Palermo L, Nevitt M, Valentin RS, Black D, Cummings SR (1996) Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. The Study of Osteoporotic Fractures Research Group. J Bone Miner Res 11:984–996
Eastell R, Cedel SL, Wahner HW, Riggs BL, Melton LJ III (1991) Classification of vertebral fractures. J Bone Miner Res 6:207–215
Bhudhikanok GS, Wang MC, Marcus R, Harkins A, Moss RB, Bachrach LK (1998) Bone acquisition and loss in children and adults with cystic fibrosis: a longitudinal study. J Pediatr 133:18–27
Lutz A, Flohr F, Reincke M, Fischer C, App E (2001) Rapid loss of bone density in lumbar spines of adult patients with cystic fibrosis. Exp Clin Endocrinol Diabetes 109:23
Chapman I, Greville H, Ebeling P, King S Kotsimbos T, Nugent P, Player R, Topliss D, Warner J, Wilson J (2008) Intravenous zoledronate improves bone density in adults with cystic fibrosis. Clin Endocrinol (Oxford) (Epub ahead of print)
Papaioannou A, Kennedy CC, Freitag A, Ioannidis G, O’Neill J, Webber C, Pui M, Berthiaume Y, Rabin HR, Paterson N, Jeanneret A, Matouk E, Villeneuve J, Nixon M, Adachi JD (2008) Alendronate once weekly for the prevention and treatment of bone loss in Canadian adult cystic fibrosis patient (CFOS trial). Chest 134:794–800
Acknowledgements
Abbott France gave an unrestricted grant to support a meta-analysis methods workshop but played no further role in the project.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Paccou, J., Zeboulon, N., Combescure, C. et al. The Prevalence of Osteoporosis, Osteopenia, and Fractures Among Adults with Cystic Fibrosis: A Systematic Literature Review with Meta-Analysis. Calcif Tissue Int 86, 1–7 (2010). https://doi.org/10.1007/s00223-009-9316-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-009-9316-9