Abstract
The purpose of the present study was to examine the effects of vitamin K2 and risedronate on bone formation and resorption, the osteocyte lacunar system, and porosity in the cortical bone of glucocorticoid (GC)-treated rats. Forty-nine female Sprague-Dawley rats, 3 months of age, were randomized into five groups according to the following treatment schedule: age-matched control, GC administration, and GC administration with concomitant administration of vitamin K2, risedronate, or vitamin K2 + risedronate. At the end of the 8-week experiment, classical bone histomorphometric analysis was performed, and the osteocyte lacunar system and porosity were evaluated on the cortical bone of the tibial diaphysis. GC administration decreased percent cortical bone area and increased percent marrow area as a result of decreased periosteal bone formation, and increased endocortical bone erosion, and increased cortical porosity. Vitamin K2 prevented a reduction in periosteal bone formation but did not affect percent cortical bone and marrow areas. Risedronate prevented a reduction in periosteal bone formation and an increase in endocortical bone erosion, resulting in prevention of alterations in percent cortical bone and marrow areas. Both vitamin K2 and risedronate increased osteocyte density and lacunar occupancy and prevented a GC-induced increase in cortical porosity. Vitamin K2 and risedronate had additive effects on osteocyte density and lacunar occupancy and a synergistic effect on cortical porosity. The present study showed the efficacy of vitamin K2 and risedronate for bone formation and resorption, the osteocyte lacunar system, and porosity in the cortical bone of GC-treated rats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Glucocorticoid (GC)-induced osteoporosis is more evident in cancellous bone than in cortical bone [1]. However, GC-induced osteoporosis increases the risk of fractures not only at skeletal sites rich in cancellous bone (the spine) but also at those rich in cortical bone (the radius and proximal femur) [1, 2]. Eventually, fractures of the long bone in the lower extremities including hip fractures caused by GC-induced cortical osteoporosis could lead to impaired mobility and increased mortality. Therefore, cortical bone tissue should be studied in more detail in subjects treated with GCs.
GC-induced bone loss has been studied using rats receiving GCs; several preclinical studies have reported GC-induced cancellous osteopenia and lower cortical bone mass compared with age-matched controls in rats [3–10]. However, cortical bone changes induced by GC administration in rats have been less studied, probably because the influence of GCs on cortical bone mass and geometry might be modest or nonsignificant. Not only cortical geometry but also the osteocyte lacunar system and cortical porosity appear to be important factors in determining mechanical properties of cortical bone [11–14]. In particular, osteocyte apoptosis may lead to a deterioration of bone quality, with a rapid increase in the risk of fracture, because osteocytes play an important role in the maintenance of bone quality [15]. Thus, it is important to study the effect of GC administration on the osteocyte lacunar system and porosity in the cortical bone.
Risedronate and vitamin K2 are widely used in the treatment of osteoporosis in Asia, and these agents could be candidates for treatment of GC-induced osteoporosis [16]. Several preclinical studies using GC-treated rats have demonstrated that risedronate suppresses bone turnover and increases cancellous bone mass [5], while vitamin K2 attenuates reductions in periosteal bone formation and cortical bone mass [4]. However, the effects of risedronate on cortical bone formation and resorption as well as the effects of risedronate and vitamin K2 on the osteocyte lacunar system and porosity in the cortical bone remain to be studied in GC-treated rats. The purposes of the present study were to compare the effects of vitamin K2 and risedronate on bone formation and resorption, the osteocyte lacunar system, and porosity in cortical bone and to explore the benefit of a combination of vitamin K2 and risedronate in GC-treated rats.
Materials and Methods
Treatment of Animals
Fifty female Sprague-Dawley rats, 3 months of age, were purchased from Hilltop Laboratory Animals (Scottdale, PA). The animals were housed under local vivarium conditions (temperature 23.8°C and 12-hour on/off light cycle) and fed with a pelleted standard chow diet containing 1.36% calcium and 2,400 IU/kg vitamin D (Rodent Diet 8604; Harlan Teklad, Madison, WI), with free access to water. Following a 1-week adaptation period to the new environment, rats were randomized by the stratified weight method into five groups of 10 rats each according to the following treatment schedule: age-matched control, CG administration, and GC administration with concomitant administration of vitamin K2, risedronate, or vitamin K2 + risedronate. Methylprednisolone sodium succinate (Pharmacia & Upjohn Company, Kalamazoo, MI), 500 mg, was reconstituted with 15 mL bacteriostatic water and then administered as the GC, at a dose of 5.0 mg/kg body weight three times a week by subcutaneous injection. Vitamin K2 (menatetrenone; Eisai, Tokyo, Japan) was suspended in 0.1 mL of 1,2-propanediol and glycerol solution at a dosage of 30 mg/kg body weight and administered by gavage into the deep mouth three times a week. Risedronate (Eisai) was dissolved in 0.1 mL of phosphate-buffered saline solution at a dosage of 10 μg/kg body weight and then administered by subcutaneous injection five times a week. The doses of vitamin K2 and risedronate were considered to be effective in rats, in accordance with previously published data [5, 6, 17]. One rat in the GC + vitamin K2 + risedronate group was deleted from the study because of a failure of GC injections during the experiment. The body weight of rats was monitored weekly, and the total experimental period was 8 weeks. The study was carried out at Winthrop-University Hospital, and the animals were maintained according to the National Institutes of Health’s Guidelines for Care and Use of Laboratory Animals. All animal experimental protocols were approved by the Laboratory Animal Care Committee of Winthrop-University Hospital.
Preparation of Specimens
All rats were labeled with 10 mg/kg of calcein (Sigma, St. Louis, MO) injected intramuscularly 10 days and 3 days before they were killed. The animals were anesthetized with ketamine injected intraperitoneally at 80 mg/kg together with xylazine at 12 mg/kg and killed by exsanguination. The left femur and right and left tibiae were collected from every animal. The femurs were stored in a freezer (−70°C) and processed later for measurement of femoral length, bone mineral content (BMC), and bone mineral density (BMD), as described below.
The right tibiae were used for classical bone histomorphometric analysis of cortical bone; the bones were fixed overnight in 40% cold ethanol and then cut into three parts using an Isomet saw (Buehler, Lake Bluff, IL). The tibial mid-diaphyses were stained with Villanueva Osteochrome (Polysciences, Warrington, PA) for 5 days. The specimens were then dehydrated sequentially in ascending concentrations of ethanol (70%, 95%, and 100%) and xylene and then embedded in methyl methacrylate (EM Science, Gibbstown, NJ) at 4°C. Cross sections of the tibial diaphysis just proximal to the tibiofibular junction were cut at 10-μm thickness using a microtome (RM 2155; Leica, Nussloch, Germany). The sections were coverslipped with Eukitt mounting medium (Calibrated Instruments, Hawthorne, NY) for classical static and dynamic histomorphometric analyses.
The left tibiae were used for the evaluation of osteocytes, lacunae, and porosity in cortical bone; the bones were fixed overnight in 70% cold ethanol and then cut into three parts using the Isomet saw. The tibial mid-diaphyses were embedded in methyl methacrylate in the same way as above. Cross sections of the tibial diaphysis just proximal to the tibiofibular junction were cut at 5-μm thickness using a microtome and then stained with Goldner’s trichrome using a modified method [18, 19]. The sections were coverslipped with Eukitt mounting medium for evaluation of osteocytes, lacunae, and porosity in cortical bone.
Histomorphometric Analysis of Cortical Bone of the Tibial Diaphysis
A digitizing morphometric system was used to measure bone histomorphometric parameters. The system consisted of an epifluorescence microscope (Nikon E-400; OsteoMetrics, Atlanta, GA), the Osteomeasure High Resolution Color Subsystem (OsteoMetrics) coupled to an IBM computer, and a morphometry program (OsteoMetrics). The measured parameters of cortical bone were total tissue area and cortical bone area, as well as periosteal and endocortical bone surface (BS, perimeter), single and double labeling surface (sLS and dLS, respectively), interlabel width, and endocortical eroded surface (ES) in accordance with the method of Chen et al. [20]. These data were used to calculate marrow area, percent cortical bone area, and percent marrow area, as well as periosteal and endocortical mineralizing surface/BS (MS/BS) [(sLS/2 + dLS)/BS], mineral apposition rate (MAR), bone formation rate/BS (BFR/BS), and endocortical ES/BS.
Measurements of Osteocyte and Lacunar Density, Lacunar Occupancy, and Cortical Porosity
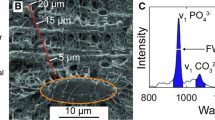
The same digitizing morphometric system was used to measure the osteocyte lacunar system and cortical porosity. The measured parameters were cortical bone area, porotic area, osteocyte number, and lacunar number in accordance with the method of Power et al. (Fig. 1) [18]. These data were used to calculate osteocyte density (total osteocyte number/cortical bone area, n/mm2), lacunar density (total lacunar number/cortical bone area, n/mm2), lacunar occupancy (total viable osteocyte number/total lacunar number, %), and cortical porosity (total canal area/cortical bone area x 100, %).
Osteocyte, lacuna, and porosity in the cortical bone of the tibial diaphysis Porotic area, osteocyte number, and lacunar number were determined in accordance with the method of Power et al. [18]
Measurements of Femoral Length, BMC, and BMD
The length of the whole left femur was measured with a dial caliper. Then, bone area, BMC, and BMD of the whole femur were determined by dual-energy X-ray absorptiometry (DXA) using the QDR-4500 Plus (Hologic, Bedford, MA). The instrument was adapted for an ultraresolution mode, with a line spacing of 0.0254 cm, resolution of 0.0127 cm, and collimation of 0.9 cm diameter. The bone was placed in a Petri dish, and to simulate soft-tissue density, tap water was poured around the bones to a depth of 1 cm. BMC and bone area were measured, and BMD of this area was calculated by dividing BMC by bone area. The coefficient of variation of these measurements at our laboratory was <1.0% [21].
Statistical Analysis
All data were expressed as means and standard deviation (SD). Comparisons of data among the groups were performed by analysis of variance (ANOVA) with Fisher’s protected least significant difference (PLSD) test. Two-way factorial ANOVA was used to examine the effects of vitamin K2 and risedronate, using the data from the GC, GC + vitamin K2, GC + risedronate, and GC + vitamin K2 + risedronate groups. All statistical analyses were performed using the StatView J-5.0 program (SAS Institute, Cary, NC) on a Macintosh (Cupertino, CA) computer. A significance level of P < 0.05 was used for all comparisons.
Results
Body Weight, Femoral Length, BMC, and BMD (Table 1)
GC administration induced reductions in body weight and femoral BMC and BMD compared with age-matched controls. Vitamin K2 attenuated a reduction in body weight but did not affect femoral BMC and BMD. Risedronate attenuated a reduction in body weight (by ANOVA with Fisher’s PLSD test) and prevented a reduction in femoral BMD without affecting femoral BMC. Femoral length was not altered by GC and vitamin K2 or risedronate. A combination of vitamin K2 and risedronate had similar effects on femoral BMC and BMD to risedronate alone.
Histomorphometric Analysis of Cortical Bone of the Tibial Diaphysis (Tables 2 and 3)
GC administration did not affect total tissue area but decreased percent cortical bone area and periosteal bone surface and increased percent marrow area and endocortical bone surface compared with age-matched controls as a result of decreased periosteal bone formation and increased endocortical bone erosion. Vitamin K2 prevented a reduction in periosteal bone formation but did not affect total tissue area, percent cortical bone and marrow areas, and periosteal and endocortical bone surfaces. Risedronate prevented a reduction in periosteal bone formation and an increase in endocortical bone erosion, leading to prevention of a reduction in percent cortical bone area and increases in percent marrow area and endocortical bone surface. Neither vitamin K2 nor risedronate affected total tissue area and periosteal bone surface. However, the combination of vitamin K2 and risedronate had similar effects to risedronate alone.
Osteocyte and Lacunar Density, Lacunar Occupancy, and Cortical Porosity (Table 4)
GC administration tended to decrease osteocyte density (statistically not significant), did not alter lacunar density, and increased cortical porosity compared with age-matched controls. Vitamin K2 increased osteocyte density and lacunar occupancy and prevented an increase in cortical porosity. Risedronate increased osteocyte density and lacunar occupancy, did not alter lacunar density, and prevented an increase in cortical porosity. A combination of vitamin K2 and risedronate had greater effects on osteocyte density and lacunar occupancy than risedronate alone, suggesting an additive effect of the two agents on osteocyte density and lacunar occupancy. In particular, vitamin K2 and risedronate had a synergistic effect on cortical porosity (by two-way factorial ANOVA).
Discussion
The present study was conducted to test the effects of vitamin K2 and risedronate on bone formation and resorption, the osteocyte lacunar system, and porosity in the cortical bone and to clarify the benefit of a combination of vitamin K2 and risedronate in GC-treated rats. The focus of the discussion is on the influence of GC on cortical bone in rats and the differential and combined effects of vitamin K2 and risedronate on the cortical bone parameters in GC-treated rats.
It has been suggested that, unlike the effects observed in humans treated with GCs, treatment of rats with GC administration not only does not result in bone loss but may exert a protective effect on the skeleton through the inhibition of bone resorption by osteoclasts [22]. In the present study, however, GC administration decreased percent cortical bone area and periosteal bone surface and increased percent marrow area and endocortical bone surface compared with age-matched controls, as a result of decreased periosteal bone formation and increased endocortical bone erosion. These results are consistent with those in previous studies [4, 9]. Because bone erosion reflects the coupling of bone formation to bone resorption, both decreased bone formation and increased bone resorption could account for increased bone erosion. However, endocortical bone formation was not affected by GC. Thus, we confirmed that GC administration induced cortical bone loss compared with age-matched controls as a result of decreased bone formation on the periosteal surface and increased bone resorption on the endocortical surface.
Vitamin K2 is known to have an anabolic action on bone; regulation of bone formation by vitamin K2 may involve its action on the γ-carboxylation of osteocalcin and may be mediated by the steroid and xenobiotic receptor [23–27]. In the present study, vitamin K2 prevented a reduction in periosteal bone formation but did not affect total tissue area, percent cortical bone and marrow areas, and periosteal and endocortical bone surfaces. Hara et al. [4] demonstrated that vitamin K2 ameliorated a reduction in periosteal bone formation in GC-treated rats, consistent with our results. However, the effect of vitamin K2 on cortical bone mass appears to be modest or nonsignificant, as has been suggested in the literature [4, 17, 28–30].
It is known that bisphosphonates inhibit osteoclast-mediated bone resorption [31]. In the present study, risedronate prevented a reduction in periosteal bone formation and an increase in endocortical bone erosion, leading to prevention of a reduction in percent cortical bone area and increases in percent marrow area and endocortical bone surface. Thus, risedronate improved the imbalance of bone formation and resorption on the endocortical bone surface and had a protective effect on bone formation on the periosteal surface, inconsistent with the previous report showing that alendronate and risedronate suppressed periosteal bone formation in rats [32].
The mechanism for the protective effect of risedronate on periosteal bone formation remains uncertain. However, one possibility for increased periosteal bone formation following administration of risedronate might be the anabolic actions of parathyroid hormone (PTH). The effect of risedronate on the serum PTH level remains to be clarified in GC-treated rats. However, the antiresorptive agent calcitonin has been reported to increase the serum PTH level and stimulate osteoblastic bone formation in GC-treated rats [3]. Another possibility might be the action of risedronate on the osteoblast because in vitro studies have demonstrated that bisphosphonates inhibit osteocyte and osteoblast apoptosis [33] and may stimulate osteoblast proliferation and differentiation [34–36], leading to increased bone formation. However, this suggestion should be further investigated by determining the number of osteoblasts on the periosteal surface because recent reports have shown that bisphosphonates inhibit bone nodule formation at nanomolar concentrations without affecting osteoblast viability, differentiation, or apoptosis in vitro [37, 38].
According to the analysis of cortical bone of the human femoral neck and related literature [18, 39], after the death of osteocytes, which are bone remodeling regulators, osteocytes disappear from lacunae. Because lacunae are believed to act as stress concentrators within tissue of compact bone, microcracks increase within cortical bone tissue and bone remodeling is increased to repair microcracks. Increased bone remodeling stimulates recruitment of osteoblasts, which differentiate into osteocytes, resulting in increased lacunar occupancy (increased osteocyte density) and increased cortical porosity (osteoid-bearing canals). Excess GCs promote apoptosis of osteoblasts and osteocytes, suppress the production of new osteoblasts and osteoclasts, and extend the life span of preexisting osteoclasts [15], which play important roles in the pathogenesis of GC-induced osteoporosis. Increased cortical porosity following GC administration in the present study, as confirmed by a previous study [9], might be attributable to increased intracortical bone resorption. Higher doses of GC or longer duration of GC administration might be required to cause a statistically significant alteration in osteocyte density and lacunar occupancy.
Vitamin K2 increased osteocyte density and thereby increased lacunar occupancy and prevented an increase in cortical porosity. These results might be attributable to the effect of vitamin K2 on osteoblasts [40, 41] and mineralization of osteoids [23–26]. Risedronate suppressed bone remodeling and prevented an increase in cortical porosity. Risedronate also increased osteocyte density with no alteration of lacunar density and thereby increased lacunar occupancy, suggesting that it might have acted on survival of osteocytes [33, 42].
Vitamin K2 and risedronate had additive effects on osteocyte density and lacunar occupancy and a synergistic effect on cortical porosity, despite lacking any beneficial effect of a combination of the two agents on parameters obtained by classical bone histomorphometric analysis. The present study confirmed the possibility that vitamin K2 and risedronate improve the osteocyte lacunar system and porosity in the cortical bone of GC-treated rats, which could lead to improvement of cortical bone quality.
In conclusion, the present study showed that vitamin K2 prevented a reduction in periosteal bone formation, while risedronate prevented a reduction in periosteal bone formation and an increase in endocortical bone erosion. Both vitamin K2 and risedronate increased osteocyte density and lacunar occupancy and prevented an increase in cortical porosity. Vitamin K2 and risedronate had additive effects on osteocyte density and lacunar occupancy and a synergistic effect on cortical porosity. Thus, we confirmed the efficacy of vitamin K2 and risedronate for bone formation and resorption, the osteocyte lacunar system, and porosity in cortical bone and the benefit of a combination of vitamin K2 and risedronate in GC-treated rats.
References
van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C (2000) Use of oral corticosteroids and risk of fractures. J Bone Miner Res 15:993–1000
van Staa TP, Leufkens HG, Cooper C (2002) The epidemiology of corticosteroid-induced osteoporosis: a meta-analysis. Osteoporos Int 13:777–787
Furuichi H, Fukuyama R, Izumo N, Fujita T, Kohno T, Nakamuta H, Koida M (2000) Bone-anabolic effect of salmon calcitonin on glucocorticoid-induced osteopenia in rats. Bio Pharm Bull 23:946–951
Hara K, Kobayasi M, Akiyama Y (2002) Vitamin K2 (menatetrenone) inhibits bone loss induced by prednisolone partly through enhancement of bone formation in rats. Bone 31:575–581
Iwamoto J, Seki A, Takeda T, Sato Y, Yamada H, Shen CL, Yeh JK (2006) Preventive effects of risedronate and calcitriol on cancellous osteopenia in rats treated with high-dose glucocorticoid. Exp Anim 55:349–355
Iwamoto J, Seki A, Takeda T, Sato Y, Yamada H, Shen CL, Yeh JK (2006) Comparative effects of risedronate and calcitriol on cancellous bone in rats with glucocorticoid-induced osteopenia. J Nutr Sci Vitaminol 52:21–27
Nitta T, Fukushima T, Nakamuta H, Koida M (1999) Glucocorticoid-induced secondary osteopenia in female rats: a time course study as compared with ovariectomy-induced osteopenia and response to salmon calcitonin. Jpn J Pharmacol 79:379–386
Noa M, Mendoza S, Mas R, Mendoza N, Leon F (2004) Effect of D-003, a mixture of very high molecular weight aliphatic acids, on prednisolone-induced osteoporosis in Sprague-Dawley rats. Drugs R D 5:281–290
Ortoft G, Oxlund H (1996) Qualitative alterations of cortical bone in female rats after long-term administration of growth hormone and glucocorticoid. Bone 18:581–590
Tanaka Y, Nakamura T, Nishida S, Suzuki K, Takeda S, Sato K, Nishii Y (1996) Effects of a synthetic vitamin D analog, ED-71, on bone dynamics and strength in cancellous and cortical bone in prednisolone-treated rats. J Bone Miner Res 11:325–336
Saha S, Hayes WC (1977) Relations between tensile impact properties and microstructure of compact bone. Calcif Tissue Res 24:65–72
Currey JD (1979) Changes in the impact energy absorption of bone with age. J Biomech 12:459–469
Dickenson RP, Hutton WC, Stott JR (1981) The mechanical properties of bone in osteoporosis. J Bone Joint Surg Br 63:233–238
Epstein S (2007) Is cortical bone hip? What determines cortical bone properties? Bone 41:S3–S8
Manolagas SC (2000) Corticosteroids and fractures: a close encounter of the third cell kind. J Bone Miner Res 15:1001–1005
Nawata H, Soen S, Takayanagi R, Tanaka I, Takaoka K, Fukunaga M, Matsumoto T, Suzuki Y, Tanaka H, Fujiwara S, Miki T, Sagawa A, Nishizawa Y, Seino Y (2005) Guidelines on the management and treatment of glucocorticoid-induced osteoporosis of the Japanese Society for Bone and Mineral Research (2004). J Bone Miner Metab 23:105–109
Iwamoto J, Yeh JK, Takeda T (2003) Effect of vitamin K2 on cortical and cancellous bones in orchidectomized and/or sciatic neurectomized rats. J Bone Miner Res 18:776–783
Power J, Noble BS, Loveridge N, Bell KL, Rushton N, Reeve J (2001) Osteocyte lacunar occupancy in the femoral neck cortex: an association with cortical remodeling in hip fracture cases and controls. Calcif Tissue Int 69:13–19
Bell KL, Loveridge N, Power J, Rushton N, Reeve J (1999) Intracapsular hip fracture: increased cortical remodeling in the thinned and porous anterior region of the femoral neck. Osteoporos Int 10:248–257
Chen MM, Yeh JK, Aloia JF, Tierney JM, Sprintz S (1994) Effect of treadmill exercise on tibial cortical bone in aged female rats: a histomorphometry and dual energy X-ray absorptiometry study. Bone 15:313–319
Prakasam G, Yeh JK, Chen MM, Castro-Magana M, Liang CT, Aloia JF (1999) Effects of growth hormone and testosterone on cortical bone formation and bone density in aged orchiectomized rats. Bone 24:491–497
Shen V, Birchman R, Liang XG, Wu DD, Lindsay R, Dempster DW (1997) Prednisolone alone, or in combination with estrogen or dietary calcium deficiency or immobilization, inhibits bone formation but does not induce bone loss in mature rats. Bone 21:345–351
Hauschka PV, Lian JB, Cole DEC, Gundberg CM (1989) Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev 69:990–1047
Koshihara Y, Hoshi K (1997) Vitamin K2 enhances osteocalcin accumulation in the extracellular matrix of human osteoblasts in vitro. J Bone Miner Res 12:431–438
Shearer MJ (1995) Vitamin K. Lancet 345:229–234
Vermeer C, Jie KSG, Knapen MHJ (1995) Role of vitamin K in bone metabolism. Annu Rev Nutr 15:1–22
Tabb MM, Sun A, Zhou C, Grun F, Errandi J, Romero K, Pham H, Inoue S, Mallick S, Lin M, Forman BM, Blumberg B (2003) Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem 278:43919–43927
Kobayashi M, Hara K, Akiyama Y (2004) Effects of vitamin K2 (menatetrenone) and alendronate on bone mineral density and bone strength in rats fed a low-magnesium diet. Bone 35:1136–1143
Otomo H, Sakai A, Ikeda S, Tanaka S, Ito M, Phipps RJ, Nakamura T (2004) Regulation of mineral-to-matrix ratio of lumbar trabecular bone in ovariectomized rats treated with risedronate in combination with or without vitamin K2. J Bone Miner Metab 22:404–414
Shiraishi A, Higashi S, Masaki T, Saito M, Ito M, Ikeda S, Nakamura T (2002) Comparison of alfacalcidol and menatetrenone for the treatment of bone loss in an ovariectomized rat model of osteoporosis. Calcif Tissue Int 71:69–79
Rogers MJ, Frith JC, Luckman SP, Coxon FR, Benford HL, Monkkonen J, Auriola S, Chilton KM, Russell RG (1999) Molecular mechanism of action of bisphosphonate. Bone 24(5 Suppl):73S–79S
Iwata K, Li J, Follet H, Phipps RJ, Burr DB (2006) Bisphosphonates suppress periosteal osteoblast activity independently of resorption in rat femur and tibia. Bone 39:1053–1058
Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T (1999) Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J Clin Invest 104:1363–1374
Giuliani N, Pedrazzoni M, Negri G, Passeri G, Impicciatore M, Girasole G (1998) Bisphosphonates stimulate formation of osteoblast precursors and mineralized nodules in murine and human bone marrow cultures in vitro and promote early osteoblastogenesis in young and aged mice in vivo. Bone 22:455–461
Im GI, Qureshi SA, Kenney J, Rubash HE, Shanbhag AS (2004) Osteoblast proliferation and maturation by bisphosphonates. Biomaterials 25:4105–4115
von Knoch F, Jaquiery C, Kowalsky M, Schaeren S, Alabre C, Martin I, Rubash HE, Shanbhag AS (2005) Effects of bisphosphonates on proliferation and osteoblast differentiation of human bone marrow stromal cells. Biomaterials 26:6941–6949
Idris AI, Rojas J, Greig IR, Van’t Hof RJ, Ralston SH (2008) Aminobisphosphonates cause osteoblast apoptosis and inhibit bone nodule formation in vitro. Calcif Tissue Int 82:191–201
Orriss IR, Utting JC, Key ML, Brandao-Burch A, Colston K, Arnett TR (2007) Zoledronate potently inhibits the growth, function and survival of normal rat osteoblasts. Calcif Tissue Int 80(Suppl 1):S84
Currey JD (2003) The many adaptations of bone. J Biomech 36:1487–1495
Igarashi M, Yogiashi Y, Mihara M, Takada I, Kitagawa H, Kato S (2007) Vitamin K induces osteoblast differentiation through pregnane X receptor-mediated transcriptional control of the Msx2 gene. Mol Cell Biol 27:7947–7954
Koshihara Y, Hoshi K, Okawara R, Ishibashi H, Yamamoto S (2003) Vitamin K stimulates osteoblastogenesis and inhibits osteoclastogenesis in human bone marrow cell culture. J Endocrinol 176:339–348
Follet H, Li J, Phipps RJ, Hui S, Condon K, Burr DB (2007) Risedronate and alendronate suppress osteocyte apoptosis following cyclic fatigue loading. Bone 40:1172–1177
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iwamoto, J., Matsumoto, H., Takeda, T. et al. Effects of Vitamin K2 and Risedronate on Bone Formation and Resorption, Osteocyte Lacunar System, and Porosity in the Cortical Bone of Glucocorticoid-Treated Rats. Calcif Tissue Int 83, 121–128 (2008). https://doi.org/10.1007/s00223-008-9146-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-008-9146-1