Abstract
Most studies that have investigated the anabolic effects of parathyroid hormone (1-84) (PTH) or PTH fragments on the skeleton of ovariectomized (OVX) rats have evaluated the short-term effects of high-dose PTH(1-34) in young animals. This study used densitometry, histomorphometry, and biomechanical testing to evaluate the effects of 12-month daily treatment with low-dose PTH (15 or 30 μg/kg) in rats that were 10 months old at baseline, 4 months after OVX. Bone mineral density (BMD) and bone strength were reduced substantially in control OVX rats. The 15 μg/kg dose of PTH restored BMD to levels similar to those in sham animals within 6 months at the lumbar spine, distal and central femur, and whole body and maintained the BMD gain from 6 to 12 months. The 30 μg/kg dose produced greater effects. Both PTH doses normalized the trabecular bone volume-to-total volume ratio (BV/TV) at lumbar vertebra 3 but not at the proximal tibia (where baseline BV/TV was very low), solely by increasing trabecular thickness. PTH dose-dependently increased bone formation by increasing the mineralizing surface, but only the 30 μg/kg dose increased resorption. PTH increased cortical BMD, area, and thickness, primarily by increasing endocortical bone formation, and restored all measures of bone strength to levels similar to those in sham animals at all skeletal sites. PTH increased bone mass safely; there was no osteoid accumulation, mineralization defect, or marrow fibrosis and there were no abnormal cells. Thus, long-term PTH therapy normalized bone strength in the aged OVX rat, a model of postmenopausal osteoporosis, through increased bone turnover and enhanced formation of both trabecular and cortical bone.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
When administered by daily injection, full-length human parathyroid hormone (1-84) (PTH) or its N-terminal fragments are potent anabolic agents in the skeleton [1, 2]. The resultant increases in bone mass are usually paralleled by increases in strength at both trabecular and cortical bone sites in animals [3–6]. These positive effects on the skeleton likely underlie the observed reduction in the incidence of fractures in humans treated with these peptides [7, 8].
The vast majority of studies that have investigated the effects of PTH or its analogs on the skeletons of rats have evaluated the effects of teriparatide, the human PTH(1-34) fragment, in young animals (<6 months old) with a high rate of bone modeling. Erben [9] reported that in female rats >9 months old, longitudinal bone growth has essentially ceased and bone remodeling processes predominate over modeling. Thus, ovariectomized (OVX) rats >9 months old represent a better model for assessing the effects of agents intended to treat human postmenopausal bone loss [10]. In most studies of teriparatide, treatment typically was initiated soon after ovariectomy, one very large dose of the peptide was tested, and the effects were followed for relatively short periods, typically <3 months [1]. Notable exceptions were 9- and 12-month studies of teriparatide and its analog SDZ PTS 893 [11–13]. Very few studies have investigated the effects of treatment with PTH, and similar to most studies with teriparatide, all studies with PTH have been of relatively short duration. Those studies have shown that PTH and teriparatide not only have similar potency at the PTH-1 receptor in vitro [14] but also increase bone mass and strength with similar efficacy in young rats [3–5, 15].
We describe the results of an in-depth investigation of the effects of daily treatment with PTH on bone turnover, mass, architecture, and strength at trabecular and cortical bone sites of 10-month-old rats that were OVX 4 months earlier. This study was conducted using the guidelines of the U.S. Food and Drug Administration (FDA) for animal testing of therapies intended for the treatment of osteoporosis. These require evaluation of 12 months of treatment at a dose considered optimal in that species and a higher dose to assess safety [16].
Materials and Methods
Animals, Diets, and Experimental Design
This study was performed at Charles River Preclinical Services (Senneville, Quebec, Canada), in accordance with the Good Laboratory Practice Regulations of the FDA. Virgin female Sprague-Dawley rats (Charles River, St. Constant, Quebec, Canada) were obtained at 5 months of age and allowed to acclimate to the facility for 1 month before study initiation and surgical procedures. They were housed individually under a 12 hour:12 hour light:dark cycle and provided throughout the study with water and PMI (Purina Mills, Incorporated) Certified Rodent Laboratory Chow FK15 (∼20 g/day). The rats were subjected to either bilateral ovariectomy or a sham operation. Surgical procedures as well as periodic densitometric scans and blood sampling were performed under isoflurane anesthesia. To maintain a comparable body weight between groups, OVX rats were provided with ∼17.5 g food/day following surgery.
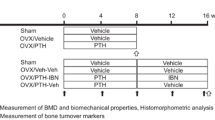
The rats were divided into two groups of sham-operated and four groups of OVX animals and left untreated for 4 months to allow bone loss to occur. One group of sham and one group of OVX rats (n = 10/group) were killed as baseline controls at 10 months of age. The remaining animals (n = 30/group) received vehicle or recombinant human PTH (15 or 30 μg/kg) by daily subcutaneous (s.c.) injection for 12 months. The 15 μg/kg dose was selected as being optimal for reversal of OVX-induced bone loss in rats based on a preliminary 28-day study [5]. The 30 μg dose assessed the safety of a higher dose and provided a dose response. To label bone-forming surfaces, each animal was injected s.c. with calcein (8 mg/kg) at 10 and 3 days before death. Animals were killed by exsanguination from the abdominal aorta following anesthesia by carbon dioxide inhalation. The right tibia and lumbar vertebra 3 (L3) were collected, cleaned of excess soft tissue, and placed in 70% ethanol for histomorphometric analysis. The right femur and L4 were cleaned of excess tissue and stored at −20°C prior to densitometry and biomechanical testing.
Biochemical Markers of Bone Turnover
Blood samples were collected from the orbital sinus before surgery, following the 4-month bone depletion period, and after 6 and 12 months of treatment. Urine was collected overnight (at least 16 hours) at the same times. Serum osteocalcin was analyzed by radioimmunoassay (Diagnostic Systems Laboratories, Webster, TX). Urine deoxypyridinoline (DPD) was quantified by high-performance liquid chromatography and normalized to urine creatinine concentrations.
Bone Densitometry
Bone mineral density (BMD) of the whole body, right femur (proximal, central, and distal regions), and vertebrae L1-L4 was measured in vivo in each animal before surgery, at the end of the 4-month bone depletion period, and after 6 and 12 months of treatment by dual-energy X-ray absorptiometry (DXA) using a QDR 2000 plus densitometer (Hologic, Bedford, MA).
Prior to the three-point bending test of the central femur, BMD, bone mineral content (BMC), and geometric properties (i.e., bone area, cross-sectional moment of inertia [CSMI], and periosteal length) were measured at the expected breaking point by peripheral quantitative computed tomography (pQCT; XCT Research SA bone scanner, version 5.40B; Stratec, Dietzenbach, Germany) using Cortmode 2, threshold 0.930 1/cm. BMD, BMC, and bone area were quantified by pQCT at the midsection of L4 using Cortmode 2, Peelmode 2, threshold 0.930 1/cm with the LOOP option and by DXA at the proximal femur.
Bone Histomorphometry
Specimens were dehydrated and defatted prior to embedding in methyl methacrylate and sectioning. Sections from each specimen were left unstained for epifluorescence microscopy or stained with toluidine blue or modified Goldner’s. The effects of OVX and PTH treatment on bone architecture and turnover were assessed in trabecular bone at L3 and the proximal tibial metaphysis and in cortical bone at the tibial diaphysis. Measurements were performed with a microscope attached to a semiautomatic system (BioQuant TCW; R&M Biometrics, Nashville, TN). Histomorphometric variables were measured and calculated as described previously [17].
Bone Biomechanics
Biomechanical testing was performed with the MTS 858 mini Bionix servohydraulic test system, with data collected by Testworks (version 3.8A) for TestStar II (version 4.0c) to produce load-displacement curves and bone fragility data (MTS Corporation, Minneapolis, MN) [18]. After completion of the three-point bending test, the femur was cut approximately 9 mm distal to the lesser trochanter for the femoral neck shear test. Prior to compression testing of L4, the vertebral arch, end plates, and spinous processes were removed to obtain a specimen with planoparallel ends. Load cells of 2.5 kN (three-point bending of the central femur and femoral neck shear at a rate of 1 mm/second) and 15 kN (compression of L4 at a load rate of 20 mm/minute) were used. The results were used to calculate a variety of bone strength parameters, including peak load, stiffness, work to failure, ultimate stress, modulus, and toughness at L4 and the central femur and peak load, stiffness, and work to failure at the femoral neck.
Statistical Analysis
Data, reported as mean ± standard error (SE), were subjected to analysis of variance followed by Fisher’s protected least significant difference test to determine the significance of differences between groups (Statview version 5.0; SAS Institute, Cary, NC).
Results
In-Life Phase
Three deaths occurred during the treatment period, two in the sham-vehicle group and the third in the OVX-15 μg/kg group. All deaths were considered unrelated to treatment. The modest dietary restriction ensured that OVX animals were not significantly heavier than sham-operated ones. Treatment with PTH did not affect body weight. At the end of the study, animals in the sham-vehicle, OVX-vehicle, OVX-15 μg/kg, and OVX-30 μg/kg groups weighed 479 ± 16, 500 ± 9, 499 ± 8, and 491 ± 11 g, respectively.
Biochemical Markers of Bone Turnover
Serum levels of the bone formation marker osteocalcin decreased in sham and OVX rats during the 4-month bone depletion period, but the decrease was much greater in sham animals (Fig. 1). Osteocalcin levels continued to decline in OVX-vehicle rats but remained significantly higher than in sham rats during the treatment phase of the study. PTH treatment caused a significant, dose-related increase in osteocalcin levels above OVX-vehicle controls after both 6 and 12 months of treatment, but the largest difference was observed at 6 months. Urinary excretion of the bone resorption marker DPD decreased markedly in sham, but not in OVX, rats during the bone depletion phase (Fig. 1). The decrease in osteocalcin and DPD in sham animals during the 4-month bone depletion phase likely reflects skeletal maturation in 10-month-old rats. DPD excretion subsequently decreased in the OVX controls during the treatment phase such that levels were no different from those of the sham-vehicle group at 12 months. When compared with OVX-vehicle controls, the 15 μg/kg dose of PTH had no effect on bone resorption whereas the 30 μg/kg dose maintained DPD excretion at a level significantly greater than in all other groups.
Densitometry by DXA
BMD decreased during the 4 months following OVX at predominantly trabecular bone sites (lumbar spine, distal femur) but tended to increase at skeletal sites containing a greater proportion of cortical bone (whole body, central femur) (Fig. 2). However, because of a greater increase in BMD in the sham-vehicle group at the central femur and whole body, BMD was significantly lower at all skeletal sites in OVX rats when treatment was initiated. BMD declined in the OVX-vehicle group at all skeletal sites during the treatment phase. In the sham-vehicle group, BMD decreased significantly between 6 and 12 months at whole body, lumbar spine, and distal femur (P < 0.001) but not at the central femur (P = 0.58). However, by 12 months the difference in BMD between the sham-vehicle and OVX-vehicle groups remained significant at all skeletal sites except whole body (Fig. 2).
Effect of daily s.c. injection of PTH (15 or 30 μg/kg) or vehicle for 12 months on BMD measured by DXA in the whole body (A), central femur (B), lumbar spine (L1-L4) (C), and distal femur (D) of OVX rats. Surgery was performed at month -4 and treatment started at month 0. Values are means ± SE (n = 18–21/group). a-c P < 0.05: significance of difference from sham-vehicle, OVX-vehicle, or OVX-15 μg/kg group, respectively.
Treatment with PTH resulted in a dose-related increase in BMD. By 6 months, the 15 μg/kg dose had restored BMD to levels similar to or significantly greater than those observed in sham-vehicle rats at all skeletal sites (Fig. 2). These increases in BMD were maintained or extended between 6 and 12 months. The 30 μg/kg dose increased BMD to levels significantly greater than those in sham-vehicle controls at most skeletal sites at 6 and 12 months. In PTH-treated animals, there was no significant decrease in BMD between 6 and 12 months at any site, in contrast to the age-related decrease observed in sham-vehicle animals during that time.
Trabecular Bone Histomorphometry
At L3, trabecular bone volume-to-total volume ratio (BV/TV) was 23% and 36% lower in OVX than in sham rats at baseline and at the end of the study, respectively (Fig. 3). The decrease in BV/TV was the result of lower trabecular number (Tb.N) and thickness (Tb.Th) and was associated with a higher trabecular separation (Tb.Sp) that was significant at the end of the study (Table 1). Similar OVX-induced changes were observed at the proximal tibial metaphysis, but the magnitude of the decrease in BV/TV was much larger (Table 2). Thus, BV/TV in the proximal tibial metaphysis was very low (2.0%) in the OVX-vehicle group at the end of the study, 16 months after OVX.
Effect of daily s.c. injection of PTH (15 or 30 μg/kg) or vehicle for 12 months on BV/TV, BFR/BS, and ES/BS in trabecular bone of L3 of OVX rats. Values are means ± SE. n = 10/group. a-e P < 0.05: significance of difference from sham-baseline, OVX-baseline, sham-vehicle, OVX-vehicle, or OVX-15 μg/kg group, respectively.
Increased bone turnover, characteristic of estrogen deficiency, occurred in OVX rats at both L3 and the proximal tibial metaphysis. Surface-referent bone formation rate (BFR/BS) was significantly higher in OVX rats than in respective sham controls at baseline but not at the end of the study (Fig. 3, Table 1). The higher BFR/BS was caused primarily by increased mineralizing surface (MS/BS). The increase in bone formation in OVX rats was usually associated with higher osteoid thickness (O.Th) and surface (OS/BS) and a longer mineralization lag time (Mlt), although these were significant only at the end of the study. Bone resorption, as assessed by eroded surface (ES/BS) and osteoclast surface (Oc.S/BS), was also higher in OVX rats, but the largest differences occurred at baseline (Fig. 3, Tables 1 and 2).
At L3, both doses of PTH restored BV/TV to the levels seen in sham controls (Fig. 3). The increase in BV/TV was solely the result of increased Tb.Th since Tb.N did not change (Table 1). Treatment with PTH caused significant dose-related increases in MS/BS, BFR/BS, ES/BS, and Oc.S/BS, although when compared with OVX-vehicle controls only the increase in MS/BS was significant with the 15 μg/kg dose (Fig. 3, Table 1). Mineral appositional rate (MAR) was unaffected by PTH treatment. Significant dose-related decreases in most osteoid measures and in Mlt were noted in PTH-treated animals (Table 1).
At the proximal tibial metaphysis, treatment with PTH caused a dose-related increase in BV/TV and Tb.Th (Table 2). Similar to L3, there was no significant change in Tb.N. However, in contrast to findings at L3, treatment with PTH was unable to restore BV/TV to sham-baseline levels but, because of the lower BV/TV in the sham-vehicle group, high-dose PTH increased BV/TV to a level similar to that in sham-vehicle rats. PTH also caused a significant dose-related increase in BFR/BS, again solely a result of increased MS/BS. PTH treatment had no significant effect on any osteoid measure or on Mlt and no consistent effects on Oc.S/BS or ES/BS (Table 2).
Cortical Bone Histomorphometry
At baseline, there were nonsignificant trends for periosteal (Ps) and endocortical (Ec) length (Lgt) and tissue (T.Ar) and cortical (Ct.Ar) area to be higher at the tibial diaphysis of OVX rats, but there was no effect on cortical thickness (Ct.Th) or marrow area (Ma.Ar) (Table 3). OVX was associated with significantly higher or a strong propensity toward higher Ps and Ec MS/BS and BFR/BS. In contrast, the increase in T.Ar in the OVX-vehicle group at study end was significant. Although Ct.Ar was the same, T.Ar and Ma.Ar were significantly higher and Ct.Th was significantly lower in the OVX-vehicle than in the sham-vehicle group (Table 3).
While there were nonsignificant trends for dose-related increases in Ps.Lgt and T.Ar, PTH treatment resulted in significantly greater (15–33%) Ct.Ar and Ct.Th relative to OVX-vehicle controls (Table 3). Moreover, with the 30 μg/kg dose, Ct.Ar and Ct.Th were increased to levels that significantly exceeded those in the sham-vehicle group. The increases in Ct.Ar and Ct.Th resulted in significant decreases in Ec.Lgt and Ma.Ar, which were maintained at sham-vehicle control levels. Bone formation was not significantly higher on the Ps and Ec surfaces of PTH-treated rats compared with OVX-vehicle controls, but the increases with the 30 μg/kg dose were significantly greater than in the sham-vehicle group on the Ec surface (Table 3).
Biomechanical Testing
There were no significant effects of OVX on bone area measured by pQCT or DXA immediately prior to biomechanical testing at any skeletal site either at baseline or at the end of the study (Fig. 4). In contrast, relative to sham controls, in OVX rats significant progressive decreases in BMC and BMD occurred at L4 (18% and 26%) and the femoral neck (9% and 18%) at baseline and at the end of the study, respectively. At the central femur, a significant decrease (9%) in BMC was observed only in OVX rats at the end of the study. The magnitude of the changes in BMD was generally similar to or slightly less than that of BMC (Fig. 4). Geometric properties at the central femur (CSMI, Ps.Lgt) were similar to or slightly greater in OVX than in sham animals, but the differences were seldom significant (Table 4).
Effects of daily s.c. injection of vehicle or PTH (15 or 30 μg/kg) for 12 months on BMD, BMC, and bone area measured by pQCT at L4 and central femur and by DXA at femoral neck of OVX rats. Values are means ± SE. n = 9–10/group. a-e P < 0.05: significance of difference from sham-baseline, OVX-baseline, sham-vehicle, OVX-vehicle, or OVX-15 μg/kg group, respectively.
Significant decreases in all parameters of bone strength were noted at baseline at L4 of OVX rats (Fig. 5, Table 4). However, because of the decrease in all measures of bone strength in sham animals during the treatment phase, only ultimate stress was significantly lower in the OVX-vehicle than in the sham-vehicle group at the end of the study. With the exception of a lower stiffness at the femoral neck of OVX rats at the end of the study, there were no significant differences between control sham and OVX animals in any strength parameter at the femur at either time point (Fig. 5, Table 4).
Effects of daily s.c. injection of vehicle or PTH (15 or 30 μg/kg) for 12 months on bone strength as assessed by peak load and stiffness at L4, central femur, and femoral neck of OVX rats. Values are means ± SE. n = 9–10/group. a-e P < 0.05: significance of difference from sham-baseline, OVX-baseline, sham-vehicle, OVX-vehicle, or OVX-15 μg/kg group, respectively.
Treatment with PTH increased bone area at all skeletal sites, although the increases were significant only at the central femur and femoral neck; the mean 8% higher area at L4 with the 30 μg/kg dose was not significant (Fig. 4). Dose-related increases in BMC and BMD occurred at all skeletal sites in PTH-treated animals, although because of the higher area, the increases in BMC were generally greater than those in BMD. At L4 and the femoral neck, the lower dose of PTH restored BMC to the level observed in the sham-baseline group, whereas at the central femur, the 15 μg/kg dose increased BMC to a level that was significantly greater than in the sham-baseline group. The 30 μg/kg dose resulted in a BMC that was significantly greater than at baseline at the central femur and femoral neck but not at L4. The effects of PTH treatment on BMD were generally smaller than those on BMC because of the greater bone area. For example, relative to OVX-vehicle controls, the increases in BMC with the 30 μg/kg dose of PTH were 66%, 29%, and 29% at L4, central femur, and femoral neck, respectively, whereas the increases in BMD were 54%, 3%, and 27%, respectively (Fig. 4). There were no significant effects of PTH treatment on geometric properties (CSMI, Ps.Lgt) at the central femur (Table 4).
Treatment of OVX rats with PTH resulted in significant, dose-related increases in all bone strength measures at L4 and prevented the progressive decreases in strength observed in the OVX-vehicle group. With the exception of a nonsignificantly higher modulus with 15 μg/kg, both doses of PTH increased bone strength at L4 to levels that were significantly greater than in sham-vehicle controls. The 30 μg/kg dose restored all bone strength parameters to levels that were similar to or significantly greater than those observed in the sham-baseline group (Fig. 5, Table 4). At the central femur, PTH treatment resulted in a significant, dose-related increase in peak load, stiffness, ultimate stress, and modulus, whereas increases in work to failure and toughness were not significant. All strength parameters at the central femur of rats receiving the 30 μg/kg dose were similar to or significantly greater than those observed in the sham-baseline group (Fig. 5, Table 4). At the femoral neck, peak load and stiffness were significantly greater in rats given the 30 μg/kg dose compared with OVX-vehicle controls. However, only stiffness was significantly greater in rats receiving 15 μg/kg. There was no consistent effect of PTH on work to failure. As observed at the other skeletal sites, PTH treatment increased all measured strength parameters to levels that were similar to or significantly greater than those in sham-baseline rats (Fig. 5, Table 4).
Safety of PTH Treatment on Bone
The newly formed trabecular and cortical bone displayed normal lamellar structure. There was no osteoid accumulation or mineralization defect. Indeed, O.Th, OS/BS, and Mlt were usually significantly lower at both L3 and the proximal tibial metaphysis of PTH-treated rats compared with OVX-vehicle controls (Tables 1 and 2). Moreover, there was no evidence of fibrosis or abnormal cells in the marrow or on the bone surfaces at any site examined by histomorphometry either at baseline or following 12 months of daily treatment with PTH (not shown).
Discussion
The anabolic effects on bone of injected bovine parathyroid extract were first described in rats by Selye [19], but it was the availability of synthetic teriparatide in the 1970s that stimulated renewed study of the bone-building potential of PTH and related peptides for the treatment of osteoporosis [14, 20, 21]. Since then, the effects of PTH, teriparatide, or other N-terminal fragments and analogs of PTH or PTH-related protein (PTHrP) have been widely studied in normal rats and in the OVX rat model of osteoporosis [3, 5, 12, 21–25].
This study determined the effects of 12 months of daily treatment of OVX rats with PTH on bone mass, structure, and strength at multiple skeletal sites. Ten-month-old rats were selected because remodeling processes prevail over modeling, making them a better model of human osteoporosis [9, 10]. This ensured that the study could be completed within their normal life span; only three rats died during the 12-month treatment period. In addition, 4 months was allowed to elapse after surgery to ensure that marked bone loss had occurred when treatment was started.
The selected doses of PTH were based on an earlier study [5] and are smaller than the ≥40 μg/kg doses typically used in studies with teriparatide or other fragments and analogs of PTH or PTHrP [21–27]. The 15 and 30 μg/kg doses of PTH correspond on a molar basis to about 6 and 12 μg/kg of teriparatide. The comparable 12-month study of teriparatide used doses of 8 and 40 μg/kg [13]. Although systemic PTH exposure was not assessed in this study, we have shown in rats that doses of 10 and 50 μg/kg result in PTH exposures 4.6- and 27-fold greater, respectively, than occur in humans given a 100 μg dose [28]. Thus, the 15 and 30 μg/kg doses should produce PTH exposures approximately seven and 15 times the clinical dose. By comparison, 5 and 75 μg/kg doses of teriparatide result in exposures approximately three- and 60-fold greater than with the clinical 20 μg dose [29].
Bone loss was well established by 4 months after OVX and continued throughout the study. It was most marked at the lumbar spine, distal femur, and proximal tibia, which contain a greater proportion of trabecular bone. BMD was also lower in OVX than in sham rats at the central femur and whole body, which are comprised of predominantly cortical bone. As occurs in women after menopause, the bone loss was accompanied by increased bone turnover, as determined by histomorphometry and biochemical markers.
PTH treatment completely reversed the effects of OVX at most skeletal sites. When assessed by DXA, the 15 μg/kg dose normalized BMD by 6 months and maintained those levels from 6 to 12 months at all skeletal sites evaluated. The 30 μg/kg dose produced more rapid and greater responses. These results extend those of the preliminary 28-day study, which showed that 15.5 μg/kg was sufficient to restore bone mass to normal whereas 155 μg/kg produced a larger effect [5]. Although the treatment regimens differed, these findings are also consistent with those from other, longer studies with PTH. Akhter et al. [30] showed that vertebral body BMD increased progressively during treatment of OVX rats with PTH (50 μg/kg) 3 or 5 days/week for 20 weeks. Iwaniec et al. [31] also reported that treatment of OVX rats with PTH (75 μg/kg) 3 days/week for 12 weeks restored distal femur BMD to levels in intact controls.
The changes observed by densitometry were also manifest when bones from PTH-treated rats were examined by histomorphometry. At L3, both doses of PTH returned BV/TV to sham levels. In contrast, at the proximal tibial metaphysis, the OVX-induced bone loss was so severe at baseline that PTH treatment was able to only partially restore BV/TV. A study with high-dose teriparatide in severely osteopenic rats showed similar results [27]. In direct contrast to studies in the iliac crest of osteoporotic women [32] and in OVX rhesus monkeys (unpublished results) in which the PTH-induced increase in BV/TV was primarily the result of increased Tb.N, the increase in BV/TV in the rat occurred solely by an increase in Tb.Th. Increases in Tb.Th have also been reported with teriparatide and SDZ PTS 893 [5, 12]. Thus, failure of PTH treatment to normalize BV/TV at the proximal tibia in the rat probably occurs because of the relatively few trabeculae on which new bone matrix could be synthesized.
PTH treatment increased bone turnover, but the increase in bone formation was greater than that of resorption. Relative to OVX controls, there were no significant effects of low-dose PTH on urinary DPD excretion or on osteoclast or eroded trabecular surfaces. The increase in BFR was primarily the result of a higher MS/BS rather than increased MAR. Wronski et al. [26] showed that treatment of 4-month-old OVX rats with high-dose teriparatide increased MAR after 5 weeks but that MAR had returned to control levels by 15 weeks. Thus, it remains possible that MAR was elevated earlier in the PTH treatment phase and escaped detection in this study.
Treatment with PTH dose-dependently increased all measures used to define bone strength, i.e., peak load, ultimate stress, stiffness, modulus, work to failure, and toughness. All parameters were quantified at L4 and the central femur, but because area at the point of breakage could not be defined at the femoral neck, only peak load, stiffness, and work to failure were quantified from the load-displacement curves at that site. Thus, PTH treatment restored bone strength of OVX rats at least to the levels observed in normal 10-month-old animals. These results are similar to those observed in studies with similar or higher doses of PTH, teriparatide, or SDZ PTS 893 [3, 4, 11, 33, 34]. The increased strength in PTH-treated animals was associated with an increase in BMC and BMD at all skeletal sites. However, because of increased bone area, the increase in BMC was greater than that of BMD. Addition of bone to the periosteal surface provides a much greater contribution to strength than bone added to endosteal (endocortical and trabecular) surfaces [35].
Increases in lumbar spine BMD, BMC, and bone area measured by DXA were also observed in postmenopausal osteoporotic women treated with PTH for 12 months [36]; and treatment with PTH for 18 months decreased the incidence of vertebral fractures by approximately 60% [8]. Increases in cortical bone volume also occurred at the hip of postmenopausal osteoporotic women treated with PTH for 12 or 18 months [37, 38]. Increases in bone size and decreases in vertebral fracture incidence have also been reported following teriparatide treatment of postmenopausal women with osteoporosis [7, 39, 40].
Results similar to those reported here were observed in a comparable study of OVX rats treated with teriparatide for 12 months, although the experimental designs differed [13]. The post-OVX bone depletion period was restricted to 5 weeks and the animals were only 5 months old at study start. However, consistent with the present study, teriparatide caused dose-related anabolic effects at trabecular and cortical bone sites and prevented or reversed the deleterious effects of OVX on bone mass and strength in lumbar vertebrae and femur.
Daily PTH treatment for 12 months safely stimulated the formation of new bone of good quality. Neither dose of PTH stimulated endocortical bone formation by an amount sufficient to reduce marrow area below sham levels. The increased bone formation was not associated with osteoid accumulation or mineralization defects. Neither was there any evidence of marrow fibrosis or abnormal cells. This is important because PTH or teriparatide treatment of normal rats for 2 years resulted in a dose-dependent increase in the incidence of bone tumors. However, while all doses of teriparatide tested (5, 30, and 75 μg/kg) induced osteosarcomas, only PTH doses ≥50 μg/kg (≅22 μg/kg teriparatide) had this effect [28, 41].
In conclusion, long-term PTH therapy reversed osteopenia at different skeletal sites in the aged OVX rat, a surrogate model of postmenopausal osteoporosis. These effects were achieved by increased bone turnover and enhanced formation of both trabecular and cortical bone. The combined increase in bone mass and improved bone quality resulted in increased bone strength at the spine and femur, suggesting that PTH has potential for the treatment of postmenopausal osteoporosis.
References
Dempster DW, Cosman F, Parisien M, Shen V, Lindsay R (1993) Anabolic actions of parathyroid hormone on bone. Endocr Rev 14:690–709
Fox J (2002) Developments in parathyroid hormone and related peptides as bone-formation agents. Curr Opin Pharmacol 2:338–344
Mosekilde L, Søgaard CH, Danielsen CC, Tørring O (1991) The anabolic effects of human parathyroid hormone (hPTH) on rat vertebral body mass are also reflected in the quality of bone, assessed by biomechanical testing: a comparison study between hPTH-(1–34) and hPTH-(1–84). Endocrinology 129:421–428
Ejersted C, Andreassen TT, Oxlund H, Jørgensen PH, Bak B, Häggblad J, Tørring O, Nilsson MHL (1993) Human parathyroid hormone (1-34) and (1-84) increase the mechanical strength and thickness of cortical bone in rats. J Bone Miner Res 8:1097–1101
Kimmel DB, Bozzato RP, Kronis KA, Coble T, Sindrey D, Kwong P, Recker RR (1993) The effect of recombinant human (1-84) or synthetic human (1-34) parathyroid hormone on the skeleton of adult osteopenic ovariectomized rats. Endocrinology 132:1577–1584
Burr DB, Hirano T, Turner CH, Hotchkiss C, Brommage R, Hock JM (2001) Intermittently administered human parathyroid hormone (1-34) treatment increases intracortical bone turnover and porosity without reducing bone strength in the humerus of ovariectomized cynomolgus monkeys. J Bone Miner Res 16:157–165
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Greenspan SL, Bone HG, Marriott TB, Zanchetta JR, Ettinger MP, Hanley DA, Drezner MK, Miller PD (2005) Preventing the first vertebral fracture in postmenopausal women with low bone mass using PTH(1-84): results from the TOP Study [abstract]. J Bone Miner Res 20(suppl 1):S56
Erben RG (1996) Trabecular and endocortical bone surfaces in the rat: modeling or remodeling. Anat Rec 246:39–46
Kimmel DB (2002) Animal models in osteoporosis research. In: Bilezekian JP, Raisz LG, Rodan GA (eds) Principles of Bone Biology, 2nd ed. Academic Press, San Diego, pp 1635–1655
Thomsen JS, Mosekilde Li, Gasser JA (1999) Long-term therapy of ovariectomy-induced osteopenia with parathyroid hormone analog SDZ PTS 893 and bone maintenance in retired breeder rats. Bone 25:561–569
Kneissel M, Boyde A, Gasser JA (2001) Bone tissue and its mineralization in aged estrogen-depleted rats after long-term intermittent treatment with parathyroid hormone (PTH) analog SDZ PTS 893 or human PTH(1-84). Bone 28:237–250
Sato M, Ma YL, Hock JM, Westmore MS, Vahle J, Villanueva A, Turner CH (2002) Skeletal efficacy with parathyroid hormone in rats was not entirely beneficial with long-term treatment. J Pharmacol Exp Ther 302:304–313
Potts JT Jr, Tregear GW, Keutmann HT, Niall HD, Sauer R, Deftos LJ, Dawson BF, Hogan ML, Aurbach GD (1971) Synthesis of a biologically active N-terminal tetratriacontapeptide of parathyroid hormone. Proc Natl Acad Sci USA 68:63–67
Oxlund H, Ejersted C, Andreassen TT, Tørring O, Nilsson MHL (1993) Parathyroid hormone (1-34) and (1-84) stimulate cortical bone formation from both the periosteum and endosteum. Calcif Tissue Int 53:394–399
FDA (1994) Guidelines for preclinical and clinical evaluation of agents used in the prevention or treatment of postmenopausal osteoporosis, http://www.fda.gov/cder/guidance/osteo.pdf
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. J Bone Miner Res 2:595–610
Turner CH, Burr DB (1993) Basic biomechanical measurements of bone: a tutorial. Bone 14:595–608
Selye H (1932) On the stimulation of new bone formation with parathyroid extract and irradiated ergosterol. Endocrinology 16:547–558
Reeve J, Tregear JW, Parsons JA (1976) Preliminary trial of low doses of parathyroid hormone 1-34 peptide in treatment of osteoporosis. Clin Endocrinol 21:469–477
Tam CS, Heersche JN, Murray TM, Parsons JA (1982) Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology 110:506–512
Hock JM, Fonseca J, Gunness-Hey M, Kemp BE, Martin TJ (1989) Comparison of the anabolic effects of synthetic parathyroid hormone-related protein (PTHrP) 1-34 and PTH(1-34) on bone in rats. Endocrinology 125:2022–2027
Mosekilde L, Danielsen CC, Gasser J (1994) The effect on vertebral bone mass and strength of long term treatment with antiresorptive agents (estrogen and calcitonin), human parathyroid hormone-(1-38), and combination therapy, assessed in aged ovariectomized rats. Endocrinology 134:2126–2134
Stewart AF, Cain RL, Burr DB, Jacob D, Turner CH, Hock JM (2000) Six-month daily administration of parathyroid hormone and parathyroid hormone-related protein peptides to adult ovariectomized rats markedly enhances bone mass and biomechanical properties: a comparison of human parathyroid hormone 1-34, parathyroid hormone-related protein 1-36, and SDZ-parathyroid hormone 893. J Bone Miner Res 15:1517–1525
Morley P, Whitfield JF, Willick GE, Ross V, MacLean S, Barbier JR, Isaacs RJ, Andreassen TT (2001) The effect of monocyclic and bicyclic analogs of human parathyroid hormone (hPTH)-(1-31)NH2 on bone formation and mechanical strength in ovariectomized rats. Calcif Tissue Int 68:95–101
Wronski TJ, Yen CF, Qi H, Dann LM (1993) Parathyroid hormone is more effective than estrogen or bisphosphonates for restoration of lost bone mass in ovariectomized rats. Endocrinology 132:823–831
Qi H, Li M, Wronski TJ (1995) A comparison of the anabolic effects of parathyroid hormone at skeletal sites with moderate and severe osteopenia in aged ovariectomized rats. J Bone Miner Res 10:948–955
Wilker CE, Jolette J, Smith SY, Doyle N, Hardisty JF, Metcalfe AJ, Marriott TB, Fox J, Wells DS (2004) An observable carcinogenic effect dose level identified in Fischer 344 rats following daily treatment with PTH(1-84) for 2 years: role of the C-terminal PTH receptor [abstract]. J Bone Miner Res 19(suppl 1):S98
Physicians Desk Reference, 59th ed. (2005) Thomson Healthcare, Montvale, NJ, p 1842
Akhter MP, Kimmel DB, Recker RR (2001) Effect of parathyroid hormone (hPTH[1-84]) treatment on bone mass and strength in ovariectomized rats. J Clin Densitom 4:13–23
Iwaniec UT, Samnegård E, Cullen DM, Kimmel DB (2001) Maintenance of cancellous bone in ovariectomized, human parathyroid hormone (hPTH[1-84])-treated rats by estrogen, risedronate, or reduced PTH. Bone 29:352–360
Dempster DW, Moreau IA, Varela A, Smith SY, Ste-Marie L-G, Fox J, Newman MK, Recker RR (2005) Treatment of postmenopausal osteoporotic women with parathyroid hormone 1-84 for 18 months improves trabecular bone architecture: a study of iliac crest biopsies using micro-computed tomography [abstract]. J Bone Miner Res 20(suppl 1):S98
Samnegård E, Akhter MP, Recker RR (2001) Maintenance of vertebral body bone mass and strength by human parathyroid hormone treatment in ovariectomized rats. Bone 28:414–422
Samnegård E, Iwaniec UT, Cullen DM, Kimmel DB, Recker RR (2001) Maintenance of cortical bone in human parathyroid hormone(1-84)-treated ovariectomized rats. Bone 28:251–260
Turner CH (2002) Biomechanics of bone: determinants of skeletal fragility and bone quality. Osteoporos Int 13:97–104
Hodsman AB, Hanley DA, Ettinger MP, Bolognese MA, Fox J, Metcalfe AJ, Lindsay R (2003) Efficacy and safety of human parathyroid hormone-(1-84) in increasing bone mineral density in postmenopausal osteoporosis. J Clin Endocrinol Metab 88:5212–5120
Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, Garnero P, Bouxsein ML, Bilezikian JP, Rosen CJ (2003) The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349:1207–1215
Bogado CE, Zanchetta JR, Mango A, Mathisen AL, Fox J, Newman MK (2005) Effects of parathyroid hormone 1-84 on cortical and trabecular bone at the hip as assessed by QCT: results at 18 months from the TOP study [abstract]. J Bone Miner Res 20(suppl 1):S22
Rehman Q, Lang TF, Arnaud CD, Modin GW, Lane NE (2003) Daily treatment with parathyroid hormone is associated with an increase in vertebral cross-sectional area in postmenopausal women with osteoporosis. Osteoporos Int 14:77–81
Zanchetta JR, Bogado CE, Ferretti JL, Wang O, Wilson MG, Sato M, Gaich GA, Dalsky GP, Myers SL (2003) Effects of teriparatide [recombinant human parathyroid hormone (1-34)] on cortical bone in postmenopausal women with osteoporosis. J Bone Miner Res 18:539–543
Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, Westmore MS, Ma YL, Nold JB (2002) Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol 30:312–321
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fox, J., Miller, M.A., Newman, M.K. et al. Daily Treatment of Aged Ovariectomized Rats with Human Parathyroid Hormone (1-84) for 12 Months Reverses Bone Loss and Enhances Trabecular and Cortical Bone Strength. Calcif Tissue Int 79, 262–272 (2006). https://doi.org/10.1007/s00223-006-0108-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-006-0108-1