Abstract
Tobacco smoking has been implicated in the development of osteoporosis and early onset of menopause in women smokers. We measured various biomechanical properties of femurs and tibiae obtained from smoke-exposed and control mice to determine cigarette smoke influences on bone mass, structure, and strength. Growing female C57BL mice were exposed to sidestream cigarette smoke in a whole-body exposure chamber, set at 30 ± 2 mg smoke particulates/m3 for 4 hours/day and 5 days/week for 12 consecutive weeks. Elevated levels of urinary cotinine and pulmonary ethoxyresorufin deethylase activity in smoke-exposed mice confirmed their effective exposure to cigarette smoke. There were no differences in body weight and physical size (length, medial-lateral and anterior-posterior widths, midshaft cortical area and thickness) of femurs and tibiae between smoke-exposed and control mice. The femoral mid-shaft yield load, stiffness, yield stress, and modulus were, respectively 8%, 13%, 10%, and 14% lower (P < 0.05) in smoke-exposed compared to control mice. The ultimate load and stress in mid-shaft femurs showed decreasing trends (P < 0.1) in smoke-exposed mice. In the femoral neck, the ultimate load and stiffness were 9% and 12% lower (P < 0.05) in smoke-exposed mice, respectively. Further, the ash-to-dry bone weight ratio was smaller (∼6%, P < 0.05), and micro-computed tomographic scanning of distal femoral bone volume/total volume (%) and trabecular thickness showed decreasing trends in smoke-exposed mice compared to the control group. We conclude that exposure to tobacco smoke deteriorates some of the biomechanical properties of bone in growing female mice.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Tobacco (cigarette) smoking is a major health risk that increases an individual’s health-care costs and decreases life expectancy [1–6]. Smoking has been strongly implicated in cancers of various organ sites (e.g., lung, bladder, pancreas, etc.) [7–9] and in various cardiovascular and respiratory diseases [10–12]. In addition, the use of tobacco is associated with low bone mass and increased fragility fracture risk [11, 13–23]. Several studies suggest that cigarette smoking exerts antiestrogenic effects in females, resulting in an increased incidence of early menopause and osteoporosis (bone fragility) in smokers [24–30]. An earlier onset of menopause by 1–2 years and a dose response has been reported in women smokers who smoke more than 10 cigarettes/day [31, 32]. On average, women are at greater risk of bone loss leading to skeletal fractures compared to men [33, 34], and smoking further increases their skeletal fragility risk [16, 23, 34, 35]. Based on a study of twin pairs discordant for cigarette use, Hopper and Seeman [14] calculated that, by the age of menopause, women who smoke one pack of cigarettes/day throughout life will have 5–8% less bone than nonsmokers. Others have observed 5–10% less bone and reduced protective effects of nutritional calcium in postmenopausal smokers than nonsmokers [15–17, 35–42]. These studies provide evidence that smoking-related bone fragility is a critical problem in women and warrants examination of the relationship between cigarette smoking and bone mechanical properties.
Nicotine is the principal pharmacologically active chemical in tobacco [43] and has been extensively studied in experimental models. However, its effects on bone strength in a noninjury animal model have been poorly defined [44–49]. Our past studies in intact (young to adult) and estrogen-replete rats given various doses of nicotine (3–9 mg/kg daily) have found some effects on the biomechanical properties of bone [44–46, 48, 49]. Although nicotine has been shown to compromise mechanical strength properties of bone during fracture healing [50], its effects on bone biomechanical properties are mixed [44–46, 48, 49]. In studies where osmotic pumps were used to continuously deliver nicotine (doses of 3.0 and 4.5 mg/kg daily) in rats, the data suggest no difference [44–46] to decreased bone strength values [48, 49] in some biomechanical properties of nicotine-treated rats compared to controls. In addition, high-dose nicotine (6 and 9 mg/kg daily) in an animal model of postmenopausal bone loss suggested a marginal effect on a few biomechanical properties of bone [48, 49]. Even with a sufficiently high intake of nicotine (6–9 mg/kg daily), the expected compromise in bone mass and strength was small [48] in the estrogen-replete (intact) and estrogen-depleted (ovariectomized) rats, a well-established animal model of postmenopausal bone loss [48, 49]. However, tobacco smoke exposure was found to be more detrimental to a bone and implant interface (in terms of bone/implant contact area) than nicotine treatment alone in the tibiae of adult Wistar rats [51]. These data suggested that tobacco smoke constituents other than nicotine might be responsible for the compromised biomechanical properties in the skeleton of smokers.
Recently, the research focus has shifted toward determining the role of genetics in skeletal health of murine models [52–57]. Therefore, our interest was in determining the effect of environmental insults, such as exposure to tobacco smoke, on bone properties in the mouse model. The main aim of the present study was to evaluate the effects of whole tobacco smoke exposure on bone mass, structure, and strength in growing virgin female mice.
Materials and Methods
Eight-week-old female C57BL (apoE−/−) mice were obtained from Jackson Laboratories (Bar Harbor, ME) and maintained as described previously [58]. After acclimatization for 2 weeks, animals were randomly divided into two groups: sham-exposed (n = 20), maintained as a control group in filtered ambient air, and smoke-exposed (n = 32), exposed to sidestream cigarette smoke as described earlier [58]. Mice were housed four to a cage and received water and a “Western type” diet (Teklad 88137, Harlan Teklad, Madison, WI, USA) ad libitum for the duration of the study.
All inhalation exposures to smoke were carried out in a whole-body Hinners-type stainless steel/glass chamber [58]. Briefly, animals were exposed to sidestream cigarette smoke in a chamber maintained at a smoke particulate concentration of about 30 mg/m3 for 4 hours/day, 5 days/week for a total of 12 weeks. Inhalation of smoke by the animals was monitored by measuring urinary cotinine during exposures and the induction of ethoxyresorufin deethylase (EROD) activity in lung microsomes. Urinary cotinine was measured by an enzyme-linked immunosorbent assay and EROD activity, by spectrofluorimetry, as described previously [58].
At the time of necropsy, femurs and tibiae were excised, cleaned of soft tissue, and stored in saline at –20°C for subsequent bone biomechanical strength, mass, and structure measurements.
Bone Strength
Femurs were tested by three-point bending with force applied to the anterior surface. The loading was such that the anterior surface of the femur (mid-shaft) was in compression and the posterior surface was in tension. After strength testing at mid-shaft, the femoral neck was tested by bending. Force was applied to the femoral head in a direction parallel to the shaft length [48, 59, 60]. All biomechanical tests were conducted at room temperature and at a rate of 3 mm/minute using a servocontrolled mechanical testing machine (Instron 5543, Canton, MA). Load-deformation curves were plotted and analyzed for structural strength parameters such as ultimate load, yield load, and stiffness [61]. Ultimate load was defined as the maximum load that a specimen (maximum height of the curve) takes before fracturing. Yield load is the load at which permanent damage/deformation is incurred in the specimen. The yield load was estimated to be the intersection point of the load displacement curve and a line parallel to the linear portion of the load-displacement curve but offset by 0.2% of the initial specimen length [61, 62]. Stiffness is the slope of the linear portion of the load-displacement curve.
Two transverse femoral cross-sections adjacent to the fracture site at the mid-shaft were first traced at ×20 on tracing papers using a profile projector (V-10; Nikon, Tokyo, Japan) and then digitized on a VaxStation 2000 computer (Digital Equipment Corp., Maynard, MA, USA) using a digitizing tablet [60]. Average radii, second moment of area/inertia about the medial-lateral axis, and cross-sectional cortical areas were determined using the program SECTION, developed at the Creighton University Osteoporosis Research Center Biomechanics Laboratory. Femoral mid-shaft apparent material strength properties (ultimate stress, yield stress, flexural modulus) were calculated as load/cross-sectional area [61, 63].
Bone Mass Measurements
After measuring length and mid-shaft widths (in the medial-lateral and anterior-posterior directions), tibial bone specimens were used to measure bone percent ash content (bone dry weight/ash weight) using standard bone ash techniques (600°C oven) [64, 65]. We used a digital caliper (Mitutoyo, Kawasaki, Japan) for the bone length and width measurements. The anatomical site was 2.9 mm proximal to the tibiofibular junction in tibiae.
Micro-Computed Tomographic Analysis
Using a micro-computed tomography (μCT) device (μ-CT-20; Scanco Medical, Bassersdorf, Switzerland), distal femurs were scanned to determine cancellous/trabecular structural properties in terms of bone volume/total volume (BV/TV), trabecular thickness, spacing, and number. The length of the distal femur scans was 4 mm from the distal end. The distal femur scans were performed at 9 μ resolution, with an integration time of 80 milliseconds, and using standard techniques as described previously [66, 67].
Statistical Analysis
Student’s t-test (SPSS, Chicago, IL; v12.0) was used to find differences in all the measured variables between smoke-exposed and control groups. The level of statistical significance was set at P ≤ 0.05. Data are reported as mean ± standard error of the mean (SEM). The pooled standard deviation (SD) was calculated to estimate the common SD for all measured variables.
Results
Biochemical and Physical Measurements
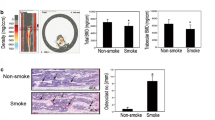
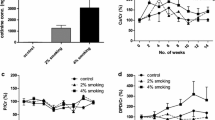
Animals appeared generally healthy, with no loss due to smoke exposure. No significant differences were observed in the body weights of control (22.82 ± 1.54) and exposed (22.24 ± 1.53) groups after 12 weeks of smoke exposure. Elevated levels of urinary cotinine and pulmonary EROD activity confirmed the exposure of mice to smoke. Urinary cotinine levels in smoke-exposed animals ranged 2.46–3.54 μg/mg creatinine in comparison to negligible levels in the control group. Similarly, EROD activity (mean ± SEM) of lung microsomes increased severalfold in smoke-exposed [23.5 ± 4 pmole/(min·mg)] compared to control [1.8 ± 0.2 pmole/(min·mg)] mice.
There were no differences in the femoral and tibial shaft physical measurements (length, width, second moment of area/inertia, cortical area, cortical thickness, etc.) between smoke-exposed and control mice (Table 1).
Bone Strength
Both the structural and apparent material strength parameters of mid-shaft femurs either were significantly lower (P < 0.05) (Figs. 1 and 2) or showed some deterioration (P < 0.1) in the smoke-exposed group (Table 2). The femoral mid-shaft (three-point bending test) yield load, stiffness, yield stress, and modulus were, respectively, 8% (SD = 0.61), 13% (SD = 0.90), 10% (SD = 0.3), and 14% (SD = 0.58) lower (P < 0.05) in smoke-exposed compared to control mice (Table 2, Figs. 1 and 2). The ultimate load and stress in mid-shaft femurs showed decreasing trends (P < 0.1) in smoke-exposed mice. In the femoral neck, ultimate load and stiffness were 9% (SD = 0.63) and 12% (SD = 0.43) lower (P < 0.05) in smoke-exposed mice, respectively (Table 2, Fig. 3).
Bone Mass/Ash
The effects of treatment on femoral bone mass/ash weight are presented in Table 3. While no differences were noted in dry bone and ash weights, the ash-to-dry bone weight ratio was smaller (∼6%, P < 0.05) in smoke-exposed mice compared to the control group (Table 3).
μCT Analysis
None of the μCT measured parameters (Table 4) was significantly different between the smoke-exposed and control groups. However, while the ratio BV/TV and trabecular thickness in the distal femur showed decreasing trends (P < 0.1) in the smoke-exposed group, there were no differences in trabecular number and spacing between the smoke-exposed and control mice (Table 4).
Discussion
This study evaluated the effects of sidestream cigarette smoke exposure (a total of 240 hours over 12 weeks) on bone biomechanical properties, bone mass, and structure in growing female mice, an animal model that has been used for the study of genetic influences on bone biomechanical properties [52–56]. The growing mouse model may allow us to examine skeletal effects in teenagers in which smoking is quite prevalent. There was a significant decrease in most of the structural strength (yield load, stiffness) and apparent material (yield stress, flexural modulus) properties of the femoral mid-shaft, while only the structural strength (ultimate and yield load) properties in the femoral neck were lower for smoke-exposed compared to control mice. In addition, measurements of tibial bone mass (ash weight/dry weight ratio) and distal femur structural properties (μCT) suggest a lower tibial bone ash weight ratio and declining trends of %BV/TV in the smoke-exposed group. These results clearly suggest deleterious effects of smoke exposure on biomechanical properties of bone in the intact (estrogen-replete) mouse model. We recognize that smoking and the depletion of estrogen in postmenopausal women create significantly lower bone mass and increase the risk of bone fragility fractures [25, 26, 29, 68]. The intent of the present study was to establish baseline data on the effect of smoking on bone properties; therefore, the estrogen status of the mice was not determined. Future studies to examine estrogen and its metabolites in intact and ovariectomized animals will be needed to examine the mechanisms of the smoke effect. Thus, further tobacco smoking studies on bone fragility are warranted. This baseline study will allow future studies to test whether compromised bone biomechanical properties are due to smoking-related defects in collagen composition, cross-linking, or modeling/remodeling of periosteal/endosteal surfaces [69, 70].
The hypothesis was that tobacco smoke exposure may cause bone-structure and apparent material strength property changes. The bone apparent material strength properties may be influenced by changes in collagen fiber composition and cross-linking. It has been shown that any blocking of the cross-linking in bone causes poor mineralization, which may lead to compromised bone-strength properties. In human tobacco smokers, the marker bone turnover N-terminal collagen cross-links (NTx) shows increased levels [20] compared to nonsmokers. In addition, these data [20] suggest that any smoking-related increased bone turnover may cause skeletal fragility.
The exposure of mice to tobacco smoke under our experimental conditions significantly raised urinary cotinine levels (2.46–3.53 μg cotinine/mg creatinine) above those in the control group. These urinary cotinine levels suggest that the smoke exposure of these mice was equivalent to the exposure reported for human smokers who smoke about 10–15 cigarettes/day [71–73]. Unlike nicotine alone [44–47, 74], the tobacco (cigarette) smoke exposure did compromise some of the biomechanical properties in this study. Previous studies of nicotine administration in rats (1.5- to 4.5-fold compared to human chronic smokers) [74] showed no effect on biomechanical properties at low doses (3–4.5 mg/kg daily) [44–47]. Further, despite the range of doses (4.5–9 mg/kg daily) used, only the higher nicotine dose (9 mg/kg daily) had limited harmful effects on vertebral bone mineral content (BMC) [49], femoral ultimate load, yield load, and yield stress [48, 49] in adult female intact and ovariectomized rats. These data [48, 49] suggest that nicotine has a minimal effect on the biomechanical properties (structural and apparent material strength) in both ovariectomized and sham-treated rats even at a high dose (9 mg/kg daily) of nicotine [48]. Similar to high doses, the lower nicotine doses (3–4.5 mg/kg daily) in growing to adult female rats that produced serum concentrations similar to those in smokers also had no effect on the biomechanical properties of bone [44–47]. However, unlike the nicotine treatment studies, the present study clearly suggests some deleterious effects of whole tobacco smoke exposure in mice on some bone biomechanical properties. While some variables (Tables 2–4, Figs. 1–3) showed significant biomechanical strength declines in the smoke-exposed group, the other variables (Tables 2–4) did not reach statistical significance; therefore, they should not be considered different between smoke-exposed and control mice. The few biomechanical properties that were significantly different (Tables 2–4, Figs. 1–3) allow us to conclude that tobacco smoking has adverse skeletal effects. However, it is likely that longer exposure to smoke may further magnify these skeletal defects in the relatively healthy bones of growing mice. In addition, exposure to tobacco smoke may evoke a differential response in rats and mice and should be considered when making a direct comparison across different species.
In studies involving determination of the harmful effects of pure tobacco smoke constituents such as nicotine and polycyclic aromatic hydrocarbons (PAHs) on biomechanical properties of bone, the route of administration may also influence the outcome. For instance, consistent with our earlier rat studies [44–46], nicotine vapor administration via inhalation (which caused plasma nicotine levels to increase to the levels of heavy smokers) did not affect femoral strength in Sprague-Dawley rats even after a 2-year exposure [47]. However, administration of nicotine in drinking water for 2 months decreased BMC and bone mineral density (BMD) in mice [75]. Similarly, studies of other smoke constituents have shown that administration of benzo[a]pyrene (BaP) and 7,12-dimethylbenz[a]anthracene (DMBA) via the subcutaneous route (250 μg/kg of BaP/DMBA weekly for 15 weeks) causes a significant decline in BMD and bone strength of adult Sprague-Dawley ovariectomized rats [76].
The negative effects of tobacco smoke and its constituents such as nicotine and PAHs on bone may result from slow healing, poor bone and implant interface, increased resorption, slower growth and lengthening, and increased bone fragility in animal models [51, 77–79]. The harmful effects of whole smoke exposure on bone in the present study are evident from the tibial bone ash ratio (Table 3) and the structural and apparent material properties of femurs (Table 2, Figs. 1–3). Both yield load (structural strength) and yield stress (apparent material strength) properties are significantly decreased in the smoke-exposed compared to control mice (Table 2), suggesting that smoking changes the material properties of bone such that the force threshold for accumulation of permanent damage declines significantly. The lower yield strength properties may result from the deterioration of collagen fiber essential for the health of bone tissue. It is well documented that smoke exposure modulates collagen fiber in lungs [80, 81]. It is, therefore, likely that it also adversely affects collagen in bones, which in turn influences their yield strength properties. Higher levels of NTx in smokers, suggesting greater bone turnover, have been reported [20]. These observations suggest that increased bone turnover by cigarette smoking may play a role in skeletal fragility and the reduction in the yield strength properties of bones.
Unlike yield load and stress, the femoral mid-shaft ultimate load and stress (Table 3) did not decrease significantly in the smoking group, suggesting that whole bone post-yield maximum strength is not as sensitive to changes in the bone properties in mice exposed to 12 weeks of cigarette smoking.
BMC and BMD in women smokers are lower, putting them at a greater risk of skeletal fractures compared to nonsmokers [16, 23, 26, 34, 38, 39]. Lower bone mass in smokers may be due to thinning of cortical bone, lower trabecular numbers, trabecular thickness, and greater trabecular spacing in cancellous bone. Although no cortical size differences (Table 1) were noted, the bone mass and declining trends (Table 3, 4) of trabecular/cancellous bone architecture in this animal study is consistent with the poor bone mass conditions observed in humans [24–30].
Lower ash-to-dry weight ratios of smoke-exposed mice bones suggest relatively lower BMC of the tibial specimens. Assuming that similar trends also exist in the femoral bone site, it could be speculated that lower BMC is responsible for the lower structural and apparent material strength properties measured in smoke-exposed mice (Table 2, Figs. 1–3). Although %BV/TV and trabecular thickness in the distal femurs showed trends of being smaller in the smoke-exposed group, trabecular number and spacing were not different (Table 4). This suggests that the overall trabecular bone in the smoke-exposed group is weaker even though μCT failed to detect defects in other parameters of bone structure. In addition, despite similar bone size at the mid-shaft femur (Table 1), either significantly lower or decreasing femoral structural strength suggests that cigarette/tobacco smoke negatively affects the apparent material properties (ultimate/yield stress, modulus; Table 3, Figs. 1–3), thus changing its intrinsic material strength properties [82]. Therefore, future experiments pertaining to tobacco smoke exposure and skeletal health should include characterization of bone intrinsic properties using techniques like nano-indentation.
Duration of cigarette smoking in humans is much longer than what has been used for animal studies. A chronic smoker smokes for an average of 18 years [83], which translates into 34% of their lifetime (average age 53 years) [84]. In animal studies (mice, 24-month life span), the human equivalent time period translates into 32 weeks of smoking. However, the 12-week duration of tobacco smoke at a concentration of 30 mg/m3 TSP (total suspended particulates) seems to be enough to negatively influence some of the bone (both cortical and trabecular) biomechanical properties. The harmful effects or changes in bone properties may be greater at higher-level exposures to tobacco smoke than those used in the present study.
The effect of smoking in postmenopausal women is even greater. It leads to considerably lower bone mass and density [68], reflecting the negative effects of both estrogen loss and tobacco smoke. The current study did not examine the effects of smoke exposure on bone health in ovariectomized mice. The combined influences of estrogen loss and tobacco smoke on bone biomechanical properties will be deleterious and should be quantified in future studies.
In summary, this study demonstrates that tobacco smoke exposure has significant detrimental effects on bone mass and bone biomechanical properties in growing female mice.
References
Secker-Walker RH, Worden JK, Holland RR, Flynn BS, Detsky AS (1997) A mass media programme to prevent smoking among adolescents: costs and cost effectiveness. Tob Control 6:207-212
Hodgson TA (1992) Cigarette smoking and lifetime medical expenditures. Milbank Q 70:81–125
Tsevat J (1992) Impact and cost-effectiveness of smoking interventions. Am J Med 93:43S-47S
Taylor DH Jr, Hasselblad V, Henley SJ, Thun MJ, Sloan FA (2002) Benefits of smoking cessation for longevity. Am J Public Health 2002;92:990–996
Nusselder WJ, Looman CW, Marang van de Mheen PJ, van de Mheen H, Mackenbach JP (2000) Smoking and the compression of morbidity. J Epidemiol Community Health 54(8):566–574
Rogers RG, Powell Griner E (1991) Life expectancies of cigarette smokers and nonsmokers in the United States. Soc Sci Med 32:1151–1159
Burns DM (2003) Tobacco-related diseases. Semin Oncol Nurs 19:244–249
Bilello KS, Murin S, Matthay RA (2002) Epidemiology, etiology, and prevention of lung cancer. Clin Chest Med 23:1–25
Lowenfels AB, Maisonneuve P (2002) Epidemiologic and etiologic factors of pancreatic cancer. Hematol Oncol Clin North Am 16:1–16
Kesteloot H (2003) Social class, all-cause, cardiovascular and cancer mortality: the importance of cigarette smoking. Acta Cardiol 58:285–287
Stabile LP, Siegfried JM (2003) Sex and gender differences in lung cancer. J Gend Specif Med 6:37–48
Centers for Disease Control and Prevention (2003) Cigarette smoking-attributable morbidity —United States, 2000. MMWR Morb Mortal Wkly Rep 52:842–844
Daniell HW (1976) Osteoporosis of the slender smoker. Vertebral compression fractures and loss of metacarpal cortex in relation to postmenopausal cigarette smoking and lack of obesity. Arch Intern Med 136:298–304
Hopper JL, Seeman E (1994) Bone density in twins discordant for tobacco use. N Engl J Med 330:387–392
Krall EA, Dawson-Hughes B (1999) Smoking increases bone loss and decreases intestinal calcium absorption. J Bone Miner Res 14:215–220
Law MR, Hackshaw AK (1997) A meta-analysis of cigarette smoking, bone mineral density and risk of hip fracture: recognition of a major efffect. BMJ 315:841–846
Pocock NA, Eisman JA, Kelly PJ, Sambrook PN, Yeates MG (1989) Effects of tobacco use on axial and appendicular bone mineral density. Bone 10:329–331
Seeman E (1996) The effects of tobacco and alcohol use on bone. In: Marcus R, Feldman D, Kelsey J (eds), Osteoporosis. Academic Press, San Diego, pp 577–597
Cornuz J, Feskanich D, Willett WC, Colditz GA (1999) Smoking, smoking cessation, and risk of hip fracture in women. Am J Med 106:311–314
Oncken C, Prestwood K, Cooney JL, Unson C, Fall P, Kulldorff M, Raisz LG (2002) Effects of smoking cessation or reduction on hormone profiles and bone turnover in postmenopausal women. Nicotine Tob Res 4:451–458
Qandil R, Sandhu HS, Matthews DC (1997) Tobacco smoking and periodontal diseases. J Can Dent Assoc 63:187–195
Moran S, Glazier G, Armstrong K (2003) Women smokers’ perceptions of smoking-related health risks. J Womens Health 12:363–371
Ward KD, Klesges RC (2001) A meta-analysis of the effects of cigarette smoking on bone mineral density. Calcif Tissue Int 68:259–270
Karamanidis D, Tamiolakis D, Koutsougeras G, Tripsanas CH, Retzos K, Karidis S, Liberis B (2001) Cigarette smoking and the degree of maturation of the vaginal squamous epithelium in postmenopausal women. Clin Exp Obstet Gynecol 28:274–276
Hardy R, Kuh D, Wadsworth M (2000) Smoking, body mass index, socioeconomic status and the menopausal transition in a British national cohort. Int J Epidemiol 29:845–851
Mueller L, Ciervo CA (1998) Smoking in women. J Am Osteopath Assoc 98(12 suppl):S7-S10
McKinlay SM (1996) The normal menopause transition: an overview. Maturitas 23:137–145
Lipsitz CM (1993) Have you come a long way, baby? Smoking trends in women. Md Med J 42:27–31
Shulman A, Ellenbogen A, Maymon R, Bahary C (1990) Smoking out the oestrogens. Hum Reprod 5:231–233
Lindquist O, Bengtsson C, Lapidus L (1985) Relationships between the menopause and risk factors for ischaemic heart disease. Acta Obstet Gynecol Scand Suppl 130:43–47
Kato I, Toniolo P, Akhmedkhanov A, Koenig KL, Shore R, Zeleniuch-Jacquotte A (1998) Prospective study of factors influencing the onset of natural menopause. J Clin Epidemiol 51:1271–1276
McKinlay SM (1996) The normal menopause transition: an overview. Maturitas 23:137–145
Welch A, Camus J, Dalzell N, Oakes S, Reeve J, Khaw KT (2004) Broadband ultrasound attenuation (BUA) of the heel bone and its correlates in men and women in the EPIC-Norfolk cohort: a cross-sectional population-based study. Osteoporos Int 15:217–225
Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PW, Kiel DP (2000) Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res 15:710–720
Payne JB, Reinhardt RA, Nummikoski PV, Dunning DG, Patil KD (2000) The association of cigarette smoking with alveolar bone loss in postmenopausal females. J Clin Periodontol 27:658–664
Galvin RJ, Ramp WEEK, Lenz LG (1988) Smokeless tobacco contains a non-nicotine inhibitor of bone metabolism. Toxicol Appl Pharmacol 95:292–300
Krall EA, Dawson-Hughes B (1991) Smoking and bone loss among postmenopausal women. J Bone Miner Res 6:331–338
Michnovicz J, Hershcopf RJ, Naganuma H, Bradlow HL, Fishman J (1986) Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. N Engl J Med 315:1305–1309
Bjarnason NH, Christiansen C (2000) The influence of thinness and smoking on bone loss and response to hormone replacement therapy in early postmenopausal women. J Clin Endocrinol Metab 85:590–596
Sirola J, Kroger H, Honkanen R, Sandini L, Tuppurainen M, Jurvelin JS, Saarikoski S (2003) Smoking may impair the bone protective effects of nutritional calcium: a population-based approach. J Bone Miner Res 18:1036–1042
Need AG, Kemp A, Giles N, Morris HA, Horowitz M, Nordin B (2002) Relationships between intestinal calcium absorption, serum vitamin D metabolites and smoking in postmenopausal women. Osteoporos Int 13:83–88
Krall EA, Dawson-Hughes B (1999) Smoking increases bone loss and decreases intestinal calcium absorption. J Bone Miner Res 14:215–220
Benowitz NL (1986) Clinical pharmacology of nicotine. Annu Rev Med 37:21–32
Fung YK, Mendlik MG, Haven MC, Akhter MP, Kimmel DB (1998) Short-term effects of nicotine on bone and calciotropic hormones in adult female rats. Pharmacol Toxicol 82:243–249
Fung YK, Iwaniec UT, Cullen DM, Akhter MP, Haven MC, Timmins P (1999) Long-term effects of nicotine on bone and calcitropic hormones in adult female rats. Pharmacol Toxicol 85:181–187
Iwaniec UT, Fung YK, Cullen DM, Akhter MA, Haven MC, Schmid M (2000) Effects of nicotine on bone and calciotropic hormones in growing female rats. Calcif Tissue Int 67:68–74
Syversen U, Nordsletten L, Falch JA, Madsen JE, Nilsen OG, Waldum HL (1999) Effects of lifelong nicotine inhalation on bone mass and mechanical properties in female rat femurs. Calcif Tissue Int 65:246–249
Akhter MP, Iwaniec UT, Haynatzki GR, Fung YK, Cullen DM, Recker RR (2003) Effects of nicotine on bone mass and strength in aged female rats. J Orthop Res 21:14–19
Iwaniec UT, Fung YK, Akhter MA, Haven MC, Nespor S, Haynatzki GR, Cullen DM (2001) Effects of nicotine on bone mass, turnover, and strength in adult female rats. Calcif Tissue Int 68:358–364
Silcox DH III, Daftari T, Boden SD, Schimandle JH, Hutton WC, Whitesides TE Jr (1995) The effect of nicotine on spinal fusion. Spine 20:1549–1553
Cesar-Neto JB, Duarte PM, Sallum EA, Barbieri D, Moreno H Jr, Nociti FH Jr (2003) A comparative study on the effect of nicotine administration and cigarette smoke inhalation on bone healing around titanium implants. J Periodontol 74:1454–1459
Akhter MP, Wells DJ, Short SJ, Cullen DM, Johnson ML, Haynatzki GR, Babij P, Allen KM, Yaworsky PJ, Bex F, Recker RR (2004) Bone biomechanical properties in LRP5 mutant mice. Bone 35:162–169
Akhter MP, Otero JK, Iwaniec UT, Cullen DM, Haynatzki GR, Recker RR (2004) Differences in vertebral structure and strength of inbred female mouse strains. J Musculoskelet Neuronal Interact 4:33–40
Kodama Y, Dimai HP, Wegedal J, Sheng M, Malpe R, Kutilek S, Beamer W, Donahue LR, Rosen C, Baylink DJ, Farley J (1999) Cortical tibial bone volume in two strains of mice: effects of sciatic neurectomy and genetic regulation of bone response to mechanical loading. Bone 25:183–190
Klein RF, Mitchell SR, Phillips TJ, Belknap JK, Orwoll ES (1998) Quantitative trait loci affecting peak bone mineral density in mice. J Bone Miner Res 13:1648–1656
Beamer WG, Donahue LR, Rosen CJ, Baylink DJ (1996) Genetic variability in adult bone density among inbred strains of mice. Bone 18:397–403
Robling AG, Turner CH (2002) Mechanotransduction in bone: genetic effects on mechanosensitivity in mice. Bone 31:562–569
Gairola CG, Drawdy ML, Block AE, Daugherty A (2001) Sidestream cigarette smoke accelerates atherogenesis in apolipoprotein E-/- mice. Atherosclerosis 156:49–55
Akhter MP, Iwaniec UT, Covey MA, Cullen DM, Kimmel DB, Recker RR (2000) Genetic variations in bone density, histomorphometry, and strength in mice. Calcif Tissue Int 67:337–349
Akhter MP, Raab DM, Turner CH, Kimmel DK, Recker RR (1992) Characterization of in vivo strain in the rat tibia during external application of a four-point bending load. J Biomech 25:1241–1246
Baumeister T, Avallone EA (1986) Marks’ standard handbook for mechanical engineers, 9th ed. McGraw-Hill, New York
Newby JR (1992) Mechanical testing handbook, vol 8. Material Information Society, American Society of Metals
Turner CH, Burr DB (1993) Basic biomechanical measurements of bone: a tutorial. Bone 14:598–604
Akpe MP, Waibel PE, Larntz K, Metz AL, Noll SL, Walser MM (1987) Phosphorus availability bioassay using bone ash and bone densitometry as response criteria. Poult Sci 66:713–720
Hill AD, Patterson KY, Veillon C, Morris ER (1986) Digestion of biological materials for mineral analyses using a combination of wet and dry ashing. Anal Chem 58:2340–2342
Kapadia RD, Stroup GB, Badger AM, Koller B, Levin JM, Coatney RW, Dodds RA, Liang X, Lark MW, Gowen M (1998) Applications of microCT and MR microscopy to study pre-clinical models of osteoporosis and osteoarthritis. Technol Health Care 6:361–372
Muller R, Hahn M, Vogel M, Delling G, Ruegsegger P (1996) Morphometric analysis of noninvasively assessed bone biopsies: comparison of high-resolution computed tomography and histologic sections. Bone 18:215–220
Johnson KC, Hu J, Mao Y (2000) Passive and active smoking and breast cancer risk in Canada, 1994-97. The Canadian Cancer Registries Epidemiology Research Group. Cancer Causes Control 11:211–221
Gerstenfeld LC, Riva A, Hodgens K, Eyre DR, Landis WJ (1993) Post-translational control of collagen fibrillogenesis in mineralizing cultures of chick osteoblasts. J Bone Miner Res 8:1031–1043
Reddi AH, Sullivan NE (1979) Inhibition of mineralization by experimental lathyrism during matrix-induced endochondral bone differentiation. Proc Soc Exp Biol Med 162:445–448
Weerd S, Thomas CMG, Kuster JETG, Cikot RJLM, Steegers EAP (2002) Variation of serum and urine cotinine in passive and active smokers and applicability in preconceptional smoking cessation counseling. Environ Res A 90:119–124
Witschi H, Espiritu I, Uyeminami D, Suffia M, Pinkerton KE (2004) Lung tumor response in strain a mice exposed to tobacco smoke: some dose-effect relationships. Inhalat Toxicol 16:27–32
Yang M, Kunugita N, Kitagawa K, Kang S-H, Coles B, Kadlubar FF, Katoh T, Matsuno K, Kawamoto T (2001) Individual differences in urinary cotinine levels in Japanese smokers relation to genetic polymorphism of drug-metabolizing enzymes. Cancer Epidemiol Biomarkers Prev 10:589–593
Benowitz NL, Kuyt F, Jacob PI (1982) Circadian blood nicotine concentrations during cigarette smoking. Clin Pharmacol Ther 32:758–764
Broulik PD, Jarab J (1993) The effect of chronic nicotine administration on bone mineral content in mice. Horm Metab Res 25:219–221
Lee LL, Lee JS, Waldman SD, Casper RF, Grynpas MD (2002) Polycyclic aromatic hydrocarbons present in cigarette smoke cause bone loss in an ovariectomized rat model. Bone 30:917–923
Yano R, Hayakawa D, Emura S, Chen H, Ozawa Y, Taguchi H, Shoumura S (2002) Effects of cigarette smoke exposure on the ultrastructure of the golden hamster parathyroid gland. Histol Histopathol 17:375–381
Ueng SW, Lee MY, Li AF, Lin SS, Tai CL, Shih C (1997) Effect of intermittent cigarette smoke inhalation on tibial lengthening: experimental study on rabbits. J Trauma 42:231–238
Nociti FH Jr, Cesar NJ, Carvalho MD, Sallum EA (2002) Bone density around titanium implants may be influenced by intermittent cigarette smoke inhalation: a histometric study in rats. Int J Oral Maxillofac Implants 17:347–352
Selman M, Montano M, Ramos C, Vanda B, Becerril C, Delgado J, Sansores R, Barrios R, Pardo A (1996) Tobacco smoke-induced lung emphysema in guinea pigs is associated with increased interstitial collagenase. Am J Physiol 271:L734-L743
Lang MR, Fiaux GW, Gillooly M, Stewart JA, Hulmes DJ, Lamb D (1994) Collagen content of alveolar wall tissue in emphysematous and non-emphysematous lungs. Thorax 49:319–326
Akhter MP, Fan Z, Rho JY (2004) Bone intrinsic material properties in three inbred mouse strains. Calcif Tissue Int 75:416–420
Pierce JP, Gilpin E (1996) How long will today’s new adolescent smoker be addicted to cigarettes? Am J Public Health 86:253–256
Boyle P (1997) Cancer, cigarette smoking and premature death in Europe: a review including the Recommendations of European Cancer Experts Consensus Meeting, Helsinki, October 1996. Lung Cancer 17:1–60
Acknowledgments
The authors thank Daniel Wells, Nick Glass, Paul Hruby, and Gleb Haynatzki for their assistance. This study was supported partially by the State of Nebraska (LB595) and the University of Kentucky.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akhter, M.P., Lund, A.D. & Gairola, C.G. Bone Biomechanical Property Deterioration Due to Tobacco Smoke Exposure. Calcif Tissue Int 77, 319–326 (2005). https://doi.org/10.1007/s00223-005-0072-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-005-0072-1