Abstract
Thiazolidinediones are insulin-sensitizing agents and in clinical use for the treatment of type II diabetes. Under specific experimental conditions, these molecules induce adipogenic differentiation of mesenchymal precursor cells at the expense of osteoblasts in vitro, suggesting possible negative effects on the skeleton. We measured effects of the thiazolidinedione BRL49653 on bone tissue of intact and estrogen-deprived skeletally mature adult female Wistar rats (6–9 months old). Weight gain and decreased plasma triglyceride levels confirmed the effectiveness of the treatment. However, no change in bone mass or fat marrow volume was observed in intact rats treated for 8 weeks with 5, 10, or 20 mg/kg of BRL49653. Study of marrow cultures established at necropsy revealed a higher responsiveness to adipogenic differentiation protocols of cultures established from the 10-mg/kg group compared to vehicle control. In a second study, the effects of thiazolidinedione treatment on the skeleton of estrogen-deprived rats were investigated. Application of 10 mg/kg of BRL49653 for 12 weeks resulted in enhanced bone loss (+31%; pQCT) and increased fat marrow volume (+117%; histomorphometry) compared to vehicle-treated OVX control. Interestingly, osteoblast number was comparable in both cases. Bone resorption parameters were significantly increased in the treatment group (+27% osteoclast number, +30% eroded surface). Enhanced bone loss due to treatment was consistently observed in the tibia, femur, and the lumbar spine. Our data indicate that thiazolidinediones may enhance bone loss induced by estrogen deprivation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The thiazolidinediones (TZDs) are antidiabetic compounds that were discovered to be potent stimulators of adipogenesis. These molecules sensitize target tissues to the action of insulin and, therefore, are widely used clinically to treat type II diabetes [1, 2]. TZDs bind and activate the nuclear receptor PPARgamma, which exerts critical control over the adipocyte differentiation process [3, 4, 5].
TZDs are routinely used today in adipogenic treatment protocols to induce fat droplet accumulation within a few days in mesenchymal precursor cultures [6, 7]. In vitro cell differentiation studies indicated that TZDs inhibit osteogenic differentiation while favoring adipogenic conversion of precursor cells [8, 9, 10], and there is evidence that lack of estrogen may potentiate TZD action [11, 12, 13]. However, the potential impact of TZDs on bone status in vivo is only poorly documented in peer-reviewed journals, despite the obvious importance of the question given their widespread clinical use.
An early animal study, which as of now is published only in abstract form [14], reported a significant reduction of tibial bone mineral density in rats treated for 28 days with pioglitazone. In contrast, Tornvig et al. [15] recently reported increased bone marrow adipose tissue, but normal bone status, in mice following a 10-month treatment with troglitazone. The authors concluded that adipogenesis and osteogenesis are regulated independently in bone marrow.
We describe here studies carried out with skeletally mature female rats, investigating also the impact of TZD treatment on bone loss following ovariectomy.
Material and Methods
Animals
Animal experimentation was carried out according to regulations effective in the Kanton of Basel–Stadt, Switzerland. Wistar rats (BRL) were housed in groups of four animals at 25°C with a 12:12-hour light–dark cycle. They were fed a standard laboratory diet containing 0.8% phosphorus and 1.1% calcium (NAFAG 890, Kliba, Basel, Switzerland). Food and water was provided ad libitum.
Study Design
Skeletally mature 6-month-old intact female virgin rats were used in the first study. The rats were evenly distributed into 4 groups (N = 8/group) according to body weight and cross-sectional total bone mineral density in the tibia as measured at baseline by pQCT. The animals were treated daily for 8 weeks with BRL49653 (produced at Novartis) at doses of 5, 10, or 20 mg/kg/day or vehicle (1% carboxymethylcellulose) by gavage. Changes in bone mineral density (DEXA, pQCT) were evaluated in vivo after 4, 8, and 12 weeks of treatment and at 8 weeks before necropsy and compared to baseline values.
In the second study a similar protocol was used on 9-month-old skeletally mature estrogen-deficient rats (N = 8/group). Bilateral ovariectomy (OVX) was carried out in all animals by a dorsolateral approach under inhalation anesthesia using Forene® (isoflurane, WOB506, Lot 55370VA, Abbott) with the exception of a sham-operated group. Animals were treated daily for 12 weeks with BRL 49653 (10 mg/kg/day) or vehicle by gavage. Changes in bone mass and geometry (DEXA, pQCT) were evaluated in vivo after 4 and 8 weeks of treatment and at 12 weeks before necropsy and compared to baseline values.
Body weight was monitored weekly in both studies. Blood was taken at necropsy. DEXA measurements were carried out after necropsy on excised tibia, femur, and lumbar vertebrae. One tibia was processed for histomorphometric analysis.
DEXA Measurements
Bone mineral density (mg/cm2) of the proximal quarter of the left tibia was measured using a regular Hologic QDR-1000 instrument adapted for measurements of small animals. A collimator with a 0.9-cm diameter and ultrahigh resolution mode (line spacing = 0.0254 cm, resolution = 0.0127 cm) was used. During measurements the animals were put under inhalation anesthesia (isoflurane, 2.5%) on a resin platform provided by Hologic for soft tissue calibration. The animals were placed in a supine position. Their hind limbs were maintained in external rotation with a tape. Hip, knee, and ankle were in 90° flexion. The left tibia was measured in total and in the proximal quarter. To control the stability of the measurements, a phantom was scanned daily.
For ex vivo experiments, the right femur and lumbar vertebrae 1–4 were collected at necropsy. For soft tissue simulation 70% ethanol was used.
pQCT Measurements
Cross-sectional bone mineral density, content, and geometry were monitored in the proximal tibia metaphysis 4.5 mm distal from the intercondylar tubercle using an adapted Stratec–Norland XCT-2000 fitted with an Oxford 50-μM X-ray tube and a collimator of 1-mm diameter. The following setup was chosen for the measurements: voxel-size: 0.2 mm × 0.2 mm × 1 mm, scan speed: scout view = 20 mm/second, final scan = 10 mm/second, 1 block, contour mode = 1, peel mode = 2, cortical threshold = 610 mg/cm3, inner threshold = 610 mg/cm3.
The animals were placed in a lateral position under inhalation anesthesia (isoflurane, 2.5%). The left leg was stretched and fixed in this position.
Blood Sampling
Blood samples of 500 μl were taken in heparin at necropsy. Plasma was separated by centrifugation and frozen until analyses. Triglyceride levels were determined on a COBAS MIRA S system using standard test kits (Roche Diagnostics).
Tissue Processing and Histomorphometric Analysis
The right proximal tibia was fixed at 4°C (Kamovsky fixative) for 24 hours, dehydrated, defattened, and embedded in resin (HistoDur Leica). A set of 4- and 10-μm nonconsecutive microtome sections was cut (Microtome 2050 Supercut, Reichert Jung) in the frontal midbody plane. Structural and cellular parameters were evaluated using a Leica DM microscope fitted with a Sony DXC-950P camera and adapted Quantimet 600 software (Leica).
Three sections per animal were sampled for all sets of parameters within a sampling area starting 0.6 mm from the lowest point of the cranial growth plate–metaphyseal junction. The mineralized bone at the 10-μm section surface was silver-stained with modified von Kossa stain, thus facilitating the determination of static structural bone parameters. Images of the stained mineralized surface layer were taken and converted into binary images. Bone volume per tissue volume (BV/TV; %), trabecular thickness (Tb.Th; μm), trabecular number (Tb.N; mm−1) and trabecular separation (Tb.S; μm) were determined at 50× (data derived from 15.3 ± 0.4-mm2 tissue area) according to the plate model [16] in the secondary spongiosa of the proximal tibia metaphysis. The 4-μm-thick sections were stained with modified Giemsa stain or tartrate-resistant acid phosphatase (TRAP) for evaluation of fat cell area and bone turnover. Microscopic images of the specimen were either evaluated using a Merz grid (fat marrow content, data derived from 4.6 ± 0.1-mm2 marrow area) or digitized and evaluated semiautomatically on screen (bone turnover; 200× data derived from bone perimeter of 38.8 ± 17.6 mm). Fat volume per marrow volume (FV/MV; %) and osteoblast number per bone surface (Obl.N/BS; mm−1) were measured on the Giemsa-stained sections, while osteoclast number (Ocl.N/BS; mm−1) and eroded surface (ES/BS; %) were determined on the TRAP-stained slides. All measurements were carried out according to the recommendation of Parfitt et al. [16].
Statistical Analysis
Results are expressed as mean ± SEM. Statistical analysis was carried out using BMDP (Version 1990 for VAX/VMS, BMDP Statistical Software Inc., Cork, Ireland). The data were subjected to one-way analysis of variance (ANOVA). Equality of variances was tested by Levene F-test and differences between groups were tested using the Bonferroni-adjusted Dunnett test. Groups were tested for difference from the vehicle-treated estrogen-competent control group. For in vitro data, Student’s t-test was used (Microsoft Excel). All statistical tests were two-tailed and unpaired.
Bone Marrow Cell Culture
At necropsy, bone marrow was prepared immediately from the left tibia and femur of each animal. Cells from each treatment group were pooled. Cell cultures were established in MEMalpha/Ham’s F12 medium (Life Technologies) supplemented with 10% fetal bovine serum (Bioconcept, Allschwill, Switzerland) and antibiotics (Bioconcept). The seeding density for primary cultures was 1.5 × 108 cells per T175 flask. After 4 days in primary culture, nonadherent cells were removed by medium change and adherent cell colonies were determined (cfu/cm2). Cultures were maintained until they reached confluence (day 16) with medium change every third day. The cultures were then split 1:4 (passage 1), grown to confluence, and part of the cells were seeded for differentiation assays, while the remaining cells were frozen for later analysis.
Osteogenic Differentiation
Cells were seeded in 12-well plates at a density of 20,000 cells per well and grown for 4 days. Osteogenic medium was then added [normal medium supplemented with ascorbic acid (50 μM), β-glycerophosphate (10 mM), and calcium adjusted to 1.8 mM final]. Cultures were maintained for 21 days (medium change twice weekly) before calcium deposited in wells was determined using the MPR2 assay kit (Roche Diagnostics).
Adipogenic Differentiation
Cells were seeded in 6-well plates at a density of 400,000 cells per well and grown for 4 days. Adipogenic medium was then added [normal medium supplemented with isobutylmethylxanthine (0.2 mM) and BRL49653 (1 μM)]. Cultures were maintained for 12 days (medium change twice weekly) before determination of adipocyte numbers (Oil Red O stain and flow cytometry). Flow cytometry using the lipophilic dye Nile Red was carried out following the method developed by Gimble et al. [6] modified as described previously [7]. We used a fluorescence/forward scatter plot of events to define a selection window called R2 in which cells with high fluorescence values were counted (adipocytes). For each sample 20,000 events were collected. The results are expressed as the percentage of cells appearing in the R2 region. All determinations were done in duplicate.
GPDH Activity Assay
For biochemical determination of GPDH activity, cell extracts were prepared in Tris/EDTA buffer. Aliquots were incubated at 37°C in 1 ml of assay solution containing 0.1 M triethanolamine, 2.5 mM EDTA, 0.1 mM β-mercaptoethanol, 125 μM NADH (Roche Diagnostics), and 200 μM dihydroxyacetonephosphate. Enzyme activity is reflected by the disappearance of NADH measured by absorption at 340 nm over 10–20 minutes [17]. The protein content of each sample was analyzed in parallel using the BioRad DO Protein Assay. Results are expressed as mU/mg protein, 1 mU being defined as 1 nmol NADH reacted/minute. All determinations were done in duplicate.
Results
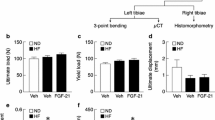
Intact animals receiving BRL49653 showed a dose-related tendency to gain weight and significantly reduced plasma triglyceride levels, as expected with such treatment (Fig. 1A, B). However, bone mineral density, as evaluated by DEXA in the entire proximal tibia and by pQCT in a proximal metaphyseal cross section, remained unchanged over the 8-week period (Fig. 1C, D). Similarly, no effect was observed on bone mineral density values in the femur (control: 281 ± 8 mg/cm2; 5-mg/kg dose: 270 ± 3 mg/cm2; 10-mg/kg dose: 285 ± 9 mg/cm2;20-mg/kg dose: 287 ± 5 mg/cm2) and in lumbar vertebral bodies (control: 257 ± 5 mg/cm2; 5-mg/kg dose: 250 ± 8 mg/cm2; 10-mg/kg dose: 249 ± 7 mg/cm2; 20-mg/kg dose: 258 ± 6 mg/cm2) as evaluated ex vivo by DEXA. Histomorphometric analysis of the tibial cancellous bone structure confirmed these findings and did not reveal differences between vehicle and BRL49653-treated animals (Fig. 2A and data not shown). Fat marrow volume was low in the proximal tibia metaphysis in these 9-month-old animals. It appeared slightly higher in the treatment groups compared to control; however, these differences did not reach statistical significance (Fig. 2B).
Weight gain (A) and plasma triglyceride levels (B) after 8 weeks of treatment of skeletally mature intact female Wistar rats. (C) Bone mineral density in the proximal tibia as determined by DEXA. (D) Change from baseline in cross-sectional bone mineral density in the tibial metaphysis as measured by pQCT; a, b, c indicate measurements at baseline and after 4 and 8 weeks, respectively. Mean ± SEM; **P< 0.01 against vehicle (ANOVA).
In mesenchymal cell cultures isolated from the bone marrow of these animals after necropsy, colony formation capacity appeared slightly reduced for the BRL49653-treated groups at 5 and 10 mg/kg; a marked reduction of about 50% was observed for the 20-mg/kg group (Fig. 3A). However, following equal expansion of cell cultures at passage 1, exposure to osteogenic conditions induced strong mineral deposition in cultures from all treatment groups (Fig. 3B). Quantification of mature adipocytes following adipogenic differentiation using Nile Red flow cytometry revealed a significant increase in adipocyte numbers in cultures established from animals treated with 10 mg/kg of BRL49653. Biochemical determination of GPDH activity produced similar results, albeit less pronounced (Fig. 4).
Analysis of marrow mesenchymal cell cultures established at necropsy. (A) Colony formation capacity; (B) mineral deposition in passage 1 cultures measured under control (white bars) or osteogenic conditions (shaded bars). Mean ± range of duplicate determinations; **P < 0.01 against vehicle (t-test).
Adipogenic differentiation of marrow mesenchymal cells. Representative photomicrographs and dot-blots from flow cytometry are shown in A. Bar = 50 μm. Corresponding quantitative results from flow cytometry are presented in B; data from enzymatic dosage of GPDH activity given in C. White bars indicate control, shaded bars indicate adipogenic treatment cell cultures. Mean ± range of duplicate determinations; *P < 0.05 against vehicle (t-test).
Following this first series of experiments we carried out a second study with ovariectomized animals, aimed at evaluating the effects of TZD treatment on bone status in a high-turnover situation. The study also appears interesting in light of recent observations that estrogen may directly inhibit adipogenesis [11, 12] and counteract PPARgamma-mediated gene transcription in specific experimental systems [13]. Estrogen deprivation induced the expected increase in body weight over the 12-week treatment period (Fig. 5A). This increase was further enhanced in the group receiving BRL49653 (borderline significance, P < 0.1). The significant drop of triglyceride levels in animals receiving the TZD confirmed the effectiveness of the treatment (Fig. 5B). Measurements of bone mineral density showed the expected significant bone loss induced by ovariectomy overtime. This bone loss was enhanced in OVX animals treated with BRL49653, reaching significance by the end of the study (Fig. 5C, D). DEXA measurements detected a slight increase in overall BMD in the proximal tibia of estrogen-competent animals during the study (+9%), while it decreased in OVX animals (−8%). This loss was significantly enhanced in estrogen-deprived animals treated with the TZD (−12%; Fig. 5C). Stable bone mass was measured by pQCT in cross sections of the proximal tibia metaphysis of sham-operated animals, while estrogen deprivation led to the expected reduction. Treatment of OVX animals with BRL49653 induced a further 30% reduction in volumetric bone mineral density (Fig. 5D). Potentiation of bone loss occurred in both the cancellous and the cortical compartments (cancellous BMD OVX/vehicle: −41.1 ± 2.4% vs. OVX/BRL49653: −48.5 ± 2.4%, P < 0.05; cortical bone mineral content at week 12 OVX/vehicle: −18.2 ± 1.7% vs. OVX/BRL49653: −24.0 ± 2.0%, P < 0.05). The latter was due to endocortical bone loss (endocortical circumference OVX/vehicle: 33.0 ± 5.2% vs. OVX/BRL49653: 44.1 ± 3.7%, P < 0.1).
Weight gain (A) and plasma triglyceride levels (B) after 12 weeks of treatment of skeletally mature sham-operated and ovariectomized (OVX) female Wistar rats, treated with vehicle or BRL49653. (C) Bone mineral density in the proximal tibia as determined by DEXA. (D) Change in cross-sectional bone mineral density in the tibial metaphysis from baseline as measured by pQCT; a,b,c,d indicate measurements at baseline and after 4, 8, and 12 weeks, respectively. Mean ± SEM; *P < 0.05, **P < 0.01 against vehicle sham; z, P < 0.1; x, P < 0.05; xx, P < 0.01 against vehicle OVX (ANOVA).
Ex vivo DEXA measurements of bone mineral density demonstrated that a similar effect had occurred in the femur (OVX/vehicle: 264 ± 5 mg/cm2 vs. OVX/BRL49653: 249 ± 5 mg/cm2, P < 0.05) and in the lumbar spine (OVX/vehicle: 236 ± 4 mg/cm2 vs. OVX/BRL49653: 208 ± 4 mg/cm2, P < 0.01).
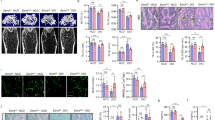
Histomorphometric analysis of the secondary spongiosa in the proximal tibia metaphysis again confirmed that cancellous bone volume was significantly reduced following ovariectomy (Fig. 6A) and more pronounced in BRL49653-treated animals. The typical structural changes due to estrogen loss were observed: Trabecular number was significantly decreased (Fig. 6B) and separation significantly increased (Fig. 6D), while trabecular thickness was only mildly decreased (Fig. 6C). The enhancement of these structural changes in response to BRL49653 treatment was visible in all three measured parameters (Fig. 6A–C).
Histomorphometric parameters (see Methods) measured in the proximal tibia metaphysis of skeletally mature sham-operated and ovariectomized (OVX) female Wistar rats treated with vehicle or BRL49653. Mean ± SEM; * P < 0.05, ** P < 0.01 against vehicle sham; z, P < 0.1; x, P < 0.05; xx, P < 0.01 against vehicle OVX (ANOVA).
Fat marrow volume was slightly higher in the 12-month-old vehicle-treated intact animals (Figs. 6E, 7A) compared to the 3-month-younger intact control animals of the first study (Fig. 2B). As expected, estrogen deficiency resulted in an increased fat marrow content (4.6-fold; Figs. 6E, 7B). This was significantly enhanced in the PPARgamma agonist-treated rats (tenfold; Fig. 6E). A substantial number of apparently mature adipocytes were visible (Fig. 7C). Osteoblast number was lower in estrogen-deficient animals than in sham-operated controls, however, this difference did not reach significance (Fig. 6F). No difference was observed between vehicle and BRL49653-treated rats. Osteoclast number (Fig. 6G) and eroded bone surface (Fig. 6H) were not different between vehicle-treated sham and OVX animals. Taken together with the data for osteoblast number, this suggests that bone turnover was not increased anymore at this late stage of estrogen deficiency. However, both parameters were still significantly increased in the estrogen-deficient animals receiving the TZD (+27% osteoclast number, Fig. 6G; +30% eroded surface, Fig. 6H).
Discussion
Treatment of intact adult rats with the TZD BRL49653 did not lead to increased fat cell differentiation in the bone marrow, and no adverse effects on bone status could be measured. However, lowered plasma triglyceride levels in treated animals demonstrated that the treatment was effective. Likewise, increased in vitro adipogenic differentiation of first-passage bone marrow mesenchymal cell cultures established from TZD-treated animals confirmed that the TZD was active.
Our observations contradict the results of a comparable study published in abstract form, where a 4-week administration of pioglitzone had led to a significant loss of bone mineral density [14]. Also, our results appear in conflict with a dog study that reported an increased fat content in bones following TZD treatment [18]. Recently, the effects of treating apolipoprotein E (apoE)-deficient mice with troglitazone for a prolonged time (10 months) were reported [15]. At the end of the treatment these animals showed strongly increased adipose tissue volume in the proximal tibia compared to control animals; however, no change of bone volume per total volume was observed in histomorphometric experiments. These data led the authors to conclude that adipogenesis and osteogenesis are regulated independently in the bone marrow. This study is in good agreement with our results with intact rats regarding overall bone status. The increased marrow fat content may be attributed to the very long treatment phase (almost lifelong). Unfortunately, the deficiency in apoE of the animals employed makes a direct comparison with our data difficult, especially as apoE may be involved in the regulation of bone mineral density [19].
No data were previously available regarding TZD effects on bone in estrogen-deficient animals. In agreement with earlier descriptions of long-term OVX rat models [20, 21], substantial bone loss occurred in our study during the 3-month period following estrogen deprivation. In the cited detailed studies, indices of cancellous bone turnover, such as osteoclast and osteoblast surfaces, were found markedly increased in OVX rats at 30 days but declined toward control levels by 90 days. The latter notion is in line with our observation that cancellous bone turnover was comparable to control levels 90 days postovariectomy. The structural changes detected in cancellous bone in the present study, namely, a predominant loss of trabecular number, are in agreement with published data [22]. In line with previous reports [23], OVX-induced deterioration of cancellous bone was associated with an increase of bone marrow fat volume.
We observed that treatment of ovariectomized rats with BRL49653 significantly enhanced bone loss and bone marrow fat content. Given the results described above for intact animals, these results were unexpected; however, recent data indicating enhanced PPARgamma transcriptional activity and effects on adipogenic differentiation [11, 12, 13] in the absence of estrogen appear in line with our observations. Interestingly, the increased marrow fat content was not paralleled by a decrease in osteoblast number as determined by histomorphometry, which is in line with the conclusion drawn by Tornvig et al. [15]. It indicates that despite increased adipogenic differentiation, the stem cell pool available for osteoblastic differentiation in the marrow is not critically depleted.
Rather, the increased bone loss observed in ovariectomized animals treated with BRL49653 is associated with increased and/or prolonged bone resorption. Thus, osteoclast number and eroded surface per bone surface were significantly increased in the TZD-treated group, and the fact that both parameters showed a roughly proportional variation indicates that increased osteoclast number, rather than activity, explains our observations. At first sight this result is again unexpected, as several recent reports demonstrated inhibition of osteoclast differentiation by PPARgamma agonists in vitro [24, 25, 26]. However, the in vivo context may be permissive for other types of regulation. It should also be noted that relatively high concentrations of TZDs (>3 μM) were required in the cited in vitro studies to induce inhibition of osteoclast differentiation, whereas effects on PPARgamma transcriptional regulation are generally observed already in the nanomolar range [3, 4, 5]. It appears possible that targets other than PPARgamma come into play at micromolar concentrations of TZDs. A specific mechanistic explanation for the increased osteoclast recruitment observed in BRL49653-treated OVX animals is lacking at present.
In summary, our data indicate that PPARgamma activators can stimulate bone resorption in estrogen-deprived animals. The effect is modest, however, as loss of estrogen is known to result in marked marrow adipogenesis, it is likely that increased signaling of PPARgamma occurs normally in bone marrow under this condition, and plays an active role in osteoclast recruitment and stimulation of bone resorption. Recent observations of cross-talk between estrogen receptor and PPARgamma-controled signaling events [11, 12, 13, 27] are in agreement with this hypothesis. Specific antagonists of PPARgamma may help to evaluate the relevance of this pathway in bone resorption stimulated by estrogen deficiency.
While this article was under review, Rzonca et al. [28] published a study demonstrating significant adverse effects of chronic treatment with BRL49653 (20 μg/kg) on the skeleton of adult mice, which are attributed to reduced osteoblastic activity. In contrast, our experiments in rats yielded no evidence for effects of the drug on bone formation.
References
HE Lebovitz JF Dole R Patwardhan EB Rappaport MI Freed (2001) ArticleTitleRosiglitazone monotherapy is effective in patients with type 2 diabetes J Clin Endocrinol Metab 86 280–288 Occurrence Handle10.1210/jc.86.1.280 Occurrence Handle1:CAS:528:DC%2BD3MXhtVGnsbo%3D Occurrence Handle11232013
F Picard J Auwerx (2002) ArticleTitlePPAR(gamma) and glucose homeostasis Annu Rev Nutr 22 167–197 Occurrence Handle10.1146/annurev.nutr.22.010402.102808 Occurrence Handle1:CAS:528:DC%2BD38XmtF2htL4%3D Occurrence Handle12055342
JM Lehmann LB Moore TA Smith–Oliver WO Wilkison TM Willson SA Kliewer (1995) ArticleTitleAn antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma) J Biol Chem 270 12953–12956 Occurrence Handle10.1074/jbc.270.50.30221 Occurrence Handle1:CAS:528:DyaK2MXmtV2itr0%3D Occurrence Handle7768881
ED Rosen CJ Walkey P Puigserver BM Spiegelman (2000) ArticleTitleTranscriptional regulation of adipogenesis Genes Dev 14 1293–1307 Occurrence Handle1:CAS:528:DC%2BD3cXjvFOgsb8%3D
TM Willson PJ Brown DD Sternbach BR Henke (2000) ArticleTitleThe PPARs: from orphan receptors to drug discovery J Med Chem 43 527–550 Occurrence Handle1:CAS:528:DC%2BD3cXht1Churk%3D Occurrence Handle10691680
JM Gimble CE Robinson X Wu KA Kelly BR Rodriguez SA Kliewer JM Lehmann DC Morris (1996) ArticleTitlePeroxisome proliferator-activated receptor-gamma activation by thiazolidinediones induces adipogenesis in bone marrow stromal cells Mol Pharmacol 50 1087–1094 Occurrence Handle1:CAS:528:DyaK28XntFCqsrw%3D Occurrence Handle8913339
V Sottile K Seuwen (2000) ArticleTitleBone morphogenetic protein-2 stimulates adipogenic differentiation of mesenchymal precursor cells in synergy with BRL 49653 (rosiglitazone) FEBS Lett 475 201–204
B Lecka–Czemik I Gubrij EJ Moemnan O Kajkenova DA Lipschitz SC Manolagas RL Jilka (1999) ArticleTitleInhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2 J Cell Biochem 74 357–371 Occurrence Handle10.1002/(SICI)1097-4644(19990901)74:3<357::AID-JCB5>3.3.CO;2-Z Occurrence Handle10412038
SM Jackson LL Demer (2000) ArticleTitlePeroxisome proliferator-activated receptor activators modulate the osteoblastic maturation of MC3T3-E1 preosteoblasts FEBS Lett 471 119–124
B Lecka–Czernik EJ Moerman DF Grant JM Lehmann SC Manolagas RL Jilka (2002) ArticleTitleDivergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation Endocrinology 143 2376–2384 Occurrence Handle10.1210/en.143.6.2376 Occurrence Handle1:CAS:528:DC%2BD38XjvFKjurg%3D Occurrence Handle12021203
ZC Dang RL Bezooijen Particlevan M Karperien SE Papapoulos CW Lowik (2002) ArticleTitleExposure of KS483 cells to estrogen enhances osteogenesis and inhibits adipogenesis J Bone Miner Res 17 394–405 Occurrence Handle1:CAS:528:DC%2BD38XitVKht78%3D Occurrence Handle11878304
R Okazaki D Inoue M Shibata M Saika S Kido H Ooka H Tomiyama Y Sakamoto T Matsumoto (2002) ArticleTitleEstrogen promotes early osteoblast differentiation and inhibits adipocyte differentiation in mouse bone marrow stromal cell lines that express estrogen receptor (ER) alpha or beta Endocrinology 143 2349–2356 Occurrence Handle10.1210/en.143.6.2349 Occurrence Handle1:CAS:528:DC%2BD38XjvFKjurw%3D Occurrence Handle12021200
X Wang MW Kilgore (2002) ArticleTitleSignal cross-talk between estrogen receptor alpha and beta and the peroxisome proliferator-activated receptor gamma1 in MDA-MB-231 and MCF-7 breast cancer cells Mol Cell Endocrinol 194 123–133 Occurrence Handle10.1016/S0303-7207(02)00154-5 Occurrence Handle1:CAS:528:DC%2BD38XntVaqu74%3D Occurrence Handle12242035
C Jennermann J Triantafillou D Cowan BGA Pennink KM Connolly DC Morris (1995) ArticleTitleEffects of the thiazolidinediones on bone turnover in the rat J Bone Miner Res 10 S241
L Tornvig J Justesen L Mosekilde E Falk M Kassem (2001) ArticleTitleTroglitazone treatment induces bone marrow adipose tissue volume but does not affect trabecular bone volume in mice Calcif Tissue Int 69 46–50 Occurrence Handle10.1007/s002230020018 Occurrence Handle1:CAS:528:DC%2BD3MXmt1egsr0%3D Occurrence Handle11685433
AM Parfitt MK Drezner FH Glorieux JA Kanis H Malluche PJ Meunier SM Ott RR Recker (1987) ArticleTitleBone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee J Bone Miner Res 2 595–610 Occurrence Handle1:STN:280:BieA3sfjsFc%3D Occurrence Handle3455637
V Sottile K Seuwen (2001) ArticleTitleA high-capacity screen for adipogenic differentiation Anal Biochem 293 124–128 Occurrence Handle10.1006/abio.2001.5121 Occurrence Handle1:CAS:528:DC%2BD3MXjslChtL0%3D Occurrence Handle11373088
A Deldar G Williams C Stevens (1993) ArticleTitlePathogenesis of thiazolidinedione-induced hematotoxicity in the dog. Diabetes 42 IssueIDSuppl 179
LM Salamone JA Cauley J Zmuda A Pasagian–Macaulay RS Epstein RE Ferrell DM Black LH Kuller (2000) ArticleTitleApolipoprotein E gene polymorphism and bone loss: estrogen status modifies the influence of apolipoprotein E on bone loss J Bone Miner Res 15 308–314 Occurrence Handle1:CAS:528:DC%2BD3cXhtlGnsrY%3D Occurrence Handle10703933
TJ Wronski LM Dann KS Scott M Cintron (1989) ArticleTitleLong-term effects of ovariectomy and aging on the rat skeleton Calcif Tissue Int 45 360–366 Occurrence Handle1:STN:280:By%2BD38bmslw%3D Occurrence Handle2509027
M Li Y Shen TJ Wronski (1997) ArticleTitleTime course of femoral neck osteopenia in ovariectomized rats Bone 20 55–61 Occurrence Handle10.1016/S8756-3282(96)00317-1 Occurrence Handle1:STN:280:ByiC2c3htFA%3D Occurrence Handle8988348
SC Miller T Wronski (1993) ArticleTitleLong-term osteopenic changes in cancellous bone structure in ovariectomized rats Anat Rec 236 433–444 Occurrence Handle1:STN:280:ByyA2s%2Fmtl0%3D Occurrence Handle8363048
D Benayahu I Shur S Ben-Eliyahu (2001) ArticleTitleHormonal changes affect the bone and bone marrow cells in a rat model J Cell Biochem 79 407–415 Occurrence Handle10.1002/1097-4644(20001201)79:3<407::AID-JCB60>3.0.CO;2-8
R Okazaki M Toriumi S Fukumoto M Miyamoto T Fujita K Tanaka Y Takeuchi (1999) ArticleTitleThiazolidinediones inhibit osteoclast-like cell formation and bone resorption in vitro Endocrinology 140 5060–5065 Occurrence Handle10.1210/en.140.11.5060 Occurrence Handle1:CAS:528:DyaK1MXmvFemsbc%3D Occurrence Handle10537132
G Mbalaviele Y Abu-Amer A Meng R Jaiswal S Beck MF Pittenger MA Thiede DR Marshak (2000) ArticleTitleActivation of peroxisome proliferator-activated receptor-gamma pathway inhibits osteoclast differentiation J Biol Chem 275 14388–14393 Occurrence Handle10.1074/jbc.275.19.14388 Occurrence Handle1:CAS:528:DC%2BD3cXjt1CnsL0%3D Occurrence Handle10799521
AC Bendixen NK Shevde KM Dienger TM Willson CD Funk JW Pike (2001) ArticleTitleIL-4 inhibits osteoclast formation through a direct action on osteoclast precursors via peroxisome proliferator-activated receptor gamma 1 Proc Natl Acad Sci U S A 98 2443–2448 Occurrence Handle10.1073/pnas.041493198 Occurrence Handle1:CAS:528:DC%2BD3MXhslKmsr4%3D Occurrence Handle11226258
H Keller F Givel M Perroud W Wahli (1995) ArticleTitleSignaling cross-talk between peroxisome proliferator-activated receptor/retinoid X receptor and estrogen receptor through estrogen response elements Mol Endocrinol 9 794–804 Occurrence Handle10.1210/me.9.7.794 Occurrence Handle1:CAS:528:DyaK2MXms1Knur0%3D Occurrence Handle7476963
SO Rzonca D Suva DC Gaddy B Montague B Lecka–Czemik (2004) ArticleTitleBone is a target for the antidiabetic compound rosiglitazone Endocrinology 145 401–406 Occurrence Handle10.1210/en.2003-0746 Occurrence Handle1:CAS:528:DC%2BD3sXhtVWgtL7O Occurrence Handle14500573
Acknowledgments
Anne Studer, Margot Brüderlin, and Reto Cortesi are gratefully acknowledged for their essential contributions to the in vivo aspects of this study. We thank Barbara Wilmering for support in cell culture.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sottile, V., Seuwen, K. & Kneissel, M. Enhanced Marrow Adipogenesis and Bone Resorption in Estrogen-Deprived Rats Treated with the PPARgamma Agonist BRL49653 (Rosiglitazone). Calcif Tissue Int 75, 329–337 (2004). https://doi.org/10.1007/s00223-004-0224-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-004-0224-8