Abstract
The purpose of this study was to (1) quantify the healing process of the human osteoporotic proximal humerus fracture (PHF) expressed in terms of callus formation over the fracture region using BMD scanning, and (2) quantify the impact of medical intervention with vitamin D3 and calcium on the healing process of the human osteoporotic fracture. The conservatively treated PHF was chosen in order to follow the genuine fracture healing without influence of osteosynthetic materials or casts. Thirty women (mean age = 78 years; range = 58–88) with a PHF, osteoporosis or osteopenia (based on a hip scan, WHO criteria), and not taking any drugs related to bone formation, including calcium or vitamin D supplementation, were randomly assigned to either oral 800 IU vitamin D3 plus 1 g calcium or placebo, in a double-blind prospective study. We measured biochemical, radiographic, and bone mineral density effect parameters to evaluate the impact on the healing process. Scanning procedures of the fractured shoulder included use of a fixation device to obtain the highest possible precision. Double scans of the fractured shoulder revealed a coefficient of variation (CV) on BMD measurements that improved from 2.8% immediately after fracture occurrence to 1.7% at 12 weeks (P = 0.003) approaching the 1.2% levels observed over the healthy shoulder. BMD was similar in the two groups at baseline (active 0.534 g/cm2 vs. placebo 0.518 g/cm2), and both increased over the 12-week observation period, with peak levels in week 6. By week 6 BMD levels were higher in the active group (0.623 g/cm2) compared with the placebo group (0.570 g/cm2, P = 0.006). Thirty seven percent of the patients presented with vitamin D levels below 30 nmol/l, indicative of mild vitamin D insufficiency. In conclusion, we have demonstrated that it is possible to quantify callus formation of the PHF with sufficiently high precision to demonstrate the positive influence of vitamin D3 and calcium over the first 6 weeks after fracture. Whether this results in more stable fractures, extends to other fracture types, or applies to other osteogenic bone agents such as bisphosphonates remains to be examined.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The formation of callus is the natural repair and fixation response to a fracture in the absence of artificial fixating devices including casts or osteosynthetic materials. Callus response is also an expression of fracture healing [1, 2], and, by numerically measuring the amount of callus, it is possible to quantify the healing process.
The conservatively treated nondisplaced proximal humerus fracture (PHF) is ideal to follow the genuine healing by callus response, since it is not fixated in a cast or by osteosynthetic materials [1, 2]. We have recently established a methodology for the measurement of bone mass with high precision using BMD scanning over the unfractured shoulder region [3]. We hypothesize that BMD scanning provides a suitable methodology to quantify changes in callus formation over the PHF, enabling us to determine the impact of medical intervention during the healing process.
Several animal studies have shown that vitamin D treatment promotes both fracture healing and mechanical strength of the callus [4, 5, 6]. However, no data on the role of vitamin D and calcium treatment in fracture healing are currently available in humans.
The aim of this study was to follow the healing process of the osteoporotic/osteopenic fracture by means of measuring changes in the calcium density (callus) over the fracture region using BMD scanning. For this purpose, only patients with a PHF and reduced BMD (T-score < −1) were included in the study, in part because reduced BMD is highly prevalent in subjects with this type of fracture, and in part to obtain a reasonably homogenic group of patients. The other aim of this study was to investigate the potential of an oral supplement of calcium and vitamin D3 mitigating some of the problems associated with the osteoporotic fracture healing process, such as delayed or insufficient healing and impeded mobility of the elderly, through nonoperative methods by means of enhanced callus formation [7, 8, 9]. For this purpose we conducted a double-blind, randomized, placebo-controlled study over a 12-week period on 30 women each with a conservatively treated PHF, who received either calcium plus vitamin D3 or placebo.
Experimental Subjects
All patients presented with an acute, X-ray confirmed, nondisplaced proximal humerus fracture (PHF), according to the Neer classification of the displaced fractures [10], and were recruited from the emergency ward of the Frederiksberg Hospital, situated in the center of Copenhagen City. Inclusion criteria were (1) women 55 years of age or older; (2) an acute nondisplaced conservatively treated PHF; (3) established osteoporosis or osteopenia as defined by a standard hip BMD (osteoporosis: T-score < −2.5 and osteopenia T-score of −1 to –2.5 according to WHO classifications [11]). Exclusion criteria were (1) suffering from any other shoulder disease or symptoms related to the shoulder region; (2) current treatment with vitamin D3, calcium, estrogen, bisphosphonate, or other medications for bone diseases within six months before the investigation; (3) any diseases affecting the liver or kidney metabolism; (4) any known diseases affecting the absorption from the gastrointestinal tract; (5) any diseases affecting thyroid gland; (6) known cancer; (7) severe dementia; (8) abnormally high serum calcium; (9) abnormal serum TSH or serum creatinine.
During a period of 20 months we identified 62 women meeting inclusion criteria (1) and (2). Eighteen declined to participate in the investigation with inconvenience cited as the primary reason, and 9 subjects were excluded due to a hip BMD T-score > −1. An additional 5 patients originally included dropped out or were subsequently excluded from the investigation due to death [N = 1 (week 0)], fracture dislocations [N = 3 (week 2)], and noncompliance [N = 1 (week 6)]. This left us with 30 Danish women (mean age = 77.7 years, range = 58–88 years) who were included and who completed the investigation.
Informed consent was obtained from all subjects and the appropriate national ethics committee approved the protocol (KF 12-049/01).
Material and Methods
A randomized, double-blind, placebo-controlled study was conducted. Half of the fracture patients received one g of calcium + 800 IU of vitamin D3, and the other half received placebo, each given in the form of chewable tablets taken twice daily for 12 weeks. Prior to entering the study, bone status of each patient was determined by a standard hip scan over the left hip. At the time of inclusion, all patients completed a questionnaire relating to physical activity and sun exposure.
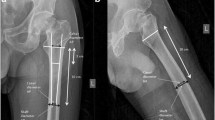
A standard X-ray image was taken over the fracture area for each participant at weeks 0, 2, 6, and 12. The X-ray images included anterior–posterior (AP) and side positions of the shoulder. For each image, the number of fracture lines (fracture grade) and the position of the fragments were described. We compared the X-rays over the observation period for each patient. Distance between the fragments was measured in millimeters, and the angling of the fragments was measured in degrees. If any changes in the location between the fragments were observed, we described the change in terms of axial distraction/consolidation, rotation, and/or angling. If the fracture evolved into a displaced type according to Neer [10], the patient was excluded from the investigation.
The Region of Interest (ROI) covered the caput humerus including the fracture area. ROI was defined as starting at the top of the caput humeri and submitted 15 mm distal over the fracture line with a breadth covering the caput. The ROI was positioned manually over each scan picture. The ROI for the hip was the femoral neck.
A Norland DEXA XR-36 BMD scanner was used for BMD measurements in the investigations. We previously described a method for BMD scanning with high precision [coefficient of variation (CV) of 1.2%] over the unfractured shoulder [3]. This technique includes use of a foam cushion device that fixes the upper arm, including the shoulder joint, and ensures an identical ROI for each patient from scan to scan. Each scanning session consisted of two separate scans over the ROI. The patient rose to a standing position on the floor between the two scans and was immediately repositioned in the foam cushion for the second scan. The method creates a scan image in the AP position equivalent to the standard AP X-ray image (Figs. 1 and 2), providing the opportunity to identify anatomical landmarks on the scanning image with support of the X-ray picture. All patients were scanned over the shoulder in duplicate at weeks 0, 2, 6, and 12, and the mean of the duplicate scans was used for statistical analysis. Scans were performed with no banding applied to the subjects during the scanning procedure.
The following parameters were measured at baseline and after 12 weeks: serum or plasma levels of: 25-OHD3 TSH, sodium, potassium, hemoglobin, sedimentation rate, and leukocytes. Serum level parameters were measured at baseline and after 2, 6, and 12 weeks for calcium (total, albumin-corrected), phosphate, alkaline phosphatase, creatinine, and albumin. To prevent bias the serum levels of calcium and vitamin D3 were blinded to the investigators during the whole investigation until the code was broken.
For initial assessment of the changes in BMD between groups, t-tests as well as the procedures outlined by Bland and Altman were used [12]. We then compared the three post-treatment BMD measurements in the two groups, using a multivariate model (due to repeated measurements on each subject) and adjusting for differences in baseline BMD by including this as a covariate. All statistical analyses were done using SYSTAT and SAS statistical software (SAS Institute, Cary, NC).
Results
Experimental Subjects
The active group consisted of 16 patients, 12 osteoporotic and 4 osteopenic. The placebo group consisted of 14 patients, 12 osteoporotic and 2 osteopenic. Time from fracture to start of treatment was a mean of 5.1 days (range = 2–11 days) for the placebo group and 6.7 days (range = 2–11 days) for the active group.
Changes in BMD over the RO1 During Fracture Healing
Both groups experienced statistically significant trends (increase) in BMD levels from week 0 over the observation period, with peak levels in week 6 (active: P = 0.00001, control: P = 0.015) (Table 1). From week 0 to week 6, the active group experienced a significantly higher trend increase in BMD than the placebo group (16.% compared with 10.0%) (P = 0.028). By week 6 we observed a significant difference between BMD levels in the active group (0.623 g/cm2) compared with the placebo group (0.570 g/cm2) (P = 0.006) (Table 1). The multivariate model, which is adjusted for repeated measurements as well as for differences in baseline BMD between the two groups, showed that the difference between the active and the placebo groups was 0.048 g/m3 (95% confidence limit 0.0060–0.0636 g/m3), which is statistically significant (P = 0.02).
Changes in Precision During Fracture Healing
The mean CV of paired double scans between week 0 and week 12 decreased significantly from 2.8% to 1.7%. (P = 0.0029). The only significant difference in precision between the active and the placebo groups was in week 6, with the active group showing a CV of 1.5% compared with 2.6% for the placebo group (P = 0.016). This compares with 1.2% on the same equipment measured over the unfractured shoulder [3].
Dislocation of the Fractures over the Observation Period
There were no significant differences in relation to changes in the location between the fragments or fracture grades (number of fracture lines) between the active and the placebo groups over the observation period. All patients were bandaged for one week in a fixated collar and cuff sling, one week in a loose collar and cuff sling, and unbandaged thereafter.
Changes in Hip BMD During the Observation Period
No statistically significant changes in hip BMD levels were observed in either group between weeks 0 and 12 (Table 2).
Changes in Biochemical Tests During Fracture Healing
Serum calcium levels were similar in the two groups at inclusion (placebo: 2.27 ± 0.09 vs. active: 2.33 ± 0.10 nmol/l); however, serum calcium levels increased in the active group [2.38 ± 0.09 nmol/l at week 6 (P < 0.001)]. Hereafter no difference was detected in otherwise unaltered serum calcium levels. Serum 25-OHD3 levels were similar in the two groups at inclusion [placebo: 39 ± 16 nmol/l (range = 22–79) vs. active: 40 ± 21 nmol/l (range = 16–87)]. Levels increased significantly in the active group from week 0 to week 12 (72 ± 17 nmol/l) (P < 0.001), whereas no changes occurred in the placebo group. Alkaline phosphatase were similar in the two groups at inclusion (placebo: 218 ± 57 vs. active: 211 ± 42 U/l), and increased equally in the two groups after 3 weeks (placebo: 265 ± 41 vs. active: 279 ± 65 U/l, both P < 0.001). Hereafter alkaline phosphophates returned to baseline values with no difference in between. At baseline, serum calcium and vitamin D levels did not correlate (N = 30).
Discussion
We have used the undislocated PHF as a model for the osteoporotic/osteopenic fracture as it provides an opportunity to investigate the genuine healing process without the interference from external or internal fixation on an analogous anatomical structure to the conservatively treated hip fracture. That osteoporosis/osteopenia indeed is prevalent in women suffering a PHF is evident, since at least 80% of women presenting with a PHF in the present study had osteoporosis or osteopenia.
The present study demonstrates that we were able to follow the increase in callus formation by means of BMD scanning on the undislocated PHF using the described procedure. In evaluating the effectiveness of the methodology for BMD measurement over the fractured shoulder, we found that within 12 weeks of fracture occurrence precision levels approached that of the unfractured shoulder [3] and were of a similar magnitude as those on other skeletal sites [13]. As highlighted in earlier work, fixation of the proximal humerus joint was essential to obtain satisfactory precision since small changes in the position of the ROI can lead to a dramatic drop in precision. Despite this, and working with an unfixated fracture, we were able to demonstrate satisfactory short-term precision, representing the ability to reposition subjects and place the measure box (for the ROI) over the identical area between successive scans. BMD increased after fracture, and the peak in week 6 expresses the end of the callus production prior to reorganization to organized bone [1, 2], which was confirmed radiographically.
The main goal of the present study was to investigate if calcium and vitamin D3 supplementation could enhance callus formation in patients with osteopenia or osteoporosis, a PHE, and not taking any drugs affecting bone formation, including calcium or vitamin D supplementation. In a randomized study we found that both active and placebo groups increased BMD over the shoulder region with a peak level in week 6, but the increase in the active group was significantly higher (16.8% vs. 10.0%) (P = 0.028) than in the placebo group. Since we have a repeated measures design and a baseline difference in BMD between the two groups, we also applied a multivariate model. We found the difference between the two groups was statistically significant (P = 0.02), with a mean BMD difference between the groups of 0.048 g/m3 (95% confidence limit 0.0060–0.0636 g/m3).
Scanning precision was similar in the two groups but showed a significant difference at week 6 with the active group showing a CV of 1.5% compared with 2.6% for the placebo group (P = 0.016). Although this may be influenced by the relatively small sample size, one possible explanation for the significant improvement in precision for the active group over the placebo group by week 6 may be the earlier stabilization of the fracture as a result of the increased callus formation.
The difference in BMD changes over the observation period between the active group and the placebo group could be interpreted as a positive impact on the fracture healing process by the vitamin D3 and calcium from a higher concentration in the cellular environments of these agents. This potentially facilitates the osteoblast in-building Ca2+ and producing callus and/or via an increase in the osteoblast/osteoclast turnover from the osteogenic cell.
There were no differences between the two groups relating to osteoporotic/osteopenic status, age, physical activity, sun exposure, or immobility of the fracture area including number, design, and location of the fracture lines.
Since none of the patients was taking calcium or vitamin D supplementation, it was expected that some degree of vitamin D insufficiency among the patients would be found. Mean vitamin D levels were approximately 40 nmol/l, and 11/30 (37%) of the patients had vitamin D levels below 30 nmol/l, a value indicative of mild vitamin D insufficiency [14]. These figures are similar to those previously found among European (including Danish) elderly people [14]. Thus, some of the positive effect on the amount of callus in the active group might be due to treating mild vitamin D insufficiency. On the other hand, the population under study represents typical Danish (and European) women, the majority of whom do not take calcium and vitamin D supplement. In a European survey on elderly people, nearly 90% did not take vitamin D supplements [14].
It is possible that the measurement of changes to and the amount of callus as an expression of the efficiency of fracture healing could be biased, since the amount of callus rises with the number of fracture lines and is inversely related to the fixation grade [15, 16]. However, we found no significant differences in fracture lines and fixation grades between the two groups when systematically studied.
Another bias could be the potential effect of calcium and vitamin D supplementation on regional disuse osteoporosis. The ROI used in our studies included both the fracture lines with subsequent callus formation and some amount of adjacent bone, both trabecular and cortical, although the amount was limited by placing the lower cut off level of the ROI as close as 15 mm to the most distal part of the fracture. This regional and fracture-close bone was probably subject to the regional acceleratory phenomenon (RAP) leading to regional osteoporosis [17]. Thus, our data might be the result of two countervailing processes: callus formation and temporary regional bone degradation. In order to elucidate this, we constructed an additional ROI measuring BMD from the original ROI and 10 mm more distal on the humerus shaft. BMD over this region did not change over time, neither was there any effect of calcium and D vitamin supplementation compared with placebo (data not shown). Therefore, this potential bias is probably of less importance.
The question remains if calcium density over the fracture area expresses the efficiency or quality of the healing process. Other studies have shown little relationship between calcium concentration in trabecular bone and bending strength [18, 19, 20, 21]. Despite its high concentration of calcium, callus has little bending strength and it is therefore possible that the vitamin D3 and calcium increases the calcium concentration over the fracture area but still yields a brittle bone. This requires further investigation into fracture strength and stability.
In conclusion, we have demonstrated that it is possible to quantify callus formation with sufficiently high precision over the first 6 weeks after fracture to establish the positive impact of vitamin D3 and calcium supplementation on the PHF. Women with reduced bone mass (osteopenia or osteoporosis) and an acute PHF might benefit from a supplementation of oral calcium plus vitamin D3 during the healing process. This is found in women not already taking calcium or vitamin D supplement. Whether this results in more stable fractures, extends to other fracture types, or applies to other osteogenic bone agents such as bisphosphonates remains to be examined.
References
Chapman, MW, Closed treatment of fractures and dislocations, In: Chapman’s Orthopaedic Surgery 3rd edition, (2001) Ed: Chapman, MW, Lippincott Williams and Wilkins, Philadelphia: pp 228–229
Mckibbin, B., Repair of fractures, In: Watson-Jones Fractures and Joint Injuries, (1982), Ed: J.N. Wilson, Churchill Livingstone, Edinburgh. pp 14–28
AM Doetsch J Faber N Lynnerup I Wätjen H Bliddal B Danneskiold–Samsøe (2002) ArticleTitleBone mineral density measurement over the shoulder region Calcif Tissue Int 71 308–314
H Omeroglu Y Ates O Akkus F Korkusuz A Bicimoglu N Akkas (1997) ArticleTitleBiomechanical analysis of the effects of single high-dose vitamin D3 on fracture healing in a healthy rabbit model Arch Orthop Trauma Surg 116 271–274
AD Delgado–Martinez ME Martinez MT Carrascal M Rodriguez–Avial L Munuera (1998) ArticleTitleEffect of 25-OH-vitamin D on fracture healing in elderly rats J Orthop Res 16 650–653
PF Brumbaugh DP Speer MJ Pitt (1982) ArticleTitle1 alpha, 25-Dihydroxyvitamin D3 a metabolite of vitamin D that promotes bone repair Am J Pathol 106 171–179
Chapman, MW, Closed treatment of fractures and dislocations, In: Chapman’s Orthopaedic Surgery, 3rd edition, (2001) Ed: Chapman, MW, Lippincott Williams and Wilkins, Philadelphia: pp 230
Chapman, MW, Osteoporosis, In: Chapman’s Orthopaedic Surgery 3rd edition, (2001) Ed: Chapman, MW, Lippincott Williams and Wilkins, Philadelphia: pp 3497
DR Steinberg RM Szabo (1995) ArticleTitleDecision making in upper extremity problems in the elderly Clin Orthop Jul . 63–69
2nd Neer CS (1970) ArticleTitleDisplaced proximal humeral fractures. I. Classification and evaluation J Bone Joint Surg Am 52 1077–1089
JA Kanis (1994) ArticleTitleAssessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group Osteoporos Int 4 368–381
JM Bland DG Altman (1986) ArticleTitleStatistical methods for assessing agreement between two methods of clinical measurement Lancet 1 307–310
Norland XR-36 Dexa Scanner Manuel, Norland, Inc, Fort Atkinson WI., (1993)
RP Wielen Particlevan der MR Lowik H Berg Particlevan den LC Groot Particlede J Haller O Moreiras WA Staveren Particlevan (1995) ArticleTitleSerum vitamin D concentrations among elderly people in Europe Lancet 346 207–210
Chapman, MW, Closed treatment of fractures and dislocations, In: Chapman’s Orthopaedic Surgery 3rd edition, (2001) Ed: Chapman, MW, Lippincott Williams and Wilkins, Philadelphia: pp 226, 229
D Paley MA Catagni F Argnani A Villa GB Benedetti R Cattaneo (1989) ArticleTitleIlizarov treatment of tibial nonunions with bone loss Clin Orthop Apr . 146–165
HM Frost (1989) ArticleTitleThe biology of fracture healing. An overview for clinicians Part I. Clin Orthop Nov . 283–293
CI Moorcroft PJ Ogrodnik PB Thomas RH Wade (2001) ArticleTitleMechanical properties of callus in human tibial fractures: a preliminary investigation Clin Biomech 16 776–782
K Choi SA Goldstein (1992) ArticleTitleA comparison of the fatigue behavior of human trabecular and cortical bone tissue J Biomech 25 1371–1381
SA Goldstein R Goulet D McCubbrey (1993) ArticleTitleMeasurement and significance of three-dimensional architecture to the mechanical integrity of trabecular bone Calcif Tissue Int 53 IssueIDSuppl 1 S127–132
SG Roberts CR Steele (2000) ArticleTitleEfficacy of monitoring long-bone fracture healing by measurement of either bone stiffness or resonant frequency: numerical simulation J Orthop Res 18 691–697
Acknowledgments
Thanks to laboratory technicians Tove Riis, Sala Hirschorn, Jette Nielsen, and secretary Helle Brandrup. Vitamin D measurements were generously provided by Peter Schwartz, M.D., Department of Biochemistry, Hvidovre University Hospital, Denmark. Statistician Lene Theil Skovgaard, Biostat, The Panum Institute, University of Copenhagen is acknowledged for her statistical contribution. The study was supported by grants from The Danish Medical Research Council, Green Flash ApS., A.V. Lykfeldt og Hustrus Legat, Nycomed A/S, and The OAK Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Doetsch, A.M., Faber, J., Lynnerup, N. et al. The Effect of Calcium and Vitamin D3 Supplementation on the Healing of the Proximal Humerus Fracture: A Randomized Placebo-Controlled Study. Calcif Tissue Int 75, 183–188 (2004). https://doi.org/10.1007/s00223-004-0167-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-004-0167-0