Abstract
The correlations between the serum levels of OPG, RANKL with age, menopause, bone markers, and bone mineral densities (BMDs) at the lumbar spine and proximal femur were studied in 504 pre- and postmenopausal Chinese women aged 20–75 years. We found that age was positively and negatively correlated with serum concentrations of OPG (r = 0.442, P < 0.001) and RANKL (r = −0.263, P < 0.001), respectively. Compared with premenopausal women, postmenopausal women showed higher serum OPG levels (107.6 ± 3.0 vs 72.0 ± 1.8 pg/ml, P < 0.001), lower serum RANKL concentrations (4.7 ± 0.4 vs. 5.8 ± 0.3 pg/ml, P < 0.001) and RANKL/OPG ratios (0.045 ± 0. 004 vs. 0.099 ± 0.008, P < 0.001). Neither serum levels of OPG nor RANKL or RANKL/OPG ratio correlated with BMDs after adjustment of age and menopause. They also showed no differences among normal, osteopenic and osteoporotic postmenopausal women. Serum levels of OPG were positively correlated with urinary excretion of NTx (r = 0.1453, P = 0.006). Serum levels of RANKL (r = −0.1928, P < 0.001) and RANKL/OPG ratio (r = −0.1303, P = 0.013) were inversely correlated with serum concentrations of OC. In multiple regression analysis, up to 20% variance (R2 = 0.106–0.224) of the OPG-RANKL system in peripheral circulation can be explained by age, menopause and bone markers.
These results suggest that although serum OPG and RANKL concentrations were unrelated with BMDs, the age– and menopause– dependent changes of serum OPG and RANKL might be a protective mechanism against the accelerated bone loss in postmenopausal women.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The receptor activator of nuclear factor-kappa B ligand (RANKL) is a transmembrane ligand expressed on osteoblasts that bind to RANK. Interaction between RANK and RANKL induces differentiation, activation and prevention of the apoptosis of osteoclastic cells, leading to enhanced bone resorption and bone loss [1,2]. The effects of RANKL can be neutralized by its decoy receptor osteoprotegerin (OPG), which plays an important inhibitory role in the regulation of osteoclastic bone resorption [3,4]. The OPG gene knockout mice demonstrated uncontrolled bone resorption and severe osteoporosis [5]. It was recently reported that homozygous deletion of the gene encoding OPG is the potential cause of juvenile Paget’s disease in some patients [6]. On the other hand, RANKL knockout mice showed a decreased number of osteoclasts, increased bone mineral density (BMD), even osteopetrosis [7]. A single intravenous injection of recombinant OPG in young growing rats [8] and postmenopausal women with osteoporosis [9] caused significant gains in bone volume and density, together with a rapid and sustained suppression of osteoclastic bone resorption and a delayed reduction of bone formation.
However, the physiological and pathophysiological significances of the variations in serum concentration of OPG, especially the serum level of RANKL, are not fully understood [10]. In human studies, the correlation between serum levels of OPG and BMDs as well as bone biochemical markers are inconsistent in different reports [11, 12, 13]. Although the changes of serum RANKL were reported in Paget’s disease [14], rheumatoid arthritis (RA) [15] and multiple myeloma (MM) [13], the studies of their associations with age and BMDs, their changes before and after menopause are rarely seen in relatively large cohorts of pre- and postmenopausal women.
The objectives of this study were to investigate the associations between serum concentrations of OPG, RANKL and age, menopause, BMD and bone biochemical markers, and to determine the factor (s) influencing serum levels of OPG and RANKL in 504 Chinese pre- and postmenopausal women aged 20–75 years.
Subjects and Methods
Subjects
We initiated our Genetic and Environmental Evaluation of Bone mass (GEEB) study in 2002. Several genetic markers, biochemical indices, and environmental factors were investigated in a cohort of healthy pre- and postmenopausal women from Shanghai, China [16].
Briefly, healthy female volunteers aged 20–75 were recruited through posters. The health status of the subjects was assessed by present and past history, as well as physical examinations and laboratory tests, including blood pressure, heart rate, fasting plasma glucose, lipid profile, and liver and kidney function. Self-reported fracture history was recorded. Those suffering diseases other than osteoporosis or taking drugs that might affect bone metabolism were excluded. A total of 504 healthy women, average age 46.6 ± 0.7 years, were eligible for the study. Menopause is recognized to have occurred after 12 consecutive months of amenorrhea, and postmenopause is defined as dating from the final menstrual period [17]. Thus, there were 282 premenopausal women with regular menstrual cycles, and 222 postmenopausal women in our cohort.
All the participants completed written informed consent before measurements. The ethical committee of Rui-jin Hospital, Shanghai Second Medical University, approved the study.
Measurement of BMDs
BMDs of lumbar spine and proximal femur, including femoral neck, Wards triangle, trochanter, total hip and L2-4 were assessed using dual-energy X-ray absorptiometry (DXA) (Lunar Expert 1313). BMD was automatically calculated from the bone area (cm2) and bone mineral content (g) and expressed absolutely in g/cm2. The postmenopausal women were divided into normal, osteopenic and osteoporotic groups according to WHO criteria [18].
Sample Collection
Blood (in the follicular phase of the menstrual cycle in premenopausal women) and a second morning urine samples were collected after an overnight fast. Serum was separated and frozen immediately after blood drawing. Both serum and urine samples were stored at −80°C until analysis.
Measurement of Bone Biochemical Markers and Serum OPG, RANKL
Serum concentration of intact osteocalcin (OC) was measured by the immunoradiometric method (Diagnositic Systems Laboratories, USA). Urinary level of N-terminal crosslinking telopeptide of type I collagen (NTx) was measured by enzyme-linked immunosorbent assay (ELISA) (Ostex International, USA). Urinary determinations were expressed in relation to creatinine excretion.
Serum concentrations of OPG and RANKL were both measured by ELISA (Biomedica, Vienna, Austria). The RANKL/OPG ratio was obtained for all individuals. The detection limit of OPG and RANKL is 2.8 pg/ml and 1.6 pg/ml, respectively. All the samples had detectable levels for OPG, while 9 were below the test limit for RANKL.
All the samples were measured in duplicate according to the manufacture’s instruction and then averaged. In cases of patients with undetectable values, the detection limit value was used for analysis. The intra- and interassay coefficients of variation (CV) for each marker, respectively, were as follow: for OC 1.4% – 3.4% and 3.3% – 5.3%, for NTx 5% – 19% and 3% – 5% for OPG <10% and <10%, for RANKL 3% – 5% and 6% – 9%.
Statistical Analysis
The results were expressed as mean ±SEM. All the data not normally distributed were logarithmically transformed before analysis. Person’s correlation and partial correlation analysis, ANOVA, covariance analysis and multiple regression analysis were used as appropriate. P values less than 0. 05 were considered significant.
Results
In the present study, serum levels of OPG showed no association with serum concentration of RANKL (r = −0.033, P = 0.514). Age was positively associated with serum level of OPG (r = 0.442, P < 0.001), but negatively correlated with that of RANKL (r = −0.263, P < 0.001) and RANKL/OPG ratio (r = 0.460, P <0.001).
Table 1 shows the significant differences of BMDs and bone biochemical markers between pre– and postmenopausal women. The serum concentration of OPG was higher, while serum level of RANKL and RANKL/OPG ratio were statistically and significantly lower in postmenopausal women.
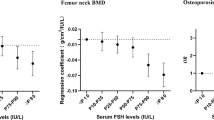
Serum concentrations of OPG, RANKL and RANKL/OPG ratios were not related to BMDs at each site after adjustment for age and menopause. There was a weak but significantly positive correlation between serum level of OPG and urinary excretion of NTx (r = 0.1453, P = 0.006). Serum RANKL level was negatively correlated with serum concentration of OC (r = −0.1928, P < 0.001). RANKL/OPG ratio showed a trend to negatively correlate with NTx (r = −0.1026, P = 0.051), but was significantly negatively associated with serum level of OC (r = −0.1303, P = 0.013).
When the postmenopausal women were divided into normal, osteopenic and osteoporotic groups according to WHO criteria [18], it was found that age, years-since-menopause (YSM), NTx and BMDs at lumbar spine and proximal femur were different among these 3 groups (Table 2). But serum concentrations of OPG, RANKL and RANKL/OPG ratio showed no difference among the groups.
Multiple regression analysis was performed to evaluate the contribution of age, menopause, NTx, OC, BMDs to serum levels of OPG, RANKL and RANKL/OPG ratio. The results revealed that menopause (r2 = 0.181, β = −0.200), age (r2 = 0.023, β = 0.267) and urinary NTx (r2 = 0.016, β = 0.130) were the independent contributors for serum OPG (R2 = 0.214). Age (r2 = 0.069, β = −0.331) and serum OC (r2 = 0.036, β = −0.203) were the independent determinants for serum RANKL (R2 = 0.106). Age (r2 = 0.201), serum OC (r2 = 0.011, β = −0.118) and urinary NTx (r2 = 0.012, β = −0.110) were the major factors influencing RANKL/OPG ratio (R2 = 0.224).
Discussion
In the present study, we have studied the serum levels of OPG and RANKL in 504 pre- and postmenopausal Chinese women. We found a increase of serum OPG with age, which was in accordance with the data from the Japanese population [19] and others [20]. It was also revealed that serum levels of OPG remained relatively constant in individuals younger than ~50 years [15], and were elevated sharply after 60 years of age [12]. Although we did not divide our samples according to age, the results also revealed a significant increase of OPG serum levels in postmenopausal women, suggesting that in addition to age, OPG serum levels might also be influenced by menopause. However, serum concentrations of OPG were also found to be age independent [21] and did not differ among premenopausal and postmenopausal women [22]. The differences in sample size, the opposing effects of estrogen deficiency and increased bone turnover on OPG production, as suggested by Eghbali-Fatourechi et al. [22], may be a cause for the discrepancies.
Serum RANKL concentration and RANKL/OPG ratio were found to be decreased with age, and the reduction was prominent in postmenopausal women in our cohort. But other studies did not observe the age-dependent [15] or menopause-related changes of RANKL serum level in healthy individuals. However, the common finding in these studies including ours, was the lack of correlation between OPG and RANKL, suggesting that different mechanisms are involved in the regulation of OPG and RANKL synthesis [15].
Since the OPG–RANKL system is very important for the regulation of bone remodeling, several studies analyzed the effects of serum OPG on BMDs and bone biochemical markers, but with inconsistent results [11, 12, 13,20]. There have been even less data on the relationship between serum RANKL and BMDs until now.
The bone mineral densities in postmenopausal women were positively or negatively correlated, or not related with serum OPG concentrations in various studies [11,12,23]. Since serum levels of OPG, RANKL and BMDs are all influenced by age and menopause, a partial correlation analysis was performed in our study after adjustment for these two factors to elucidate the associations between serum levels of OPG, RANKL, RANKL/OPG ratio and BMDs. None of these 3 parameters was correlated with BMDs at lumbar spine or proximal femur after adjustment for age and menopause. Serum levels of OPG, RANKL and RANKL/OPG ratio also showed no differences among normal, osteopenic and osteoporotic postmenopausal women. Yano et al. [19] reported an increase of OPG in osteoporotic versus nonosteoporotic women, but other studies do not confirm such a result [12,23]. In Paget’s disease, serum RANKL concentration and RANKL/OPG ratios were not different between patients and controls, although the mean OPG levels in patients were higher [14].
The mechanisms and pathophysiological role of high serum OPG levels and low RANKL concentrations in postmenopausal women are not clear. It is suggested that the rise of serum OPG levels with age and bone turnover [1, 24, 25], especially in osteoporotic women [20], and their decrease in Paget’s disease patients with higher baseline values after antiresorptive treatment [14] may reflect a paracrine mechanism of the skeleton to compensate for increased bone resorption. The positive correlation of serum OPG levels with urinary NTx and higher concentrations of serum OPG in postmenopausal women in our study also support such a hypothesis. On the other hand, lower serum levels of OPG may present as a risk factor for osteoporotic fracture [20].
RANKL is an essential factor for osteoclasts’ activities [1], but it is somewhat difficult to explain our results that serum levels of RANKL and OC were both reduced in postmenopausal women, and were negatively associated.
Many studies demonstrated higher serum OC levels in postmenopausal women than in premenopausal ones [26, 27, 28]. But similar to our study, some studies found lower serum levels of OC in osteoporotic postmenopausal women [29] and in women who had sustained a fracture [30]. Preanalytical variability of this marker may be one of the factors accounting for these discordant results [31, 32]. However, since the blood samples were drawn, stored, and tested with the same protocol and under the same conditions in our study, such factors, if any, should generally have an impact on all samples, not only to postmenopausal women, thus are unlikely to be the major attributable factor for this difference.
Although there is some new understanding for the function of circulating OC [33], it is generally considered to cause osteoblast activity [32] and its level showed no difference between serum and cancellous bone [34]. So, the changes of serum OC levels in postmenopausal women may reflect a reduced bone formation. A negative association between serum concentration of RANKL and OC found in our study seems to indicate that the lower the RANKL (less osteoclastic activity), the higher the OC (more osteoblastic function). If this is true, then the suppressed level of RANKL may also be a limiting factor to impaired bone formation and excessive resorption in our postmenopausal women, as indicated by a lower concentration of serum OC and higher levels of urinary NTx: it will not further aggravate osteoclastic activities, and may even provide more posibilities for osteoblasts to restore their function. However, our hypothesis is only inferred from a marker of late osteoblastic activities, which may not be synthesized simultaneously with other bone formation parameters [35]. Measurement of a more stable and reproducible form of osteocalcin, such as N-terminal midpeptide, and/or other bone formation markers, and observing their changes longitudinally may further examine and modify this speculation.
It should also be noted that RANKL is made as a membrane anchored molecule, and can be cleaved from the cell surface as a soluble molecule [36]. The RANKL test kits available now are designed to detect soluble RANKL in biological fluids, such as serum. Whether the amount and activities of soluble RANKL are related to their membrane-bound forms, and whether the presence of OPG in serum will interfere with the detection of RANKL are still under investigation [2, 37].
In order to further explore the relative contribution of age, menopause, bone biochemical markers and BMDs to serum OPG, RANKL, and RANKL/OPG ratio, we performed multiple regression analyses. The results revealed that up to nearly 20% variance of OPG-RANKL system in peripheral circulation can be explained by age, menopause and bone biochemical markers, but bone mineral densities were not the independent determinants. These results suggested that although serum OPG and RANKL are not specific markers for bone mineral densities, several possibilities may account for this.
First, besides bone, a variety of other tissues, such as lung, kidney and heart all can express OPG mRNA [4]. Serum OPG has also been involved in vascular calcification [38], diabetic microvascular complications [39] and is associated with the cause of mortality in elderly women [23]. RANKL/OPG ratio is also a prognostic index for survival in MM patients [13]. So, although changes in the OPG-RANKL system may not be specific to bone metabolism, they might function as a compensatory self-protective mechanism against age-associated diseases [12].
Second, serum OPG and RANKL levels may not consistently reflect the local milieu in the bone. The changes of OPG-RANKL system in bone microenvironment seems to be different from those in peripheral circulation. For example, in contrast to the common findings of age–related increase of serum OPG, the expressions of OPG in human bone marrow cells decreased with age [40]. The OPG mRNA levels in mice osteoblast–like cells also declined with age, while the expressions of RANKL mRNA in adult and old mice were higher than those in the young mice [41]. The surface concentration of RANKL per bone marrow mononuclear cells was increased by 2–3 fold in early postmenopausal women over premenopausal and estrogen–treated postmenopausal women, and showed no correlation with its serum value [22]. These findings highlight the importance of assessing the levels of these factors directly in the bone microenvironment [22].
In conclusion, the present study showed that in Chinese women, age and menopause were the major contributors to serum OPG and RANKL concentrations; bone biochemical markers also had some effects. Although serum OPG and RANKL levels were unrelated to BMDs and were not different among normal, osteopenic and osteoporotic postmenopausal women, the age–dependent increase of serum OPG concentration is very likely a compensatory response for accelerated bone resorption. Whether the age–dependent decrease of serum RANKL level is also beneficial for restoring the reduced bone formation in postmenopausal women needs to be further studied in different populations, especially in longitudinal studies.
References
S Khosla (2001) ArticleTitleMinireview: the OPG/RANKL/RANK system Endocrinol 142 5050–5055 Occurrence Handle10.1210/en.142.12.5050 Occurrence Handle1:CAS:528:DC%2BD3MXovVSntb8%3D Occurrence Handle11713196
DL Lacey E Timms HL Tan (1998) ArticleTitleOsteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation Cell 93 165–176 Occurrence Handle10.1016/S0092-8674(00)81569-X Occurrence Handle1:CAS:528:DyaK1cXivVyhtrg%3D Occurrence Handle9568710
PJ Kostenuik V Shalhoub (2001) ArticleTitleOsteoprotegerin: a physiological and pharmacological inhibitor of bone resorption Curr Pharm Res 7 613–635 Occurrence Handle1:CAS:528:DC%2BD3MXjsVyis78%3D
WS Simonet DL Lacey CR Dunstan (1997) ArticleTitleOsteoprotegerin: a novel secreted protein involved in the regulation of bone density Cell 89 309–319 Occurrence Handle10.1016/S0092-8674(00)80209-3 Occurrence Handle1:CAS:528:DyaK2sXislCgtLc%3D Occurrence Handle9108485
A Mizuno N Amizuka K Irie (1998) ArticleTitleSevere osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin Biochem Biophys Res Commun 247 610–615 Occurrence Handle10.1006/bbrc.1998.8697 Occurrence Handle1:CAS:528:DyaK1cXkt12htr8%3D Occurrence Handle9647741
MP Whyte SE Obrecht PM Finnegan (2002) ArticleTitleOsteoprotegerin deficiency and juvenile Paget’s disease N Engl J Med 347 175–184 Occurrence Handle10.1056/NEJMoa013096 Occurrence Handle1:CAS:528:DC%2BD38Xlt12jsL0%3D Occurrence Handle12124406
YY Kong H Yoshida I Sarosi (1998) ArticleTitleOPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis Nature 397 315–323
C Capparelli S Morony K Warmington (2003) ArticleTitleSustained antiresorptive effects after a single treatment with human recombinant osteoprotegerin (OPG): a pharmacodynamic and pharmacokinetic analysis in rats J Bone Miner Res 18 852–858 Occurrence Handle1:CAS:528:DC%2BD3sXjslahsrk%3D Occurrence Handle12733724
PJ Bekker D Holloway A Nakanishi (2001) ArticleTitleThe effect of a single dose of osteoprotegerin in postmenopausal women J Bone Miner Res 16 348–360 Occurrence Handle1:CAS:528:DC%2BD3MXptlOquw%3D%3D Occurrence Handle11204435
KA Buckley WD Fraser (2002) ArticleTitleReceptor activator for nuclear factor kappa B ligand and osteoprotegerin: regulators of bone physiology and immune responses/potential therapeutic agents and biochemical markers Ann Clin Biochem 39 551–556 Occurrence Handle10.1258/000456302760413324 Occurrence Handle1:CAS:528:DC%2BD3sXhtFGltbk%3D Occurrence Handle12564836
A Rogers G Saleh RA Hannon (2002) ArticleTitleCirculating estradiol and osteoprotegerin as determinants of bone turnover and bone density in postmenopausal women J Clin Endocrinol Metab 87 4470–4475 Occurrence Handle10.1210/jc.2002-020396 Occurrence Handle1:CAS:528:DC%2BD38XnvFCku74%3D Occurrence Handle12364420
S Kudlacek B Schneider W Woloszczuk (2003) ArticleTitleSerum levels of osteoprotegerin increase with age in a healthy adult population Bone 32 681–686 Occurrence Handle10.1016/S8756-3282(03)00090-5 Occurrence Handle1:CAS:528:DC%2BD3sXks1WksLc%3D Occurrence Handle12810175
E Terpos R Szydlo JF Apperley (2003) ArticleTitleSoluble receptor activator of nuclear factor kappa B ligand (RANKL)/osteoprotegerin (OPG) ratio predicted survival in multiple myeloma. Proposal for a novel prognostic index Blood 102 1064–1069 Occurrence Handle10.1182/blood-2003-02-0380 Occurrence Handle1:CAS:528:DC%2BD3sXlvFCmt7Y%3D Occurrence Handle12689925
L Alvarez P Peris N Guanabens S Vidal I Res F Pons X Filella A Monegal J Munoz-Gomes AM Ballesta (2003) ArticleTitleSerum osteoprotegerin and its ligand in Paget’s disease of bone: relationship to disease activity and effect of treatment with bisphosphonates Arthritis Rheum 48 824–828 Occurrence Handle10.1002/art.10834 Occurrence Handle1:CAS:528:DC%2BD3sXislKgurg%3D Occurrence Handle12632438
M Ziolkowska M Kurowska A Radzikowska (2002) ArticleTitleHigh levels of osteoprotegerin and soluble receptor activator of nuclear factor κB ligand in serum of rheumatoid arthritis patients and their normalization after anti-tumor necrosis factor α treatment Arthritis Rheuma, 46 1744–1753
JM Liu HY Zhao Ning Guang (2004) ArticleTitleRelationship between body composition and bone mineral density in healthy young and premenopausal Chinese women Osteoporos Int 15 238–242 Occurrence Handle10.1007/s00198-003-1536-7 Occurrence Handle14727013
WHO Scientific Group Research on the menopause in the 1990’s (1996) A report of the WHO Scientific Group. World Health Organization, Geneva, Switzerland. 866:1–79
WHO Assessment of fracture risk, its application to screening for postmenopausal osteoporosis (1994) Report of a WHO study Group. WHO Technical Report Series, No. 843
K Yano E Tsuda N Washida (1999) ArticleTitleImmunological characterization of circulating osteoprotegerin/osteoclastogenesis inhibiting factor: increased serum concentrations in postmenopausal women with osteoporosis J Bone Miner Res 14 518–527 Occurrence Handle1:STN:280:DyaK1M3ltVygsw%3D%3D Occurrence Handle10234572
A Fahrleitner-Pammer H Dobnig C Piswanger-Soelkner (2003) ArticleTitleOsteoprotegerin serum levels in women: correlation with age, bone mass, bone turnover and fracture status Wien Klin Wochenschr 115 291–297 Occurrence Handle1:CAS:528:DC%2BD2cXltVen Occurrence Handle12793029
K Junk M Lein K Hosslin (2002) ArticleTitleOsteoprotegerin and receptor activator of nuclear factor-Kappa B ligand (RANKL) in the serum of healthy adults Int J Biol Markers 17 177–181 Occurrence Handle1:CAS:528:DC%2BD3sXlt1aguw%3D%3D Occurrence Handle12408468
G Eghbali-Fatourechi S Khosla A Sanyal (2003) ArticleTitleRole of RANK ligand in mediating increased bone resorption in early postmenopausal women J Clin Invest 118 1221–1230 Occurrence Handle10.1172/JCI200317215
WS Browner LY Lui SR Cummings (2001) ArticleTitleAssociations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women J Clin Endocrinol Metab 86 631–637 Occurrence Handle10.1210/jc.86.2.631 Occurrence Handle1:CAS:528:DC%2BD3MXht1Knsbc%3D Occurrence Handle11158021
S Khosla HM Aggighi LJ Melton (2002) ArticleTitleCorrelates of osteoprotegerin levels in women and men Osteoporos Int 13 394–399 Occurrence Handle10.1007/s001980200045 Occurrence Handle1:CAS:528:DC%2BD38XlsF2gtLo%3D Occurrence Handle12086350
K Yano O Shibata A Mizuno (2001) ArticleTitleImmunological study on circulating murine osteoprotegerin/osteoclastogenesis inhibitory factor (OPG/OCIF): possible role of OPG/OCIF in the prevention of osteoporosis in pregnancy Biochem Biophys Res Commun 288 217–224 Occurrence Handle10.1006/bbrc.2001.5745 Occurrence Handle1:CAS:528:DC%2BD3MXnsVGntrs%3D Occurrence Handle11594776
H Resch P Pietschmann S Kudlacek (1994) ArticleTitleInfluence of sex and age on biochemical bone metabolism parameters Miner Electrolyte Metab 20 117–121 Occurrence Handle1:CAS:528:DyaK2cXmsFahsb8%3D Occurrence Handle7815999
PR Ebeling LM Atley JR Guthrie (1996) ArticleTitleBone turnover markers and bone density across the menopausal transition J Clin Endocrinol Metab 81 3366–3371 Occurrence Handle10.1210/jc.81.9.3366 Occurrence Handle1:CAS:528:DyaK28Xls1OrtLk%3D Occurrence Handle8784098
SM Kakonen J Hellman M Karp (2000) ArticleTitleDevelopment and evaluation of three immunofluorometric assays that measure different forms of osteocalcin in serum Clin Chem 46 332–337 Occurrence Handle1:CAS:528:DC%2BD3cXjvFyksrg%3D Occurrence Handle10702519
P Pietschmann H Resch E Krexner (1991) ArticleTitleDecreased serum osteocalcin levels in patients with postmenopausal osteoporosis Acta Med Austriaca 18 114–116 Occurrence Handle1:STN:280:By2C1c%2FmsVU%3D Occurrence Handle1796722
K Akesson S Ljunghall B Jonsson (1995) ArticleTitleAssessment of biochemical markers of bone metabolism in relation to the occurrence of fracture: a retrospective and prospective population-based study of women J Bone Miner Res 10 1823–1829 Occurrence Handle1:STN:280:BymC2MrmsF0%3D Occurrence Handle8592961
MJ Power PR Fottrell (1991) ArticleTitleOsteocalcin. Diagnostic methods and clinical application Crit Rev Clin Lab Sci. 28 287–335 Occurrence Handle1:CAS:528:DyaK38XlsVOmsw%3D%3D Occurrence Handle1930680
PD Delmas R Eastell P Garneo MJ Seibel J Stepan (2000) ArticleTitleThe use of biochemical markers of bone turnover in osteoporosis Osteoporos Int 11 (Suppl 6) S2–17
KK Ivaska TA Hentunen J Vaaraniemi (2004) ArticleTitleRelease of intact and fragmented osteocalcin molecules from bone matrix during bone resorption in vitro J Biol Chem 279 18361–18369
CE Ber A Kroner E Thomas (2002) ArticleTitleComparison of biochemical markers of bone metabolism in serum and femur aspirates Clin Orthop 395 174–179 Occurrence Handle11937878
ED Dieo MA Martin C Piedra (1995) ArticleTitleLack of correlation between levels of osteocalcin and bone alkaline phosphatase in healthy control and postmenopausal osteoporotic women Horm Metab Res 27 151–154
L Lum BR Wong R Josien (1999) ArticleTitleEvidence for a role of a tumor necrosis factor-alpha (TNF-alpha)-converting enzyme-like protease in shedding of TRANCE, a TNF family member involved in osteoclastogenesis and dentritic cell survival J Biol Chem 274 13613–13618
T Nakashima Y Kobayashi S Yamasaki (2000) ArticleTitleProtein expression and functional difference of membrane-bound and soluble receptor activator of NF-kappaB ligand: modulation of the expression by osteotropic factors and cytokines Biochem Biophys Res Commun. 275 768–775 Occurrence Handle10.1006/bbrc.2000.3379 Occurrence Handle1:CAS:528:DC%2BD3cXmtFGlsL8%3D Occurrence Handle10973797
LC Hofbauer M. Schoppet Osteoprotegerin (2001) ArticleTitleA link between osteoporosis and arterial calcification? Lancet 358 257–259 Occurrence Handle10.1016/S0140-6736(01)05494-0 Occurrence Handle1:STN:280:DC%2BD3MvltFanuw%3D%3D Occurrence Handle11498208
ST Knudsen CH Foss PL Poulsen (2003) ArticleTitleIncreased plasma concentrations of osteoprotegerin in type 2 diabetic patients with microvascular complications Eur J Endocrinol 149 39–42 Occurrence Handle1:CAS:528:DC%2BD3sXlslGgsbs%3D Occurrence Handle12824864
HA Makhluf SM Mueller S Mizuno (2000) ArticleTitleAge related decline in osteoprotegerin expression by human bone marrow cell cultured in three-dimensional collagen sponges Biochem Biophys Res Commun 268 669–672 Occurrence Handle10.1006/bbrc.2000.2182 Occurrence Handle1:CAS:528:DC%2BD3cXhtFGju7c%3D Occurrence Handle10679262
J Cao L Venton T Sakata (2003) ArticleTitleExpression of RANKL and OPG correlates with age-related bone loss in male C57BL/6 mice J Bone Miner Res 18 270–277 Occurrence Handle1:CAS:528:DC%2BD3sXht1Sktr4%3D Occurrence Handle12568404
Acknowledgment
This study was supported by the Key Instructor Sponsor Program, Ministry of Education, China (OOTPJS111); Medical Star Program, Shanghai Health Bureau; Young and Middle-age Outstanding Instructor Training Program, Shanghai Second Medical University, and partly from Key Discipline Construction Fee, Shanghai Education Committee.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Liu, J., Zhao, H., Ning, . et al. Relationships Between the Changes of Serum Levels of OPG and RANKL with Age, Menopause, Bone Biochemical Markers and Bone Mineral Density in Chinese Women Aged 20-75. Calcif Tissue Int 76, 1–6 (2005). https://doi.org/10.1007/s00223-004-0007-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-004-0007-2