Abstract
Because of the increasing interest on stimulating fracture healing, knowledge about the role and chronology of growth factors during the healing process is important. The purpose of this study was to quantify the protein concentration of IGF-I and TGF-β1 during rat tibial fracture healing 5, 10, and 15 days after fracture using ELISA methods and to analyze the distribution of the proteins and the related mRNA expression in the fracture callus by immunohistochemistry and in situ hybridization. The following three groups were analyzed: Fractured tibiae intramedullary stabilized with K-wires coated with IGF-I and TGF-β1 compared with fractures stabilized with uncoated K-wires and unfractured tibiae. The weight of the callus increased during the healing process in both experimental groups. The protein concentration of IGF-I and TGF-β1 in the fracture callus showed significant changes between the investigated time points and treatment groups compared with the unfractured tibia. IGF-I increased with healing time whereas TGF-β1 revealed a constantly elevated level at the investigated time points. Mesenchymal cells, osteoblasts, osteocytes, proliferating and immature chondrocytes, and osteoclasts expressed both growth factors. No differences in the expression and localization pattern of the growth factors were detectable among the groups. Using the different methods for quantification and visualization of the growth factors, no differences (except the increased IGF-I concentration at day 15 in the growth factor group) were seen between the normal and the growth factor-stimulated fracture healing as an indication for physiological healing after exogenous growth factor treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Stimulation of bone healing by locally applied growth factors such as insulin-like growth factor-I (IGF-I) and transforming growth factor-β1 (TGF-β1) has successfully been demonstrated in different studies [1, 2, 3]. Both growth factors play a crucial role during several developmental events such as establishment of the left–right body axis (member of the TGF-β family) [4], embryonic skeletal growth, and bone healing [5, 6, 7]. It has been shown that TGF-β1 regulates different cell types that are directly involved in bone formation such as mesenchymal cells, chondrocytes, osteoblasts, and osteoclasts [8, 9], and IGF-I stimulates replication of osteoblasts and the synthesis of bone matrix [10, 11]. Previous studies investigated the expression pattern of these growth factors during skeletal growth, fracture healing, and distraction osteogenesis. TGF-β1 is detectable in inflammatory cells and extracellular matrix within the fracture gap, periosteum, peripheral soft tissues, and newly formed bone matrix and areas of remodeling [12]. Flanders et al. [13] used two self-made antibodies raised against different TGF-β1 epitopes and visualized extracellular or intracellular TGF-β1 production in different tissues from mice, cattle, and human with a high specifity of the antibody in the different species indicating the high conservation of the protein. IGF-I and IGF receptor staining reveals a widely spread distribution within the callus in most cell types and differentiation stages of the osteoblastic cell line, in the newly proliferating and early hypertrophic chondrocytes, and mesenchymal cells [14, 15]. Furthermore, the protein amount of IGF-I and TGF-β1 was quantified in normal and pathological bone tissue [16, 17, 18, 19]. Based on this knowledge, several animal models successfully used growth factors to stimulate bone growth or healing [20, 21, 22, 23, 24]. However, until now little is known about the effect of exogenously applied growth factors on the endogenous production and expression of the growth factors. The temporal expression and localization of growth factors during different phases of fracture healing has not been well investigated until now.
The purpose of the present study was to obtain more information on the changes in growth factor concentration, mRNA expression and protein localization during normal and growth factor-stimulated fracture healing. This knowledge is necessary for the therapeutic use of growth factors in the clinic. Therefore, the hypothesis of the study is that the exogenously applied growth factors do not alter the cellular expression pattern of the growth factors as visualized by immunohistochemistry and in situ hybridization but might influence the amount of synthesized growth factors. Until now, there were no investigations that analyzed the effect of locally applied growth factors on endogenous production. To investigate this, a well-described poly(D,L-lactide) implant coating (PDLLA) for controlled local growth factor release was used [25]. Previous studies revealed a stimulating effect on rat and porcine bone healing using this coating technique for local growth factor application without a systemic effect of the applied growth factors [1, 3, 26]. In the early phase of fracture healing (days 5, 10, and 15 after fracture), changes in the proliferation pattern due to the locally applied growth factors were observed indicating an enhanced callus maturation [27].
Materials and Methods
A standardized tibial fracture of 5-month-old female Sprague–Dawley rats (Haarlan–Winkelmann, Germany) was performed and then intramedullary stabilized with titanium Kirschner wires (K-wires) as described previously [1, 26]. Three different time points were investigated (days 5, 10, and 15 after fracture). All experiments were approved by the local authority. The unfractured tibiae served as control for normal levels.
-
Group 1: uncoated K-wire (N = 39)
-
Group 2: IGF-I and TGF-β1 coated K-wire (N = 39)
-
Control: unfractured tibia (N = 8 for ELISA)
-
ELISA and protein quantification: N = 8 each group and time point
-
Immunohistochemistry and in situ hybridization: N = 5 each group and time point
Coating Technology and Growth Factors
For local growth factor application a previously described [25] coating technique of implants was used. Poly(D,L-lactide) (Boehringer Ingelheim, Germany) (30-kD molecular weight) was dissolved in chloroform and recombinant human IGF-I (5% w/w, 50 µg; R&D Systems, Minneapolis, MN, USA) and recombinant human TGF-β1 (1% w/w, 10 µg; R&D Systems) were incorporated [25]. Titanium Kirschner wires (1.0-mm diameter, Synthes, Paull, PA, USA) were coated two times and dried under sterile conditions. The coating was approximately 10 µm thick and showed a high mechanical stability. In vitro tests revealed a release of approximately 50% of the incorporated IGF-I and TGF-β1 within the first 48 hours. The remaining growth factors were released in a sustained manner. The properties of the PDLLA coating were described previously in more detail [25]. Because of the conserved structure and function of the growth factors and receptors in vertebrate radiation, human growth factors can be used for rodent studies [4, 28]. The homology of the active proteins is IGF-I is 95.7% and TGF-β1 is 99.1% (according to Swiss-Prot/TrEML; http://us.expasy.org/sprot/ ).
Protein Extraction Procedure
Eight animals of each group were sacrificed 5, 10, and 15 days after fracture. The animals were perfused with 0.9% NaCl at sacrifice to clear the bone from blood. The tibiae were dissected and freed from surrounding tissue under sterile conditions and immediately cooled in liquid nitrogen. The “fresh” callus weight was determined immediately after preparation of the callus by using a precision balance. The bone/callus was cut into the requested length (0.5 mm proximal and distal from the fracture) and the weight was determined. Using a cooled mill (Retsch, Haan, Germany) the bone was pulverized and the powder was diluted for 2 hours in 10 ml 1 M acetic acid/g callus at 4°C (pH 3.8) to avoid IGF-binding protein artifacts [29]. Previous studies were able to show that the optimum pH for the extraction of growth factors from tissue ranges between 3.6 and 4.2 [30]. Approximately 100% more IGF was measurable after extraction at pH 4.5 compared with a neutral pH of 7.5 or a basic pH of 10.5 [31]. Comparable results were obtained for the dilution of TGF-β1 from the latent complex [32, 33, 34]. After extraction the samples were centrifuged at 12,000 rpm and the supernatant was stored at −80°C.
Protein, IGF-I, and TGF-β1 Quantification
To quantify the total protein concentration, a Coomassie Plus Protein Assay (Pierce, Perbio Science Rockford, IL, USA) was used. IGF-I and TGF-β1 concentrations in the extract were quantified using ELISA methods (both kits Brenzel Bioanalytic, Lahntal/Marburg, Germany). IGF-I was analyzed using a competitive ELISA and TGF-β1 using an ELISA based on the sandwich principle. The standards and the samples for the IGF ELISA were diluted with 1 M acetic acid at approximately pH 4.0. Control experiments to validate the ELISA tests revealed no differences due to the use of acidic or neutral buffer. Because of the higher dilution of the samples for the TGF ELISA, the assay buffer was used, resulting in a neutral pH. The analyses were performed according to the instructions of the manufacturer. The sensitivities of the ELISA kits were TGF-β1 = 1.9 ng/l and IGF-I = 0.15 µg/l. The TGF-β1 kit showed no cross reactivity to TGF-β2 or TGF-β3, and the IGF-I kit showed no cross reactivity to IGF-II. Interassay coefficient of variation (CV) was 7.5% (TGF-β1) or 6.7% (IGF-I) and intraassay CV was 1% (TGF-β1) or 2.31% (IGF-I).
Histology and Immunohistochemistry
Five animals of each group were sacrificed 5, 10, and 15 days after fracture. The tibiae were dissected, fixed in 10% buffered formaldehyde, decalcified in EDTA for 14 days, and paraffin-embedded. For immunohistochemical staining, deparaffinized 5-µm sections were used. After dehydration the sections were incubated in 5% normal horse serum to block nonspecific binding. After blocking the sections were incubated with either goat polyclonal TGF-β1 antibody (raised against human TGF-β1, Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:100) or goat polyclonal IGF-I antibody (raised against human IGF-I, R&D-Systems, Minneapolis, MN, USA; 1:50). A biotinylated horse anti-goat IgG secondary antibody (Vector Laboratories, Burlingame, CA, USA) was used to detect the primary antibody. The Avidin–Biotin-Complex detection system (ABC method; Vector Laboratories) coupled with alkaline phosphatase was used. After blocking of the endogenous alkaline phosphatase with Lavamisole (Vector), Vector® Red used as chromogen visualized the binding. The sections were counterstained with methyl green (1% methyl green in 20% ethanol) to detect cell nuclei and cartilage. Incubation of slices without the primary antibody or goat IgG or goat serum in the concentration of the primary antibody served as negative control. No section showed any staining in these control experiments. Safranin Orange/Light Green was used for histological overview stain (8 min 1% Safranin O, 10 min in picric acid, wash in 1% acetic acid, 8 min methyl green, wash in 1% acetic acid). The slices were analyzed using an image analysis system (Zeiss KS 400).
In situ Hybridization
The same sections used for immunohistochemistry were used for in situ hybridization. A 0.7-kb cDNA fragment encoding mouse IGF-I [35] was kindly provided by Dr. G.I. Bell (Howard Hughes Medical Institute, Chicago, IL, USA). A mouse-derived TGF-β1 cDNA fragment [36] of 0.41-kb size was supplied by Dr. E. Vuorio (Department of Medical Biochemistry and Molecular Biology, University of Turku, Turku, Finland). DIG-labeled UTP single-stranded RNA probes for in situ hybridization were generated using a DIG RNA labeling kit (Roche Diagnostics GmbH, Mannheim, Germany) according to the manufacturer’s protocol. To obtain sense (control) and antisense, riboprobe templates were transcribed using the respective T7 and T3 RNA polymerases (Roche Diagnostics GmbH). Transcription results were gel-checked and riboprobes were then applied to hybridization buffer or stored upon usage.
Prior to the hybridization procedures, glassware was cooked for 2 hours at 180°C to minimize the risk of RNAse contamination. Sections were deparaffinized with xylol and rehydrated in freshly prepared solutions of decreasing alcohol concentrations. For deproteinization, slides were kept for 20 minutes in 0.2 M HCl, then slides were washed for 15 minutes with 0.3% Triton X-100 to improve permeabilization. Digestion of remaining proteins was performed by incubating slides for 30 minutes in TE buffer containing 5 µg/ml proteinase K at 37°C. Slides were then rinsed in 0.2% glycine for 5 minutes. To reduce nonspecific background staining, sections were acetylated twice for 5 minutes with 0.25% acetic anhydride in 0.1 M triethanolamine. Finally postfixation was carried out for 15 minutes in 4% paraformaldehyde at 4°C. Slides were then rinsed with 2× SSC to equilibrate for prehybridization. Prehybridization was carried out by overlaying slides with 2× SSC in 50% deionized formamide and incubating in a moist chamber at 50°C. Hybridization buffer was prepared containing 50% deionized formamide, 200 mM Tris-HCl (pH 7.4), 1 mM EDTA, 0.33 M NaCl, 0.2 M DTT, 5 µg/µl tRNA, 1 µg/µl herring sperm DNA, 10×Denhardt’s solution, 10% dextran sulfate, and DIG-labeled riboprobes at concentrations of 4–8 ng/µl hybridization mixture. Slices were covered with hydrophobic coverslips (HybriSlips, Grace Bio Labs, Bend, Or, USA) and incubated at 50°C for 16–18 hours. Excess riboprobes were removed the next day by high-stringency washing at 47°C. Slides were kept 30 minutes in 2× SSC, 30 minutes in 1× SSC containing 50% formamide, and another 30 minutes in 0.5× SSC/50% formamide. Unbound RNA was digested with 2 U/µl RNase T1 in TE buffer at 37°C for 15 minutes. Finally, slides were rinsed in 0.1× SSC at 47°C for 30 minutes and for 10 minutes in 0.2× SSC at room temperature. Hybridized riboprobes were visualized by incubating slides with an anti-DIG-alkaline phosphatase-conjugated antibody overnight at 4°C (1:750 in blocking medium, Roche Diagnostics). Subsequent color reaction was performed using NBT as a substrate for alkaline phosphatase.
To verify specificity of hybridization, sections were also probed with sense transcripts of the related cDNA, or either antisense probe or antibody was omitted. All control experiments yielded no or minor nonspecific staining.
Statistical Analysis
Statistical analyses were performed for quantitative data of the ELISA. The data were expressed as mean ± SD and related to the total tissue weight or to the total protein concentration. Mann–Whitney U-test and Bonferroni–Holms test were performed for statistical analysis (SPSS 9.0.1 software; SPSS, Chicago, IL).
Results
Callus Weight and Quantification of Growth Factors
Both experimental fracture groups 1 and 2 showed a significant increase in callus weight over the experimental period compared with the unfractured tibiae. At day 15 after fracture the callus weight was increased approximately threefold in both fracture groups compared with the unfractured tibia (Table 1).
The total protein content of the callus compared with the unfractured tibia was significantly increased up to 30% in group 1 at days 5 and 10 with a further significant increase at day 15. A consistently elevated protein content of 30% was measurable in the growth factor-treated group 2 at the investigated time points. At day 15 after fracture the protein content was significantly increased in group 1 compared with the growth factor-treated group 2 (Fig 1, Table 2).
The results of (a) the weight and (b) the total protein content of the callus were used to normalize the results of the IGF-I and TGF-β1 quantification (Figs. 2 and 3: correlation to the callus weight; Tables 3 and 4: correlation to the total callus protein). All data were compared with the values of the unfractured tibia.
The IGF-I analyses revealed significant differences depending on the correlation to callus weight or protein content. The concentration of IGF-I/mg callus increased significantly in the uncoated K-wire group 1 from day 5 to day 15 (Fig. 2). Correlating the IGF-I values with the total protein content, a significant increase was detectable only from day 5 to day 10 (Table 3). The growth factor-treated group 2 showed a significant increase in the measured IGF-I/mg callus between days 5 and 10 without a further increase at day 15 (Fig. 2). However, analyzing the IGF-I/protein, a significant increase was seen up to day 15 (Table 3). At day 15 after fracture the amount of IGF-I/protein was significantly enhanced in the growth factor-treated group 2 compared with the uncoated K-wire group 1 (Table 3).
The measurement of the TGF-β1 content in the fractured bones revealed a significantly enhanced level of TGF-β1/mg callus compared with the unfractured tibia. No significant differences between the experimental fracture groups were seen depending on the type of TGF-β1 correlation (per weight or protein) and no significant differences were seen between the groups and the three time points (Fig. 3). The growth factor-treated group 2 revealed a significant increase in TGF-β1 after normalizing to the protein content from day 10 to day 15 (Table 4).
Histology
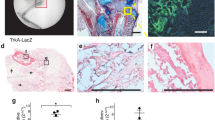
In the unstimulated fracture healing a fibrous callus was formed and intramembraneous ossification occurred under the periosteum at a distance to the fracture side at day 5. The callus size increased and strong cartilage formation was detectable at day 10 postfracture. The newly formed woven bone had the characteristic nonlamellar structure (days 10 and 15). Endochondral ossification occurred in the outer margin of the cartilage 15 days postfracture (Fig. 4 a–c). The morphology of the calluses showed changes as a result of the local application of growth factors with signs of progressed callus healing. More cartilaginous tissue was present in the fracture callus of the growth factor group 2 at day 5 after fracture (Fig. 4d′). At day 15 hematopoietic cells were detectable in the woven bone of the growth factor-treated group (Fig. 4f′). Because of enchondral ossification the cartilaginous callus was reduced in this group (Fig. 4).
Histological stain (Safranin Orange/Light Green) of fractured tibiae 5, l0, and 15 days after fracture stabilized with a titanium implant (a–c, a′, c′) or with a growth factor-coated implant (d–f,d′, f′). The mineralized tissue stains green and the cartilage red. Because of the low magnification the small island of newly formed cartilage is not clearly detectable in (d) but in the higher magnification (d′). Newly formed bone marrow (f′) with erythrocytes {*}. Scale bars: (a–f) 2 mm, (a′, d′) 100 µm, (c′, f′) 100 µm. Ca: cartilage, Ct: corticalis, St: soft tissue, Wb: woven bone.
Localization and Expression of Growth Factors
The TGF-β1 and IGF-I antibodies stained primarily the cytoplasm. TGF-β1 immunoreactivity (IR) was detectable in mesenchymal cells in the fracture callus at day 5. At a distance to the fracture site osteoblasts were stained positive for TGF-β1 in the newly formed woven bone. The newly formed chondrocytes in the callus of the growth factor group 2 were also positive for TGF-β1. At days 10 and 15 immunoreactivity was found in proliferating chondrocytes and chondrocytes in the margin of the cartilaginous callus. No or only a few hypertrophic chondrocytes were positive. Osteoblasts, osteocytes, and osteoclasts showed an intense staining at the investigated time points and in both groups (Fig. 5 a–c). In addition, the endothelial cells of new invading blood vessels showed high immunoreactivity.
TGF-β1 immunoreactivity (IR, red color) (a–c), TGF-β1 in situ hybridization (ISH, black color) (d–f), IGF-IR (red color) (g–i), and IGF-I ISH (black color) (j–l). Shown are sections from tibiae 5 (a, d, g, j), 10 (b, e, h, k), and 15 (c, f, i, l) days after fracture and stabilization with uncoated titanum implants. At day 5 an intense staining is detectable in mesenchymal cells of the newly formed soft callus (St) (a, d, g, j). Hypertrophic chondrocytes are mostly negative, whereas proliferating cells reveal an intense staining (b, c, e, f, h, i, k, l). Scale bar: 500 µm. Ca: cartilage, St: soft tissue, Wb: woven bone, arrows indicate staining at the margin of the cartilage.
The results of the IGF immunohistochemistry were comparable to the TGF-β1 staining. During the different phases of fracture healing IGF-I immunoreactivity was detectable in mesenchymal cells, osteoblasts, young chondrocytes (but not in hypertrophic chondrocytes), and endothelial cells of blood vessels (Fig. 5 g–i). No temporal changes in the protein detection were seen. The different cell types showed comparable staining independent from the investigated time point.
The expression of the mRNA reflects in general the protein localization (Fig. 5 d–f,j–l showing the TGF-β1 and IGF-I in situ hybridization). The immunohistochemical staining and the expression pattern of IGF-I and TGF-β1 mRNA in the fracture callus revealed no major differences between the two investigated groups. Almost the same cell types stained positive in the immunohistochemistry and the in situ hybridization (Fig. 6).
Higher magnification of TGF-β1 and IGF-I in situ hybridization. (a,b) Osteoclasts and osteoblast expressing TGF-β1 mRNA. (c–e) Osteocytes, chondrocytes, and osteoclasts expressing IGF-I. (f) Control ISH with the sense transcript of IGF-I. (a, b, d, e, f) 15 days postfracture, (c) 5 days postfracture. Scale bars: (a–e) 100 µm, (f) 500 µm. Ct: cortical bone, OB: osteoblast, OC: osteoclast, Wb: woven bone, hCH: hypertrophic chondrocytes, pCH: proliferating chondrocytes.
Neither the immunohistochemistry nor the in situ hybridization revealed differences in the expression pattern between the groups and the different time points.
Discussion
This study used different methods to investigate the temporal expression and localization of IGF-I and TGF-β1 during different phases of fracture healing. In addition to the normal fracture healing, the influence of locally applied IGF-I and TGF-β1 on these parameters was analyzed. The growth factor quantity was normalized to the callus weight or to the total protein content of the callus. The amount of IGF-I per mg callus showed a significant increase during the healing process, whereas the TGF-β1 level was significantly enhanced at day 5 and then revealed a steady state throughout. No significant differences were measurable between the two experimental fracture groups. A previous study revealed also temporal changes in the expression of another growth factor during fracture healing. A significant increase in the VEGF level was measured at day 5 after fracture decreasing at day 10 [37].
However, normalizing the growth factor quantity to the total protein content revealed different results. At day 15 significantly more IGF-I/protein was measurable in the growth factor-treated group 2 compared with the uncoated K-wire group 1 and the amount of TGF-β1/protein increased significantly in the group 2 from day 10 to day 15. The dimension of measured IGF-I and TGF-β1 in bone was comparable to concentrations found in other studies [17, 38, 39, 40, 41]. The IGF-I concentration was higher than the TGF-β1 concentration in the analyzed bony tissue. However, the measured concentration in the tissue depends in a high degree on the tissue preparation (e.g., pH of the extraction buffer) and the quantification methods used [32, 33, 34].
The localization of the growth factors on the level of the protein and the mRNA revealed a high coincidence between the methods used. The stained cell types in both methods were in accordance with other studies investigating the distribution of IGF-I and TGF-β1 during bone formation and healing [12, 14, 15, 42, 43, 44, 45, 46]. No differences in the localization of the protein or the mRNA were seen between the groups and time points suggesting a good correlation between the two methods. However, differences between the groups in the callus morphology were found. Enhanced callus maturation was detectable because of the local growth factor application. Cartilaginous tissue was detectable earlier (day 5) in the growth factor-treated calluses compared with the titanium K-wire-treated calluses, followed by an earlier appearance of newly formed medullary cavities in the growth factor group (day 15). No differences were found in the progression of callus modeling and remodeling which is an indication for normal healing (described in further detail in [27]).
Because of the high homology of the locally delivered human growth factors to the endogenously produced rat growth factors [IGF-I: 95.7% and TGF-β1: 99.1% (Swiss-Prot/TrEMBL)], it was not possible to distinguish between exogenously applied and endogenously produced growth factors. Both the antibodies for the immunohistochemistry and the ELISA kits detected human and rat growth factors. Furthermore, the specificity of the immunohistochemical staining is supported by the mRNA analysis using in situ hybridization. Comparing both methods, similar cytoplasmatic detection patterns of the individual growth factors were seen in the callus tissue.
The concentration of IGF-I and TGF-β1 locally released from the coating during healing is very low. Approximately 50 µg IGF-I and 10 µg TGF-β1 were incorporated in the coating of each wire. Previous studies revealed a release of 54% of IGF-I and 48% of TGF-β1 within the first 48 hours followed by a sustained release of an additional 30% in the next 40 days [25]. The half-life period of growth factors in vivo is very short. For rats, a half-life of 240 minutes of injected IGF-I [47] and a half-life of 60–163 minutes for TGF-β1 were found [48]. These might explain the results from the ELISA analysis. Approximately 50% of the incorporated growth factors were released and metabolized before the first investigated time point: day 5. After this initial peak only a small release of growth factors occurred in the following days. Therefore, we expected no significant changes in the growth factor concentration in the callus due to the exogenously released factors from the coated implants. However, the released factors seem to influence the endogenous production of IGF-I and TGF-β1. Differences were seen in subject to the reference value, pointing out the necessity to consider the reference value. Depending on changes in callus weight or protein content between the treatment groups or fracture healing stages, the reference value might influence the results. Correlating the IGF-I and TGF-β1 concentration to the callus weight (mg) or to the total protein (µg) yielded different results. No significant differences between the groups were seen after correlation with the callus weight. However, correlation of the IGF-I concentration to the total protein content revealed a significant difference 15 days after fracture. The IGF-I concentration was significantly elevated in the growth factor group 2 compared with the uncoated K-wire group 1. This might be explained by the significantly reduced total protein content in the callus in growth factor group 2 compared with uninfluenced healing, indicating an enhanced healing of the callus as a result of the remodeling of the cartilage and soft tissue. In the histological sections a smaller cartilaginous callus was seen in the growth factor group.
The results of this study demonstrate that the concentration of TGF-β1 increases earlier than the IGF-I concentration during fracture healing. However, the IGF-I concentration shows a further increase from day 10 to day 15, whereas TGF-β1 shows a constantly elevated level from day 5 to day 15. The IGF-I concentration is much higher in the bony tissue than the TGFβ1 concentration, and there was increase of approximately 20% (IGF-I) or 50% (TGF-β1) during healing. The immunohistological and hybridization methods revealed no differences in the cell types expressing the investigated growth factors, indicating that the local application of growth factors does not lead to an alteration of the cell physiology over an elevated time period. Changes in the amount of expressed growth factors cannot be detected with both used methods. The amount of growth factors quantified in the unfractured tibiae by ELISA was on average 7.6 ng/mg bone IGF-I and 132.8 pg/mg bone TGF-β1. Even the maximum changes between both experimental fracture groups resulted in a difference of approximately 0.8 ng/mg bone (IGF-I per callus, day 15) or 35 pg/mg bone (TGF-β1 per callus, day 10). These very small changes were not detectable with the immunohistochemical and hybridization methods used.
Besides the investigated growth factors IGF-I and TGF-β1, additional growth factors, cytokines, and hormones are important for fracture healing. It is known that the action of growth hormone is mediated by IGF-I and the action of IGF-I is mediated by binding proteins. Therefore, not only the total amount of the growth factors is important for their action but also the binding proteins. This issue should be addressed in further studies.
The binding proteins and latency complex make it necessary to extract the growth factors in acid. This was previously described for IGF-I and TGF-β1 [30, 32, 33]. Approximately 95% more IGF-I was extracted from bone at pH 4.5 compared with pH 7.5 [31]. In pilot experiments of this study, the standards of the ELISAs were diluted with acetic acid or buffer to test a possible influence of the pH on the proteins. No differences in the measured amount were detectable between the dilution with buffer or with acid.
Taken the results together, the callus composition changed as a result of the local growth factor application as shown by the callus morphology and measured protein content. The amount of IGF-I and TGF-β1 showed slight changes due to the growth factor treatment. However, the growth factor application did not change the cellular expression pattern of the growth factors. Comparing these results with those of previous studies showing a positive effect of the locally applied growth factors on fracture healing using biomechanical testing, histomorphometry, and immunohistochemistry [1, 3, 26], we conclude that the applied dosage and the application method of the growth factors is effective to stimulate healing without altering the physiological expression of the factors. It is clinically relevant for the safe use of exogenously applied growth factors for stimulation of bone healing that they do not alter the physiological expression pattern of the growth factors and the healing pattern.
References
G Schmidmaier B Wildemann H Bail M Lucke T Fuchs A Stemberger A Flyvbjerg NP Haas M Raschke (2001) ArticleTitleLocal application of growth factors (insulin-like growth factor-1 and transforming growth factor-ß1) from a biodegradable poly(D,L-lactide) coating of osteosynthetic implants accelerates fracture healing in rats. Bone 28 341–350 Occurrence Handle10.1016/S8756-3282(00)00456-7 Occurrence Handle1:CAS:528:DC%2BD3MXjtFGgtro%3D Occurrence Handle11336914
G Schmidmaier B Wildemann T Gäbelein J Heeger F Kandziora N Haas M Raschke (2003) ArticleTitleSynergistic effects of IGF-I and TGF-1 on fracture healing in rats—Single versus combined application of IGF-I and TGF-β1. Acta Orthop. Scand 74 604–610 Occurrence Handle10.1080/00016470310018036 Occurrence Handle14620984
M Raschke B Wildemann P Inden H Bail A Flyvbjerg J Hoffmann NP Haas G Schmidmaier (2002) ArticleTitleInsulin-like growth factor-1 and transforming growth factor-betal accelerates osteotomy healing using polylactide-coated implants as a delivery system: a biomechanical and histological study in minipigs. Bone 30 144–151 Occurrence Handle10.1016/S8756-3282(01)00640-8 Occurrence Handle1:CAS:528:DC%2BD38XktFCjtA%3D%3D Occurrence Handle11792577
SJ Newfeld RG Wisotzkey S Kumar (1999) ArticleTitleMolecular evolution of a developmental pathway: phylogenetic analyses of transforming growth factor-beta family ligands, receptors and Smad signal transducers. Genetics 152 783–795 Occurrence Handle1:CAS:528:DyaK1MXktFGgurs%3D Occurrence Handle10353918
ME Bolander (1992) ArticleTitleRegulation of fracture repair by growth factors. Proc Soc Exp Biol Med 200 165–170 Occurrence Handle1:CAS:528:DyaK38XktVGitLg%3D Occurrence Handle1374563
S Mohan DJ Baylink (1991) ArticleTitleBone growth factors. Clin Orthop 263 30–48 Occurrence Handle1993386
S Trippel R Coutts T Einhorn R Mundy R Rosenfeld (1996) ArticleTitleGrowth factors as therapeutic agents. J Bone Joint Surg 78A 1272–1286
JM Hock E Canalis M Centrella (1990) ArticleTitleTransforming growth factor-beta stimulates bone matrix apposition and bone cell replication in cultured fetal rat carvariae. Endocrinology 126 421–426
M Joyce AB Roberts M Sporn ME Bolander (1990) ArticleTitleTransforming growth factor-beta and the initiation of chondrogenesis and osteogenesis in the rat femur. J Cell Biol 110 2195–2207 Occurrence Handle2351696
J Hock M Centrella E Canalis (1988) ArticleTitleInsulin like growth factor I has independent effects on bone matrix formation and cell replication. Endocrinology 122 254–260 Occurrence Handle1:CAS:528:DyaL1cXltVShtg%3D%3D Occurrence Handle3335207
E Canalis (1980) ArticleTitleEffect of insulin like growth factor I on DNA and protein synthesis in cultured rat calvaria. J Clin Invest 66 709–719 Occurrence Handle1:CAS:528:DyaL3cXmtFGkuro%3D Occurrence Handle6252249
DS Steinbrech BJ Mehrara NM Rowe ME Dudziak JS Luchs PB Saadeh GK Gittes MT Longaker (2000) ArticleTitleGene expression of TGF-beta, TGF-beta receptor, and extracellular matrix proteins during membranous bone healing in rats. Plast Reconstr Surg 105 2028–2038 Occurrence Handle1:STN:280:DC%2BD3c3ps1aisg%3D%3D Occurrence Handle10839400
KC Flanders NL Thompson DS Cissel SE Van-Obberghen CC Baker ME Kass LR Ellingsworth AB Roberts MB Sporn (1989) ArticleTitleTransforming growth factor-beta 1: histochemical localization with antibodies to different epitopes. J Cell Biol. 108 653–660 Occurrence Handle1:CAS:528:DyaL1MXhsVyltLk%3D Occurrence Handle2465297
K Okazaki S Jingushi T Ikenoue K Urabe H Sakai Y Iwamoto (2003) ArticleTitleExpression of parathyroid hormone-related peptide and insulin-like growth factor I during rat fracture healing. J Orthop Res 21 511–520 Occurrence Handle10.1016/S0736-0266(02)00161-4 Occurrence Handle1:CAS:528:DC%2BD3sXivFCkt78%3D Occurrence Handle12706025
C Eingartner S Coerper J Fritz C Gaissmaier G Koveker K Weise (1999) ArticleTitleGrowth factors in distraction osteogenesis. Immuno-histological pattern of TGF-beta 1 and IGF-I in human callus induced by distraction osteogenesis. Int Orthop 23 253–259 Occurrence Handle1:STN:280:DC%2BD3c7hslSisQ%3D%3D Occurrence Handle10653288
B Schumacher J Albrechtsen J Keller A Flyvbjerg I Hvid (1996) ArticleTitlePeriosteal insulin-like growth factor I and bone formation. Changes during tibial lengthening in rabbits. Acta Orthop Scand 67 237–241 Occurrence Handle1:STN:280:BymA3cvptlY%3D Occurrence Handle8686459
J Lammens Z Liu J Aerssens J Dequeker G Fabry (1998) ArticleTitleDistraction bone healing versus osteotomy healing: a comparative biochemical analysis. J Bone Miner Res 13 279–286
T Ueland J Bollerslev TB Hansen EN Ebbesen L Mosekilde K Brixen A Flyvbjerg O Djoseland (1999) ArticleTitleIncreased cortical bone content of insulin-like growth factors in acromegalic patients. J Clin Endocrinol Metab 84 123–127 Occurrence Handle1:CAS:528:DyaK1MXmslWmuw%3D%3D Occurrence Handle9920071
RK Aaron S Wang DM Ciombor (2002) ArticleTitleUpregulation of basal TGF beta1 levels by EMF coincident with chondrogenesis—implications for skeletal repair and tissue engineering. J Orthop Res 20 233–240 Occurrence Handle10.1016/S0736-0266(01)00084-5 Occurrence Handle1:CAS:528:DC%2BD38XjtVGgu7w%3D Occurrence Handle11918302
A Nilsson J Isgaard A Lindahl L Peterson O Isaksson (1987) ArticleTitleEffects of unilateral arterial infusion of GH and IGF-I on tibial longitudinal bone growth in hypophysectomized rats. Calif Tissue Int 40 91–96 Occurrence Handle1:CAS:528:DyaL2sXhtFChtbo%3D
HM Nielsen TT Andreassen T Ledet H Oxlund (1994) ArticleTitleLocal injection of TGF-beta increases the strength of tibial fractures in the rat. Acta Orthop Scand 65 37–41 Occurrence Handle1:STN:280:ByuB3crjs1c%3D Occurrence Handle8154281
HD Zegzula DC Buck J Brekke JM Wozney JO Hollinger (1997) ArticleTitleBone formation with use of rhBMP-2 (recombinant human bone morphogenetic protein-2). J Bone Joint Surg Am 79 1778–1790 Occurrence Handle1:STN:280:DyaK1c%2Fnt1Cnuw%3D%3D Occurrence Handle9409791
M Lind (1998) ArticleTitleGrowth factor stimulation of bone healing. Effects on osteoblasts, osteomies, and implants fixation. Acta Orthop Scand Suppl 283 2–37 Occurrence Handle1:STN:280:DyaK1M%2Fntlejtw%3D%3D Occurrence Handle9856074
ML Bouxsein TJ Turek CA Blake D D’Augusta X Li M Stevens HJ Seeherman JM Wozney (2001) ArticleTitleRecombinant human bone morphogenetic protein-2 accelerates healing in a rabbit ulnar osteotomy model. J Bone Joint Surg Am 83 1219–1230
G Schmidmaier B Wildemann A Stemberger NP Haas M Raschke (2001) ArticleTitleBiodegradable poly(D,L-lactide) coating of implants for continuous release of growth factors. J Biomed Mater Res 58 449–455 Occurrence Handle11410904
G Schmidmaier B Wildemann F Cromme F Kandziora NP Haas M Raschke (2002) ArticleTitleBMP-2 coating of titanium implants increases biomechanical strength and accelerates bone remodeling in fracture treatment. Bone 6 618–622
B Wildemann G Schmidmaier S Ordel R Stange NP Haas M Raschke (2003) ArticleTitleCell proliferation and differentiation during fracture healing are influenced by locally applied IGF-I and TGF-1: Comparison of two proliferation markers, PCNA and BrdU. J Biomed Mater Res 65 150–156 Occurrence Handle10.1002/jbm.b.10512
Z Upton CA Yandell BG Degger SJ Chan S Moriyama GL Francis FJ Ballard (1998) ArticleTitleEvolution of insulin-like growth factor-I (IGF-I) action: in vitro characterization of vertebrate IGF-I proteins. Comp Biochem Physiol B Biochem Mol Biol 121 35–41 Occurrence Handle10.1016/S0305-0491(98)10111-6 Occurrence Handle1:STN:280:DyaK1M7jvVahuw%3D%3D Occurrence Handle9972282
J Pfeilschifter J Erdmann S Storch R Ziegler M Weinreb (1999) ArticleTitleChanges in the concentration of insulin-like growth factor I and transforming growth factor beta 1 in rat femoral bone during growth. Calcif Tissue Int 64 78–82 Occurrence Handle10.1007/s002239900582 Occurrence Handle1:CAS:528:DyaK1MXktVyqsQ%3D%3D Occurrence Handle9868288
AJ D’Ercole AD Stiles LE Underwood (1984) ArticleTitleTissue concentrations of somatomedin C: further evidence for multiple sites of synthesis and paracrine or autocrine mechanisms of action. Proc Natl Acad Sci USA 81 935–939 Occurrence Handle1:CAS:528:DyaL2cXhs1ylsL8%3D Occurrence Handle6583688
MR Benedict DC Ayers JD Calore RA Richman (1994) ArticleTitleDifferential distribution of insulin-like growth factors and their binding proteins within bone: relationship to bone mineral density. J Bone Miner Res 9 1803–1811
RM Lyons J Keski–Oja HL Moses (1988) ArticleTitleProteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol 106 1659–1665 Occurrence Handle2967299
DA Lawrence R Pircher P Jullien (1985) ArticleTitleConversion of a high molecular weight latent beta-TGF from chicken embryo fibroblasts into a low molecular weight active beta-TGF under acidic conditions. Biochem Biophys Res Commun 133 1026–1034 Occurrence Handle1:CAS:528:DyaL28Xks1elsA%3D%3D Occurrence Handle3866579
LM Wakefield DM Smith KC Flanders MB Sporn (1988) ArticleTitleLatent transforming growth factor-beta from human platelets. A high molecular weight complex containing precursor sequences. J Biol Chem 263 7646–7654 Occurrence Handle1:CAS:528:DyaL1cXktFOlur0%3D Occurrence Handle3163692
S Gonzalez–Parra J Argente JA Chowen M van Kleffens JW van Neck DI Lindenbeigh–Kortleve SL Drop (2001) ArticleTitleGene expression of the insulin-like growth factor system during postnatal development of the rat pituitary gland. J Neuroendocrinol 13 86–93 Occurrence Handle10.1046/j.1365-2826.2001.00590.x Occurrence Handle1:CAS:528:DC%2BD3MXotVyrsQ%3D%3D Occurrence Handle11123518
P Virolainen K Elima M Metsaranta HT Aro E Vuorio (1998) ArticleTitleIncorporation of cortical bone allografts and autografts in rats: expression patterns of mRNAs for the TGF-betas. Acta Orthop Scand 69 537–544 Occurrence Handle1:STN:280:DyaK1M%2FntVyrsA%3D%3D Occurrence Handle9855240
T Pufe B Wildemann W Petersen R Mentlein M Raschke G Schmidmaier (2002) ArticleTitleQuantitative measurement of the splice variants 120 and 164 of the angiogenic peptide vascular endothelial growth factor in the time flow of fracture healing: a study in the rat. Cell Tissue Res 309 387–392 Occurrence Handle10.1007/s00441-002-0605-0 Occurrence Handle1:CAS:528:DC%2BD38XmsVChurY%3D Occurrence Handle12195295
RD Finkelman TA Linkhart S Mohan KH Lau DJ Baylink NH Bell (1991) ArticleTitleVitamin D deficiency causes a selective reduction in deposition of transforming growth factor beta in rat bone: possible mechanism for impaired osteoinduction. Proc Natl Acad Sci USA 88 3657–3660 Occurrence Handle1:CAS:528:DyaK3MXisVKhtrk%3D Occurrence Handle2023915
RD Finkelman NH Bell DD Strong LM Demers DJ Baylink (1992) ArticleTitleOvariectomy selectively reduces the concentration of transforming growth factor beta in rat bone: implications for estrogen deficiency-associated bone loss. Proc Natl Acad Sci USA 89 12190–12193 Occurrence Handle1:CAS:528:DyaK3sXhsVSju7w%3D Occurrence Handle1465458
CJ Rosen HP Dimai D Vereault LR Donahue WG Beamer J Farley S Linkhart T Linkhart S Mohan DJ Baylink (1997) ArticleTitleCirculating and skeletal insulin-like growth factor-I (IGF-I) concentrations in two inbred strains of mice with different bone mineral densities. Bone 21 217–223 Occurrence Handle10.1016/S8756-3282(97)00143-9 Occurrence Handle1:CAS:528:DyaK2sXmtVGmurc%3D Occurrence Handle9276086
T Ueland EN Ebbesen JS Thomsen L Mosekilde K Brixen A Flyvbjerg J Bollerslev (2002) ArticleTitleDecreased trabecular bone biomechanical competence, apparent density, IGF-II and IGFBP-5 content in acromegaly. Eur J Clin Invest 32 122–128 Occurrence Handle10.1046/j.1365-2362.2002.00944.x Occurrence Handle1:STN:280:DC%2BD387mtlyrtQ%3D%3D Occurrence Handle11895459
M Joyce S Jingushi M Bolander (1990) ArticleTitleTransforming growth factor-beta in the regulation of fracture repair. Orthop Clin North Am 21 199–209 Occurrence Handle1:STN:280:By%2BC3MvpsVM%3D Occurrence Handle2296458
J Andrew J Hoyland S Andrew A Freemont D Marsh (1993) ArticleTitleDemonstration of TGF-beta 1 mRNA by in situ hybridization in normal human fracture healing. Calcif Tissue Int 52 74–78 Occurrence Handle1:CAS:528:DyaK3sXisVynsL4%3D Occurrence Handle8443694
J Andrew J Hoyland A Freemont D Marsh (1993) ArticleTitleInsulin like growth factor gene expression in human fracture callus. Calcif Tissue Int 53 97–102 Occurrence Handle1:CAS:528:DyaK2cXpslWluw%3D%3D Occurrence Handle8402329
K Tatsuyama Y Maezawa H Baba Y Imamura M Fukuda (2000) ArticleTitleExpression of various growth factors for cell proliferation and cytodifferentiation during fracture repair of bone. Eur J Histochem 44 269–278 Occurrence Handle1:CAS:528:DC%2BD3cXos12hurg%3D Occurrence Handle11095098
Y Yu JL Yang PJ Chapman–Sheath WR Walsh (2002) ArticleTitleTGF-beta, BMPS, and their signal transducing mediators, Smads, in rat fracture healing. J Biomed Mater Res 60 392–397
J Zapf C Hauri M Waldvogel ER Froesch (1986) ArticleTitleAcute metabolic effects and half-lives of intravenously administered insulinlike growth factors I and II in normal and hypophysectomized rats. J Clin Invest 77 1768–1775 Occurrence Handle1:CAS:528:DyaL28XktF2ns78%3D Occurrence Handle3711334
TF Zioncheck SA Chen L Richardson M Mora–Worms C Lucas D Lewis JD Green J Mordenti (1994) ArticleTitlePharmacokinetics and tissue distribution of recombinant human transforming growth factor beta 1 after topical and intravenous administration in male rats. Pharm Res 11 213–220
Acknowledgements
This work was supported by a grant to Dr. G. Schmidmaier by the Deutsche Forschungsgemeinschaft (DFG Schm 1436 1-2) and to Dr. Britt Wildemann by the Berliner Sparkassen Stiftung. We thank Ms. C. Bergmann for her excellent technical help with the in situ hybridization.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wildemann, B., Schmidmaier, G., Brenner, N. et al. Quantification, Localization, and Expression of IGF-I and TGF-β1 During Growth Factor-Stimulated Fracture Healing . Calcif Tissue Int 74, 388–397 (2004). https://doi.org/10.1007/s00223-003-0117-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-003-0117-2