Abstract
Antiresorptive drugs are widely used to prevent osteoporotic fractures in men and women. Large clinical trials have shown vertebral fracture risk reductions up to 50%, resulting from relatively small increases of 3–8% in bone mineral density (BMD). We developed a computer model that mimics bone turnover in human vertebral cancellous bone during menopause and antiresorptive treatment. This model links cell activity in trabeculae to changes in bone volume and mechanical properties. We asked whether treatment started shortly after menopause is better than treatment started late after menopause. In order to answer this question we used the model to simulate menopause and 5 years of anti-resorptive treatment with two different agents: one incorporated in the tissue, one not incorporated. We found that late treatment can result in almost the same bone mass as early treatment, but early treatment is much better in conserving the strength and stiffness of the cancellous bone. The effect of the incorporation of drugs in the tissue (giving the drugs a long half-life) was small. After discontinuation of treatment, bone was lost slower, but after 20 years the difference between the incorporated and the not incorporated drug in stiffness and bone volume was below 3%. This kind of simulation model may be used to preclinically test new pharmaceuticals and treatment protocols and to predict long-term effects of treatment before patient data become available.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Pharmacological agents that reduce bone resorption are widely used nowadays in osteoporosis treatment. These antiresorptive agents primarily increase bone mineral density (BMD) by reducing osteoclast activity and bone turnover [1, 2, 3]. In the first three years of treatment, fast increases in spinal BMD of 2–8% have been reported and ongoing slow increases up to 7 years after start of treatment have been shown [4, 5]. These treatments reduced vertebral fracture risk by up to 50% [4, 6, 7, 8, 9]. However, this large reduction cannot be explained by the small increases in BMD [10].
The most important effects of antiresorptive treatment take place in cancellous bone [11]. However, DXA and biochemical markers of bone turnover do not provide information about changes in architecture and stiffness of cancellous bone. More knowledge about the relation between the effects of antiresorptive treatment at the trabecular level and changes in global properties of cancellous bone could elucidate the efficacy of these drugs and the large anti-fracture effects resulting from small increases in BMD.
Our goal, therefore was to develop a computer model of remodeling during menopause and antiresorptive treatment to investigate changes in BMD, anisotropy and most importantly, the mechanical properties of the bone. As basis for this model, a previously published model of age-related remodeling was used [12]. This model links changes at the trabecular level to changes in architecture and strength of cancellous bone using three-dimensional models of human cancellous bone and remodeling parameters known from histology. Other simulations of bone remodeling during menopause and treatment have been performed, but these looked at the total bone balance or used artificial models of cancellous bone [13, 14, 15, 16]. In contrast, we use models of human cancellous bone specimens and simulate bone remodeling at the trabecular level.
As input for our model we used clinical and biological data from the literature. Increases in bone remodeling markers over menopause varied from 20 to 150% in literature [17, 18, 19]. During the perimenopausal years, bone resorption increased more than bone formation, resulting in a negative bone balance and bone loss in this period [18, 20]. Some reported data are conflicting: some studies showed ongoing high turnover, others showed a decrease during the postmenopausal years [17, 18, 19]. Using histomorphometry, it was shown that the bone formation rate in postmenopausal women was a factor 1.1 to 2 higher than in premenopausal women [21, 22]. Several studies reported changes in spinal BMD resulting from these changes in remodeling ‘[19, 23, 24, 25, 26]. Premenopausal bone losses were small, typically below 1% per year. Bone loss increased before the last menses and reached a maximum of 2.3–3.8% per year on average. After this period, the rate of bone loss decreased and became very small during the 5th–8th postmenopausal year. The postmenopausal bone loss rate was similar to or slightly higher than the premenopausal loss rate. The rates of bone loss varied widely, for example, the maximum rate of bone loss varied between 0.3 and 6% per year.
We aimed to simulate menopause and treatment with two types of antiresorptive agents; one that is incorporated in the bone tissue and therefore has a long half-life and one that is not incorporated, acts directly on the bone cells and has a quick treatment response. Specifically, we wanted to compare the effects of these treatments on bone volume, age and mechanical properties and to investigate whether treatment started shortly after menopause is better than treatment started years after menopause.
Methods
As input for the model we used a three-dimensional computer model of a human cancellous bone specimen (Fig. 1a), made using a micro-CT scanner (Scanco Medical, Zurich, Switzerland). The specimen was taken from an autopsy L4 vertebra of a 37-year-old donor. This donor was part of the European Union BIOMED1 project “Assessment of Bone Quality in Osteoporosis” and did not suffer any osteoporotic fractures. The model, of 4*4*4 mm cancellous bone, consisted of cubic elements of 14*14*14 µm. The bone volume fraction was 12.9%.
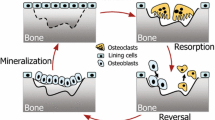
(A) Three-dimensional reconstruction of 4 × 4 × 4 mm cancellous bone made using a micro-CT scanner. (B) Detail of the reconstruction shown in (A). All elements are cubes with a side length of 14 µm. (C) Two-dimensional representation of the three steps of the remodeling process performed in three dimensions in this study.
The simulation model of menopause and antiresorptive treatment was based on a previously published model of age-related remodeling [12]. In this model, ongoing remodeling was simulated by repeating three steps. In the first step spherical resorption cavities with a specified depth were created, randomly distributed over the surface of the trabeculae. In step 2 it was checked whether resorption cavities breached trabeculae. Cavities that breached a trabecula were not refilled, which resulted in permanent bone loss. All other cavities were refilled in step 3, except for one or more surface elements, which were removed from the model in order to simulate the formation deficit (Fig. 1c). In the new model these remodeling parameters could be changed gradually during the simulation: (1) the number of resorption cavities, (2) the resorption depth, and (3) the bone balance per cavity. A negative balance is known as a formation deficit, a positive bone balance we call a formation surplus: osteoblasts make more tissue than osteoclasts resorbed and bone volume increases.
Bone tissue deposited during simulated treatment with a drug that was incorporated in the tissue was marked as drug-containing tissue, because it has been shown that antiresorptive drugs are mostly incorporated in active resorption cavities [27]. The simulations were performed on an SGI Origin3800, 25 years of simulated remodeling took approximately 25 minutes of one CPU.
Model of Menopause
Using information from the literature [17, 18, 19, 20, 21, 22, 23, 24, 25, 26], we developed a model of menopause. The reported changes in bone remodeling vary widely, therefore, we used changes in remodeling parameters in the range of reported values that resulted in changes in bone mass similar to changes in BMD measured in clinical studies.
The simulation started with normal remodeling [12]. Resorption depth was 42 µm, the formation deficit was 3.6% (2 elements in the model) per cavity [28, 29]. Remodeling space, the amount of bone resorbed but not yet replaced at any timepoint, was 4 or 6% of the bone volume [29, 30]. This remodeling space was created by making resorption cavities until the total volume of the cavities was 4 or 6% of the bone volume. We varied remodeling space and the changes in remodeling space and formation deficit during and after menopause. During the perimenopausal years, we increased the remodeling space by 33, 66 or 100% by creating more resorption cavities, using the increases in bone remodeling markers during this period as a guideline. Bone turnover was gradually increased over a period of 1, 2 or 3 years, to mimic slow changes in remodeling shown in clinical data [19] (Fig. 2a shows an increase over 2 years). The formation deficit was increased to mimic the more negative bone balance during the perimenopausal years [18].
The effect of three remodeling rates after menopause was investigated: the bone remodeling rate was kept at the high perimenopausal level (Fig. 2a, dashed line), returned to the premenopausal value (Fig. 2a, dotted line), or decreased to a value in between the pre- and the perimenopausal remodeling rate (Fig. 2a, continuous line). The remodeling rate was decreased over a period of 3, 4 or 5 years (Fig. 2 shows a decrease over 4 years).
Because of the wide biological variation, simulations with varying remodeling parameters resulted in changes in bone mass in the same range as changes in bone mass reported in the literature [19, 23]. These literature studies used BMD measurements to determine changes in bone mass over time. For our model of antiresorptive treatment we used a simulation model that resulted in changes in bone mass corresponding to the average changes in bone mass reported in clinical studies.
The effect of simulated menopause on the mechanical properties of the cancellous bone was determined by calculating the stiffness of the specimen at several timepoints. This stiffness, which is a good predictor of the strength of cancellous bone [31], was calculated using finite element analyses [32].
Simulation Antiresorptive Treatment
We simulated treatment with two drugs: one that was incorporated in the bone tissue and a drug that was not incorporated. The first drug was incorporated in newly formed bone tissue and reduced the resorption depth of cavities made in drug-containing tissue. The second drug was not incorporated in the tissue and reduced resorption depth by directly acting on the osteoclasts. Besides this, both drugs changed the formation deficit to a formation surplus and decreased the number of resorption cavities. The changes in remodeling are summarized in Table 1.
Five years of simulated treatment were started early (1 year after the start of the perimenopausal period), or late (10 years after the start of this period). The effects of the treatment regimens on bone age, volume and stiffness were determined.
Results
Simulation of Menopause
The simulated premenopausal remodeling resulted in rates of bone loss of 0.6 and 1% of the bone volume per year, for remodeling spaces of 4 and 6% of the bone volume. The rates of bone loss resulting from the increased turnover during the perimenopausal period are shown in Table 2. The rates of bone loss in our simulation model are within the range of bone loss rates measured in perimenopausal women [19, 23, 24, 25, 26].
A variation in the periods over which the rate of turnover in our model was changed during the perimenopausal years had only small effects. Increasing bone remodeling over a period of 1, 2 or 3 years, and decreasing over a period of 3, 4 or 5 years resulted in variations of bone volume of less than 3% from 10 to 20 years after the start of the perimenopausal period.
Ongoing turnover at the high remodeling rate (dashed line in Fig. 2A) resulted in complete destruction of the bone architecture within 10–30 years, which is not in agreement with clinical BMD data. The rates of bone loss resulting from decreased remodeling after the perimenopausal period (dotted and continuous line in Fig. 2A) were both in the biological range. Because clinical studies indicate that the postmenopausal remodeling rate is higher than the premenopausal remodeling rate [17, 33, 34], we included this in our menopause model (continuous line in Fig. 2). The bone loss in this model corresponded to the average rate of bone loss in the spine determined with BMD measurements. In this model, the premenopausal remodeling space was 4%, the remodeling space was increased by 66% and the formation deficit was doubled in the perimenopausal period.
The transient increase in turnover resulted in loss of 12% of the bone volume 10 years after menopause (Fig. 2b), which is in the range of reported clinical data [19, 23]. The stiffness decreased 2% in the first year of high turnover and was decreased by almost 35% 20 years after the start of the perimenopausal period (Fig. 2b).
Simulation of Antiresorptive Treatment
The drug not incorporated resulted in a fast increase in bone volume, up to 5% after 5 years of treatment. The incorporated drug resulted in a slower increase in bone mass and a maximal increase of 4% (Fig. 3). The incorporated drug resulted in a slower increase in bone mass because this drug only reduced resorption depth in drug-containing tissue. Stopping treatment with the not incorporated drug resulted in faster bone loss than stopping treatment with the incorporated drug, because the incorporated drug still exerted a protective effect after treatment was stopped. The increases in stiffness resulting from simulated antiresorptive treatment were larger in the transversal directions than in the main load-bearing (superior-inferior) direction (Table 3).
Although 9% of the bone volume was lost between 1 and 10 years after the start of menopause (Fig. 2b) the long-term difference in bone volume between early and late treatment was only 3% (Fig. 3). The difference in stiffness was considerably larger, as shown in Table 4.
The increases in bone volume in the simulations were in the range of increases in BMD seen in clinical studies [5, 35]. Simulated antiresorptive treatment increased the mean age of the tissue by 9–13% 20 years after the start of the perimenopausal period.
Discussion
In this study, we developed a computer simulation model of remodeling in human vertebral cancellous bone during menopause and antiresorptive treatment. This computer model incorporates remodeling parameters in 3D cancellous bone models and predicts changes in bone volume, age and stiffness resulting from menopause and subsequent drug treatment.
Simulated antiresorptive treatment resulted in changes in bone volume, in agreement with clinical data [5, 35]. We showed increases in stiffness of the cancellous bone, which were larger than the increases in bone volume. The transversal stiffness increased more than the stiffness in the main load-bearing direction. The large increase in transversal stiffness could contribute to the large anti-fracture effects of small increases in BMD, because the bones are often loaded in the transversal direction in falls. Moreover, the reduced turnover during treatment results in an increase in the average age and mineral content of the bone tissue [36, 37], which leads to a concomitant increase in stiffness of the bone tissue [38]. In the simulations we determined the age of the bone tissue, but the relation between age and mineral content of bone tissue is not well known. Therefore, we presented the results as changes in bone volume, rather than BMD. A constant bone volume in our simulation model could correspond to a slow increase in BMD due to prolonged secondary mineralization in reality. This would increase the stiffness of the cancellous bone even more than our model predicts.
Like all simulation models, this model is a simplification of reality. In reality, resorption and refill of a cavity take several weeks, and in the simulation model cavities are completely resorbed or completely refilled instantly. However, this has no effects on the long-term changes in bone volume and stiffness [12]. As input for our model we used a specimen from a 37-year-old male donor. It has been shown that at this age there is no significant difference in vertebral cancellous bone volume fraction and mechanical properties between males and females [39, 40]. Besides this the effects on bone of antiresorptive treatment in osteoporotic men and women are similar [5, 41]. Another simplification in our model is that the effects of mechanical loads on the bone structure were not taken into account. Mechanical loading probably affects bone remodeling: high loads are assumed to induce bone formation, loads below a certain threshold bone resorption [15]. The exact mechanisms are not known, but we can speculate on the expected effects on the architecture. For example, trabeculae that are breached, through which no load is transferred anymore, are expected to be resorbed rather fast. Including this in the model would lead to slightly faster bone loss, but it would have no effects on the stiffness, since the breached trabeculae do not contribute to the load bearing in the specimen. On the other hand, high local loads could induce extra bone formation. As a result of this, the thinning of the trabeculae in the load-bearing direction could be slowed down, or the remaining trabeculae might even increase in thickness to compensate for loss of other trabeculae.
A 3D simulation of bone remodeling as presented here, which also includes the effects of mechanical loads, is not feasible at the moment, but less detailed simulations and simulations in 2D have been performed [15, 42]. As computer technology develops further and the effect of loading on remodeling is better understood, such a detailed simulation will be possible in the future. Another improvement to the model that can be made, for example, by making a parallel version of our code, is the use of smaller elements in the model. This would enable investigation of the effects of small changes in, e.g., resorption depth during normal remodeling and during menopause, which is not possible with the current elements of 14 µm cubed.
Previously, other simulation models of bone remodeling during aging, menopause and antiresorptive treatment have been performed [13, 14, 16, 43, 44, 45]. Some models used artificial models of trabecular bone or 2D models [13, 44]; the other models did not include the bone architecture. Using these last models, the contributions of the change in remodeling space, the bone balance and mineralization changes to the total changes in BMD were estimated [45, 46]. The changes in BMD found in clinical studies could be explained from the changes in remodeling space and bone balance or from a change in remodeling space combined with an increase in tissue mineralization. In our model, we changed the remodeling space and bone balance during menopause and antiresorptive treatment and found results in agreement with the changes in remodeling found in Heaney et al. [46].
Our simulations of menopause and antiresorptive treatment were based on biological and clinical data [17, 18, 19, 20, 21, 22, 23, 24, 25, 26]. However, the reported changes in the biological and clinical studies vary widely. Moreover, there is no exact relation between the changes in markers in serum or urine and changes in bone remodeling. Therefore, we varied the parameters in our model in the range of reported biological values and compared the results to reported changes in BMD. Obviously, the parameters we used for the menopause simulation also affect the simulations of treatment. However, changing these parameters did not change our conclusions about the differences between the incorporated and the nonincorporated drug, early and late treatment and the gain in stiffness in transversal and longitudinal directions.
Most clinical studies have been performed in osteoporotic patients or in patients who were postmenopausal for at least 2 years [4, 5, 7, 35]. Here, we investigated the difference between treatment started ‘early’ and ‘late’ (1 and 10 years after the start of menopause). Late treatment resulted in an approximately 3% lower bone volume, a 6–9% lower stiffness in the main load-bearing direction and a 15–22% lower stiffness in transversal directions than early treatment, 20 years after menopause. This indicates that late treatment can result in almost the same bone mass as early treatment, but early treatment is better than late treatment when mechanical properties are considered: when bone is lost, some trabeculae are breached and the architecture deteriorates. These trabeculae are not made again when bone mass is increased. This is in agreement with previous investigations of the effects of the transient increase in remodeling during menopause: even a couple of years of high turnover can increase the fracture risk later in life [47]. Also, early treatment largely reduces the number of years that a patient is at risk of fracture. This strongly supports early treatment, even if the bone mass that can be obtained with late treatment is almost the same.
We compared a drug that was incorporated and one that was not incorporated. These two types are comparable to treatments used frequently nowadays: estrogens and SERM’s are not incorporated in the bone tissue, but bisphosphonates are incorporated [27]. In our simulation, the long-term effect of incorporation of a drug in the bone tissue was small: stopping treatment resulted in a slower bone loss than stopping treatment with the nonincorporated drug. This difference is in agreement with clinical data [48, 49, 50]. However, 20 years after menopause the difference in bone volume was below 3% in our simulation, so the long-term effects are very small.
According to our simulation model, the bone turnover probably decreases after the perimenopausal years. Previously, a decrease in turnover after menopause, as well as elevated turnover up to 40 years after menopause, have been reported [17, 18]. Our results show that ongoing high turnover with a formation deficit would result in an unrealistic total destruction of the bone within 10–30 years after menopause. The decrease in turnover in our simulation resulted in changes in bone volume in agreement with BMD data from clinical studies [19, 23, 24]. Another possibility, that we cannot exclude based on our results, is that remodeling does continue at a high rate, but that the formation deficit decreases: perfect remodeling, with equal resorption and formation, would not result in destruction of the bone. However, we think it is unlikely that after years of remodeling with a formation deficit, remodeling at old age would be perfect remodeling without bone loss. In our opinion, the reduced rate of bone loss 5–8 years after the start of the perimenopausal period is a result of a partial refilling of the excess remodeling space created during the perimenopausal period, not of unequal bone resorption and formation. This should be realized when prescribing antiresorptive treatment: the constant bone mass should not be confused with an effect of the treatment.
In conclusion, the presented model mimics bone remodeling during menopause and antiresorptive treatment, and predicts changes in bone volume in agreement with data from clinical follow-up studies. We found that the rate of bone turnover reaches a peak during the perimenopausal years and probably decreases thereafter. During the first years after stopping treatment, an incorporated drug still exerted a protective effect on the bone. It might be possible to take advantage of this by applying intermittent treatment [51]. The presented model could be used as a guideline for the development of such treatment regimens. Early treatment was found to be better, not in terms of bone mass, but it preserved the mechanical properties of the bone better than late treatment. This kind of simulation model of biological systems may be used in the future to predict the effects of drug treatments before patient data become available.
References
FP Coxon MH Helfrich R Van’t Hof S Sebti SH Ralston A Hamilton MJ Rogers (2000) ArticleTitleProtein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res 15 1467–1476 Occurrence Handle1:CAS:528:DC%2BD3cXlvVSjsLw%3D Occurrence Handle10.1359/jbmr.2000.15.8.1467 Occurrence Handle10934645
DJ Rowe LA Etre MJ Lovdahl DJ Pietrzyk (1999) ArticleTitleRelationship between bisphosphonate concentration and osteoclast activity and viability. Vitro Cell Dev Biol Anim 35 383–388 Occurrence Handle1:CAS:528:DyaK1MXls1yjt7w%3D Occurrence Handle10.1007/s11626-999-0112-7
S Vedi JE Compston (1996) ArticleTitleThe effects of long-term hormone replacement therapy on bone remodeling in postmenopausal women. Bone 19 535–539 Occurrence Handle1:CAS:528:DyaK28XnsVaktLw%3D Occurrence Handle10.1016/S8756-3282(96)00227-X Occurrence Handle8922654
B Ettinger DM Black BH Mitlak RK Knickerbocker T Nickelsen HK Genant C Christiansen PD Delmas JR Zanchetta J Stakkestad CC Gluer K Krueger FJ Cohen S Eckert KE Ensrud LV Avioli P Lips SR Cummings (1999) ArticleTitleReduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. JAMA 282 IssueID22 2124 Occurrence Handle10.1001/jama.282.22.2124
RP Tonino PJ Meunier R Emkey JA Rodriguez-Portales CJ Menkes RD Wasnich HG Bone AC Santora M Wu R Desai PD Ross (2000) ArticleTitleSkeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. Phase III Osteoporosis Treatment Study Group. J Clin Endocrinol Metab 85 3109–3115 Occurrence Handle1:CAS:528:DC%2BD3cXmsVKru70%3D Occurrence Handle10999794
UA Liberman SR Weiss J Broll HW Minne H Quan NH Bell J Rodriguez-Portales RW Downs Jr J Dequeker M Favus (1995) ArticleTitleEffect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med 333 1437–1443 Occurrence Handle1:CAS:528:DyaK28XkslehtA%3D%3D Occurrence Handle10.1056/NEJM199511303332201 Occurrence Handle7477143
DM Black DE Thompson DC Bauer K Ensrud T Musliner MC Hochberg MC Nevitt S Suryawanshi SR Cummings (2000) ArticleTitleFracture risk reduction with alendronate in women with osteoporosis: the Fracture Intervention Trial. FIT Research Group. J Clin Endocrinol Metab 85 4118–4124 Occurrence Handle1:CAS:528:DC%2BD3cXotlWnurw%3D Occurrence Handle10.1210/jcem.85.11.6953 Occurrence Handle11095442
J Reginster HW Minne OH Sorensen M Hooper C Roux ML Brandi B Lund D Ethgen S Pack I Roumagnac R Eastell (2000) ArticleTitleRandomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 11 83–91 Occurrence Handle1:CAS:528:DC%2BD3cXhvFGgsr0%3D Occurrence Handle10.1007/s001980050010 Occurrence Handle10663363
HA Pols D Felsenberg DA Hanley J Stepan M Munoz-Torres TJ Wilkin G Qin-sheng AM Galich K Vandormael AJ Yates B Stych (1999) ArticleTitleMultinational, placebo-controlled, randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Foxamax International Trial Study Group. Osteoporos Int 9 461–468 Occurrence Handle1:CAS:528:DyaK1MXkt1CktrY%3D Occurrence Handle10.1007/PL00004171 Occurrence Handle10550467
SR Cummings DB Karpf F Harris HK Genant K Ensrud AZ LaCroix DM Black (2002) ArticleTitleImprovement in spine bone density and reduction in risk of vertebral fractures during treatment with antiresorptive drugs. Am J Med 112 281–289 Occurrence Handle1:CAS:528:DC%2BD38XhvVWkurk%3D Occurrence Handle10.1016/S0002-9343(01)01124-X Occurrence Handle11893367
BL Riggs LJ Melton (1995) Osteoporosis: etiology, diagnosis, and treatment, 2nd ed. Lippincott-Raven Hagerstown, MD, USA
JC van der Linden JAN Verhaar H Weinans (2001) ArticleTitleA three-dimensional simulation of age-related remodeling in trabecular bone. J Bone Miner Res 16 688–696 Occurrence Handle1:STN:280:DC%2BD3MvlvFKqsg%3D%3D Occurrence Handle10.1359/jbmr.2001.16.4.688 Occurrence Handle11315996
S Tayyar PS Weinhold RA Butler JC Woodard LD Zardiackas KR St. John JM Bledsoe JA Gilbert (1999) ArticleTitleComputer simulation of trabecular remodeling using a simplified structural model. Bone 25 733–739 Occurrence Handle1:STN:280:DC%2BD3c%2FlvV2qsA%3D%3D Occurrence Handle10.1016/S8756-3282(99)00218-5 Occurrence Handle10593419
JS Thomsen L Mosekilde RW Boyce E Mosekilde (1994) ArticleTitleStochastic simulation of vertebral trabecular bone remodeling. Bone 15 655–666 Occurrence Handle1:STN:280:DyaK2M7nvVahsg%3D%3D Occurrence Handle10.1016/8756-3282(94)90314-X Occurrence Handle7873294
R Huiskes R Ruimerman GH van Lenthe JD Janssen (2000) ArticleTitleEffects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature 405 704–706 Occurrence Handle1:CAS:528:DC%2BD3cXktlGnsbo%3D Occurrence Handle10.1038/35015116 Occurrence Handle10864330
J Reeve (1986) ArticleTitleA stochastic analysis of iliac trabecular bone dynamics. Clin Orthop 213 264–278 Occurrence Handle3780102
P Garnero E Sornay-Rendu MC Chapuy PD Delmas (1996) ArticleTitleIncreased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J Bone Miner Res 11 337–349 Occurrence Handle1:CAS:528:DyaK28Xit1Cgu74%3D Occurrence Handle10.1002/jbmr.5650110307 Occurrence Handle8852944
PR Ebeling LM Atley JR Guthrie HG Burger L Dennerstein JL Hopper JD Wark (1996) ArticleTitleBone turnover markers and bone density across the menopausal transition. J Clin Endocrinol Metab 81 3366–3371 Occurrence Handle1:CAS:528:DyaK28Xls1OrtLk%3D Occurrence Handle8784098
R Recker J Lappe K Davies R Heaney (2000) ArticleTitleCharacterization of perimenopausal bone loss: a prospective study. J Bone Miner Res 15 1965–1973 Occurrence Handle1:STN:280:DC%2BD3cvnslSkuw%3D%3D Occurrence Handle10.1359/jbmr.2000.15.10.1965 Occurrence Handle11028449
RP Heaney RR Recker PD Saville (1978) ArticleTitleMenopausal changes in bone remodeling. J Lab Clin Med 92 964–970 Occurrence Handle1:STN:280:DyaE1M7htVOqtA%3D%3D Occurrence Handle739174
R Eastell PD Delmas SF Hodgson EF Eriksen KG Mann BL Riggs (1988) ArticleTitleBone formation rate in older normal women: concurrent assessment with bone histomorphometry, calcium kinetics, and biochemical markers. J Clin Endocrinol Metab 67 741–748 Occurrence Handle1:CAS:528:DyaL1cXlvVOrurc%3D Occurrence Handle10.1210/jcem-67-4-741 Occurrence Handle3262119
ZH Han S Palnitkar DS Rao D Nelson AM Parfitt (1997) ArticleTitleEffects of ethnicity and age or menopause on the remodeling and turnover of iliac bone: implications for mechanisms of bone loss. J Bone Miner Res 12 498–508 Occurrence Handle1:STN:280:DyaK2s3ls1Sgsg%3D%3D Occurrence Handle10.1359/jbmr.1997.12.4.498 Occurrence Handle9101361
H Okano H Mizunuma M Soda I Kagami S Miyamoto M Ohsawa Y Ibuki M Shiraki T Suzuki H Shibata (1998) ArticleTitleThe long-term effect of menopause on postmenopausal bone loss in Japanese women: results from a prospective study. J Bone Miner Res 13 303–309 Occurrence Handle1:STN:280:DyaK1c7lsV2isQ%3D%3D Occurrence Handle10.1359/jbmr.1998.13.2.303 Occurrence Handle9495525
G Mazzuoli M Acca D Pisani D Diacinti A Scarda L Scarnecchia MT Pacitti E D’Erasmo S Minisola G Bianchi G Manfredi (2000) ArticleTitleAnnual skeletal balance and metabolic bone marker changes in healthy early postmenopausal women: results of a prospective study. Bone 26 381–386 Occurrence Handle1:STN:280:DC%2BD3c7osFelsA%3D%3D Occurrence Handle10.1016/S8756-3282(00)00242-8 Occurrence Handle10719282
JR Guthrie PR Ebeling JL Hopper E Barrett-Connor L Dennerstein EC Dudley HG Burger JD Wark (1998) ArticleTitleA prospective study of bone loss in menopausal Australian-born women. Osteoporos Int 8 282–290 Occurrence Handle1:STN:280:DyaK1M%2FhsVKnug%3D%3D Occurrence Handle10.1007/s001980050066 Occurrence Handle9797914
JM Pouilles F Tremollieres C Ribot (1993) ArticleTitleThe effects of menopause on longitudinal bone loss from the spine. Calcif Tissue Int 52 340–343 Occurrence Handle1:STN:280:DyaK3s3nvVKitA%3D%3D Occurrence Handle10.1007/BF00310195 Occurrence Handle8504369
M Sato W Grasser N Endo R Akins H Simmons DD Thompson E Golub GA Rodan (1991) ArticleTitleBisphosphonate action. Alendronate localization in rat bone and effects on osteoclast ultrastructure. J Clin Invest 88 2095–2105 Occurrence Handle1:CAS:528:DyaK38XjsVGgtA%3D%3D Occurrence Handle10.1172/JCI115539 Occurrence Handle1661297 Occurrence Handle295810
JE Compston PI Croucher (1991) ArticleTitleHistomorphometric assessment of trabecular bone remodelling in osteoporosis. Bone Miner 14 91–102 Occurrence Handle1:STN:280:DyaK38%2FgsVajsA%3D%3D Occurrence Handle10.1016/0169-6009(91)90086-F Occurrence Handle1912765
HM Frost (1985) ArticleTitleThe pathomechanics of osteoporoses. Clin Orthop 200 198–225 Occurrence Handle3905111
AM Parfitt (1983) The psychiological and clinical significance of bone histomorphometric data. Bone histomorphometry: techniques and interpretation. CRC Press Boca Raton, FL 143–223
FJ Hou SM Lang SJ Hoshaw DA Reimann DP Fyhrie (1998) ArticleTitleHuman vertebral body apparent and hard tissue stiffness. J Biomech 31 1009–1015 Occurrence Handle1:STN:280:DyaK1M%2FpsVKltA%3D%3D Occurrence Handle10.1016/S0021-9290(98)00110-9 Occurrence Handle9880057
B Van Rietbergen A Odgaard J Kabel R Huiskes (1996) ArticleTitleDirect mechanics assessment of elastic symmetries and properties of trabecular bone architecture. J Biomech 29 1653–1657 Occurrence Handle1:STN:280:DyaK2s7gtFWnsw%3D%3D Occurrence Handle10.1016/S0021-9290(96)80021-2 Occurrence Handle8945668
JC Gallagher D Goldgar A Moy (1987) ArticleTitleTotal bone calcium in normal women: effect of age and menopause status. J Bone Miner Res 2 491–496 Occurrence Handle1:STN:280:DyaL1czjvVensw%3D%3D Occurrence Handle10.1002/jbmr.5650020605 Occurrence Handle3455633
JJ Stepan J Pospichal J Presl V Pacovsky (1987) ArticleTitleBone loss and biochemical indices of bone remodeling in surgically induced postmenopausal women. Bone 8 279–284 Occurrence Handle1:STN:280:DyaL1c7gslWqtg%3D%3D Occurrence Handle10.1016/8756-3282(87)90002-0 Occurrence Handle3501301
I Fogelman C Ribot R Smith D Ethgen E Sod JY Reginster (2000) ArticleTitleRisedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trial. BMD-MN Study Group. J Clin Endocrinol Metab 85 1895–1900 Occurrence Handle1:CAS:528:DC%2BD3cXlt1Wlsb4%3D Occurrence Handle10843171
GY Boivin PM Chavassieux AC Santora J Yates PJ Meunier (2000) ArticleTitleAlendronate increases bone strength by increasing the mean degree of mineralization of bone tissue in osteoporotic women. Bone 27 687–694 Occurrence Handle1:CAS:528:DC%2BD3cXosFygtL8%3D Occurrence Handle10.1016/S8756-3282(00)00376-8 Occurrence Handle11062357
CP Jerome (1989) ArticleTitleEstimation of the bone mineral density variation associated with changes in turnover rate. Calcif Tissue Int 44 406–410 Occurrence Handle1:STN:280:DyaL1MzltlOrsw%3D%3D Occurrence Handle10.1007/BF02555969 Occurrence Handle2504453
JD Currey (1984) ArticleTitleEffects of differences in mineralization on the mechanical properties of bone. Philos Trans R Soc Lond B Biol Sci 304 509–518 Occurrence Handle1:STN:280:DyaL2c7ksFWnug%3D%3D Occurrence Handle10.1098/rstb.1984.0042 Occurrence Handle6142490
L Mosekilde (1989) ArticleTitleSex differences in age-related loss of vertebral trabecular bone mass and structure—biomechanical consequences. Bone 10 425–432 Occurrence Handle1:STN:280:DyaK3c7ms12ntw%3D%3D Occurrence Handle10.1016/8756-3282(89)90074-4 Occurrence Handle2624823
RW McCalden JA McGeough CM Court-Brown (1997) ArticleTitleAge-related changes in the compressive strength of cancellous bone. The relative importance of changes in density and trabecular architecture. J Bone Joint Surg Am 79 421–427 Occurrence Handle1:STN:280:DyaK2s3itl2msA%3D%3D Occurrence Handle9070533
JD Ringe H Faber A Dorst (2001) ArticleTitleAlendronate treatment of established primary osteoporosis in men: results of a 2-year prospective study. J Clin Endocrinol Metab 86 5252–5255 Occurrence Handle1:CAS:528:DC%2BD3MXos1Kqs7c%3D Occurrence Handle10.1210/jcem.86.11.7988 Occurrence Handle11701687
M Mullender B van Rietbergen P Ruegsegger R Huiskes (1998) ArticleTitleEffect of mechanical set point of bone cells on mechanical control of trabecular bone architecture. Bone 22 125–131 Occurrence Handle1:STN:280:DyaK1c7js1WjsA%3D%3D Occurrence Handle10.1016/S8756-3282(97)00251-2 Occurrence Handle9477235
RP Heaney (1994) ArticleTitleThe bone-remodeling transient: implications for the interpretation of clinical studies of bone mass change. J Bone Miner Res 9 1515–1523 Occurrence Handle1:STN:280:DyaK2M7hslGmsQ%3D%3D Occurrence Handle10.1002/jbmr.5650091003 Occurrence Handle7817796
Z Tabor E Rokita (2002) ArticleTitleStochastic simulations of remodeling applied to a two-dimensional trabecular bone structure. Bone 31 413–417 Occurrence Handle1:STN:280:DC%2BD38vntVCrtA%3D%3D Occurrence Handle10.1016/S8756-3282(02)00837-2 Occurrence Handle12231415
CJ Hernandez GS Beaupre R Marcus DR Carter (2001) ArticleTitleA theoretical analysis of the contributions of remodeling space, mineralization, and bone balance to changes in bone mineral density during alendronate treatment. Bone 29 511–516 Occurrence Handle1:CAS:528:DC%2BD3MXos1Kntrw%3D Occurrence Handle10.1016/S8756-3282(01)00613-5 Occurrence Handle11728920
RP Heaney AJ Yates AC Santora (1997) ArticleTitleBisphosphonate effects and the bone remodeling transient. J Bone Miner Res 12 1143–1151 Occurrence Handle1:STN:280:DyaK2svgtlektg%3D%3D Occurrence Handle10.1359/jbmr.1997.12.8.1143 Occurrence Handle9258743
J Reeve (1987) ArticleTitleBone turnover and trabecular plate survival after artificial menopause. Br Med J (Clin Res Ed) 295 757–760 Occurrence Handle1:STN:280:DyaL1c%2FltFKksQ%3D%3D Occurrence Handle10.1136/bmj.295.6601.757
FA TremoIlieres JM Pouilles C Ribot (2001) ArticleTitleWithdrawal of hormone replacement therapy is associated with significant vertebral bone loss in postmenopausal women. Osteoporos Int 12 385–390 Occurrence Handle10.1007/s001980170107
B Orr-Walker DJ Wattie MC Evans IR Reid (1997) ArticleTitleEffects of prolonged bisphosphonate therapy and its discontinuation on bone mineral density in post-menopausal osteoporosis. Clin Endocrinol (Oxf) 46 87–92 Occurrence Handle1:CAS:528:DyaK2sXhslOgsbc%3D Occurrence Handle10.1046/j.1365-2265.1997.d01-1741.x
JL Stock NH Bell CH Chesnut KE Ensrud HK Genant ST Harris MR McClung FR Singer RA Yood S Pryor-Tillotson L Wei AC Santora (1997) ArticleTitleIncrements in bone mineral density of the lumbar spine and hip and suppression of bone turnover are maintained after discontinuation of alendronate in postmenopausal women. Am J Med 103 291–297 Occurrence Handle1:STN:280:DyaK1c%2Fis1Cntw%3D%3D Occurrence Handle10.1016/S0002-9343(97)00130-7 Occurrence Handle9382121
BJ Riis J Ise T von Stein Y Bagger C Christiansen (2001) ArticleTitleIbandronate: a comparison of oral daily dosing versus intermittent dosing in postmenopausal osteoporosis. J Bone Miner Res 16 1871–1878 Occurrence Handle1:CAS:528:DC%2BD3MXnsVKqtLo%3D Occurrence Handle10.1359/jbmr.2001.16.10.1871 Occurrence Handle11585352
Acknowledgements
J.C. van der Linden was supported by the Dutch Foundation for Research (NWO/MW), the National Computing Facilities Foundation (NCF) provided computing time. The authors thank Prof. Ruegsegger for providing the CT-scan data from the European Union project “Assessment of bone quality in osteoporosis.”
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van der Linden, J., Verhaar, J., Pols, H. et al. A Simulation Model at Trabecular Level to Predict Effects of Antiresorptive Treatment after Menopause . Calcif Tissue Int 73, 537–544 (2003). https://doi.org/10.1007/s00223-002-2151-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-002-2151-x