Abstract
We investigated the relation between lifetime physical activity and bone mineral density (BMD) in South African women using data collected in a case-control study of breast cancer in relation to BMD. Subjects (n = 144) were of black African or mixed ancestral origin, and <60 years of age (mean age 42.6 ± 8.9 years). Cases had newly diagnosed breast cancer (n = 62) and controls were referred for conditions unrelated to BMD or breast cancer (n = 82). Physical activity data consisting of household, occupational and leisure-time activity, and activity for transport, were collected via questionnaire at 4 life stages (epochs), viz. 14–21, 22–34, 35–50, and 50+ years of age. Total energy (MET hrs) and peak strain scores were calculated. Lumbar spine and total proximal femur BMD were measured using dual-energy x-ray absorptiometry. BMD measures were similar between groups, therefore data were combined. BMD measures were unrelated to total lifetime physical activity. However, the major determinants of total proximal femur BMD included age, transport activity including walking and bicycling between the ages of 14 and 21 years, and current weight (adjusted r2 = 0.33, P < 0.0001). The major determinants of lumbar spine BMD included age, household energy expenditure between the ages of 14 and 21 years, and current weight (adjusted r2 = 0.23, P < 0.0001). Total peak bone strain score for activities between 14–21 years of age was also significantly correlated with lumbar spine BMD (r = 0.18, P < 0.05). Intraclass correlation coefficients to assess tracking of activity through epochs 1, 2, and 3 were high for total energy expenditure (0.96; 95%CI: 0.94–0.97), household (0.98; 95%CI: 0.97–0.99) and occupational activity (0.78; 95%CI: 0.71–0.84) and activity for transport (0.92; 95%CI: 0.89–0.94). These data suggest that walking or activities resulting in impact loading at a young age are associated with higher BMD in later years. In addition, our findings suggest tracking of physical activity over time.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Studies that have examined the relationship between bone mineral density (BMD) and current physical activity levels have found a positive relationship [1, 2, 3, 4]. Other studies have also found past activity to be associated with current BMD in older women [5, 6]. The most detailed study of historical physical activity [7] found a consistent dose-response relationship between increasing quartiles of historical physical activity, excluding walking, and bone area and density in postmenopausal women. However, in a more recent study of Swedish men and women up to 85 years of age [8], lifetime occupational and sport activities, as well as bed rest, were not associated with BMD. Few longitudinal cohort studies have assessed the relationship between lifetime physical activity and BMD, and none have extended through to middle age. In the Amsterdam Growth and Health Longitudinal Study [9], both lumbar spine and hip BMD (at different sites) measured at 28 years of age was significantly associated with physical activity measured during adolescence. In addition, physical activity patterns of the subjects were monitored over the same period [10], and low-to-moderate tracking was observed for daily physical activity.
In addition to physical activity, weight-bearing exercise and high impact loading have been shown to be positively associated with increased BMD in men and women [11, 12, 13, 14, 15]. A range of peak strain scores for different physical activities have previously been developed [16]. In their study of adolescents and adults, peak strain scores were positively related to lumbar spine BMD. It may, therefore, be important to quantify weight-bearing activity in the assessment of historical physical activity in relation to BMD. Furthermore, most of the studies that have focused on the relationship between physical activity patterns and BMD, have involved middle-aged, Caucasian groups.
The aim of the present study was to evaluate the relationship between lifetime physical activity patterns, both in terms of total energy expenditure and bone impact loading, and bone mineral density in middle and older-age women of black or of mixed ancestral origin in the Western Cape of South Africa.
Materials and Methods
Subjects
The sample of 144 subjects comprised black women (n = 17) and women of mixed ancestral origin (n = 127) aged 22–59 years, residing in Cape Town, South Africa. All subjects had been participants in a case-control study of bone mineral density and breast cancer conducted in 1998. Informed consent was obtained from all subjects and the study was approved by the Ethics Committee at the University of Cape Town. Cases (n = 62) were women who presented with newly diagnosed incident primary cancer of the breast at Groote Schuur Hospital; only women who had not yet received treatment with chemotherapy or radiation were included. Controls (n = 82) were women who had been admitted to the same hospital for non-gynecologic illnesses unrelated to risk of breast cancer or contraceptive use. Cases and controls with bone diseases were excluded. Physical activity data were not collected on 17 of the subjects because the physical activity questionnaire was incomplete.The cases (76%) and the controls (53%) were aged 40 years or older; the proportion of women of mixed ancestral origin was 90% among the cases and 87% among the controls.
Medical History and Lifestyle Factors
All participants were administered standard questionnaires by nurse-interviewers to obtain information on risk factors for breast cancer, including reproductive history contraceptive history, and family history of breast cancer. Other information collected included years of current or past cigarette smoking, as well as current and past alcohol and coffee consumption. Demographic and socioeconomic data collected included education, employment and marital status.
Physical Activity History
Historical information was obtained on levels of physical activity for 4 age groups—14–21, 22–34, 35–50, and 50 or more years. The physical activity questionnaire was adapted and customized to this population of South African women from that of Kriska et al. [7]. A cue card with 34 activities divided into household, occupational, leisure-time and transport activities was used. Only activities over 3 METs (metabolic equivalents) according to the classification of activities by Ainsworth et al. [17] were included (one MET is defined as the energy expenditure for sitting quietly, which for the average adult is approximately 3.5 ml of oxygen/kg body weight/min). Each subject, under the guidance of the nurse-interviewer, selected activities in which she had participated for at least 6 months. Subjects then recalled the total number of years within each epoch that they engaged in this activity, as well as months/year, sessions/month and average min/session. We assigned each activity a MET level according to the Compendium of Physical Activities [17]. Lifetime physical activity data were divided into ‘absolute’ MET hrs, as well as an average of MET hrs/week for each epoch. In addition, MET hrs and MET hrs/week were calculated for household, occupational, leisure-time and transport activities within each epoch. Walking was quantified by separate questions: total time walking, walking for leisure, and walking for transport. Peak strain score was calculated for each epoch based on the peak strain score for each activity developed by Groothausen et al. [16]. This scoring system reflects impact loading and allocates a score of 1–5 for activities ranging from non-weight-bearing to those involving jumping actions.
Milk Intake
Milk/maas (a local sour milk drink) intake during the four epochs (adapted from Sandler et al. [18]) was used as a proxy for calcium intake. Subjects were asked whether they consumed milk with every meal; frequently/not with every meal; sometimes; rarely or never. Responses were categorized as 1 for every meal, 2 if frequently/not with every meal, 3 if sometimes, and 4 if rarely or never. Due to respondent burden and the homogeneity of the population, additional dietary information was not collected.
Bone Densitometry and Anthropometric Measures
On completion of the interview, each of the subjects had an osteodensitometry scan of the lumbar spine and the left proximal femur. The nurse-interviewer obtained signed informed consent from each subject. Scans were performed in the Department of Nuclear Medicine, Groote Schuur Hospital using a Hologic QDR-1000 (version 4.20) dual-energy x-ray bone densitometer (Hologic Inc., Waltham, MA). Average bone mineral densities (g/cm2) were determined for lumbar vertebrae 1 through 4 and the total proximal femur, Ward’s triangle, femoral neck, greater trochanter, and the intertrochanteric area. We used the World Health Organisation criteria [19] based on the T-score (the subject’s BMD expressed in relation to the young adult reference mean) to classify osteoporosis and osteopenia.
The nurse interviewers measured waist and hip circumferences and weight and height, according to standardized methods outlined in the study manual, at the time of the bone density scan. We calculated body mass index (BMI) as weight (kg) divided by height (m) squared.
Statistical Analysis
The StatsoftTM (Statistica v5.0, 1999) statistical package was used. Descriptive analyses were performed to determine mean values and standard deviations. Continuous data were compared between cases and controls using the one-way analysis of variance, covaried for age, weight and ethnicity. In addition, a chi-square test was used to compare categorical data such as smoking and alcohol intake between the groups. Pearson correlation coefficients for the relation of BMD to the various lifestyle and physical activity variables were calculated for the total cohort (cases and controls combined). These variables were then entered into a multiple stepwise regression in order to determine the influence of possible confounders. Transformation of the energy expenditure data, which was not normally distributed, did not improve the distribution of data and therefore was not used. Intraclass correlation coefficients were calculated for all subjects who had physical activity data for epoch’s 1, 2 and 3 (n = 96) to determine whether any of the physical activity measures tracked over time.
Results
The only significant difference in subject characteristics between the cases and controls was age (45.3 ± 8.7 vs 40.5 ± 8.6 yrs, respectively, P < 0.01). We found no differences between cases and controls in physical characteristics such as weight, height and BMI; reproductive factors such as parity and months of breastfeeding; and lifestyle factors, including past and present smoking and milk intake. All comparisons between groups were adjusted for ethnicity and age. As shown in Table 1, BMD of the lumbar spine, total proximal femur and femoral neck were similar in the cases and controls. There was also no difference between the cases and controls in the prevalence of osteoporosis and osteopenia. Because the cases and controls were similar in BMD measures and other characteristics, we combined the groups for all further analyses. Subject characteristics of the group (n = 144) are presented in Table 2.
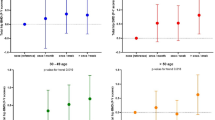
Total proximal femur (F) BMD and femoral neck (FN) BMD were lower in current and ex-smokers (n = 80) than in never-smokers (n = 64); (F BMD: 0.952 ± 0.132 vs 0.996 ± 0.124 g/cm2, P < 0.05; FN BMD: 0.838 ± 0.128 vs 0.906 ± 0.202 g/cm2, P < 0.01), after adjusting for weight, age and ethnicity (Fig. 1). No differences were found in BMD between women who had consumed alcohol (n = 44) and women who had not (n = 100) or between women who were past or present coffee drinkers (n = 133) and women who never drank coffee (n = 11).
Bivariate analysis was used to describe the relationship between the BMD measures and various subject characteristics (Table 2). The BMD of the lumbar spine, total proximal femur and femoral neck were inversely correlated with age, but only the association with femoral neck BMD was statistically significant (r = 0.26, P < 0.01). All three BMD measures were significantly correlated with weight, height, BMI and waist and hip circumferences. The strongest correlation was seen with total proximal femur BMD, where 20–25% of the variance in BMD was explained by a variance in the anthropometric measures. Age at menarche was significantly correlated with femoral neck BMD (r = 0.18; P < 0.05) but not with any of the other BMD sites.
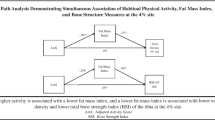
Means of the various physical activity parameters for epochs 1, 2, 3 and 4 are included in Table 3. Using bivariate analysis, total energy expenditure (TEE) and household (HH) energy expenditure, in average MET hrs/week, at ages 14–21 years were both significantly correlated with lumbar spine BMD (TEE: r = 0.18, P < 0.05; HH: r = 0.22, P < 0.05). Transport activity (TR), including walking and bicycling, and average time spent walking per day (W) were significantly correlated with total proximal femur BMD at this age (TR: r = 0.19, P < 0.05; W: r = 0.22, P < 0.05). Total peak bone strain score for activities at ages 14–21 was significantly correlated with lumbar spine BMD (r = 0.18, P < 0.05).
Using multivariate analysis, the variables, age, total energy expenditure for epoch 1, and current weight were included in the model that accounted for 21% of the variance in lumbar spine BMD (P < 0.0001, standard error of the estimate, SEE = 0.126 (Table 4). When total energy expenditure for epoch 1 was replaced with household energy expenditure for epoch 1, the same variables accounted for 23% of the variance in lumbar spine BMD (P < 0.0001, SEE = 0.124 (Table 4)).
The statistical model that best predicted total proximal femur BMD included age, transport activity including walking and bicycling between the ages of 14 and 21 years, and current weight. These variables accounted for 33% of the variance in total proximal femur BMD (P < 0.0001, SEE = 0.109, Table 5). When transport activity was replaced by average time spent walking per day between the ages of 14 and 21 years, 29% of the variance in total proximal femur BMD was accounted for (P < 0.0001, SEE = 0.105) (Table 5). BMD was not significantly correlated to any measures of physical activity in epochs 2, 3 or 4.
Intraclass correlation coefficients were calculated to determine tracking of activity through epochs 1, 2 and 3. For total energy expenditure an intraclass correlation coefficient of 0.96 (95%CI: 0.94–0.97) was obtained. For household activity and occupational activity, the intraclass correlation coefficient was 0.98 (95%CI: 0.97–0.99) and 0.78 (95%CI: 0.71–0.84), respectively. Similarly, activity for transport had an intraclass correlation coefficient of 0.92 (95% CI: 0.89–0.94), however, the intraclass correlation coefficient for leisure time activity was poor (−0.14; 95%CI: −0.22– −0.03).
Discussion
In the present study we found that the maximum influence of lifetime physical activity on adult BMD was between the ages of 14 and 21 years. Total walking and activity for transport were positively associated with total proximal femur BMD only in the first epoch. In addition, household activities, which were mostly weight-bearing tasks such as mopping and sweeping, were correlated with lumbar spine BMD during the same age period. Our results agree with earlier findings [5, 6, 7] that physical activity during early age periods is positively associated with BMD in later life.
The women in our study differed from those studied previously in that they were non-Caucasian and from socioeconomically disadvantaged backgrounds, where leisure time physical activity was low, whereas the participants in previous studies were Caucasian women, generally from the middle class [7, 8, 20]. Another difference is that our study incorporates household and occupational activity, as well as walking for transport. Had we only characterized leisure time activity, we might have failed to find the association with transport and household activities, highlighting the importance of physical activity measurement tailored to the lifestyle of the population under study. The finding that the relationship between physical activity and BMD was strongest in the earliest age period provides indirect evidence that mechanical loading resulting from physical activity during the adolescent years may result in a higher peak bone mass later in life. In addition, the relationship that we found between walking and BMD of the total proximal femur highlights the importance of impact loading and weight-bearing activity on bone accretion. Our findings support the data from Ulrich et al. [20] who found that total weight-bearing exercise during childhood and the teenage years was associated with BMD in American women.
The method used by Ulrich et al. [20] to classify weight-bearing activity did not take into account the impact loading of the activity. In our study we used the classification system of Groothausen et al. [16] to calculate a total peak bone strain score and we found that the total peak strain score during the first epoch (14–21 years) was also significantly associated with BMD of the lumbar spine. In contrast, Groothausen et al. found that peak strain physical activity during both adolescence and adulthood was positively related to lumbar spine BMD. These findings suggest that it is important to measure impact loading when investigating the relationship between BMD and physical activity.
Others have found that daily physical activity patterns track over time [10]. In our study, household activity accounted for most of the total energy expenditure in all four epochs and this, together with total energy expenditure, tracked throughout epochs 1, 2 and 3. Our study, however, only found activity during adolescence to be related to current BMD, therefore suggesting that physical activity is most effective during the period of bone accretion, as shown by Kriska et al. [7], and the relationship between past physical activity and current physical activity may actually confound this relationship.
With respect to factors other than physical activity, it is well known that weight and BMI are associated with increased BMD [21, 22]. Our findings are consistent with these studies and also show a strong relationship between waist circumference and BMD of the lumbar spine, which may just be a function of increased weight.
While Sandler et al. [18] found that women who reported drinking milk with every meal during childhood and adolescence had significantly higher bone densities than women who reported drinking milk less frequently, we failed to confirm such a relationship. However, most of the women in our study reported that they consumed milk rarely, and our failure to show an association between milk consumption and BMD may be due to decreased variability in the response to questions on milk consumption.
An unexpected finding was the significant positive correlation between age at menarche and femoral neck BMD. Delayed menarche has previously been associated with reduced bone mineral density [23]. We have no apparent explanation for this association in our study.
The present data were collected in a case-control study of breast cancer risk in relation to BMD. A criterion for subject selection was the absence of bone disease. The successful application of this criterion is indicated by the low prevalence of osteoporosis in the cases and controls. The study was not primarily designed to assess correlates of BMD. However, insofar as the appropriate data for such an assessment were collected, and the BMD measures and variables related to physical activity were similar in the cases and controls, we used the combined data for this purpose. The physical activity patterns of our study population included a large amount of household activity and daily walking as a means of transport and there was limited participation in leisure-time activity. The physical activity questionnaire that we used was specially adapted for this population. All BMD measurements were done at the same facility using internationally accepted procedures. Information bias is not a concern as the women were interviewed about their physical activity before their bone measurements were taken. However, there were undoubtedly errors in reporting physical activity, which if random could have weakened the relationship between BMD and physical activity. This questionnaire has also been found to be reproducible in a population of a similar age to ours [24].
As the current study was part of a much larger study, it was impossible to collect sufficient detail on some important confounders such as nutritional intake, particularly calcium intake. A more sensitive proxy for Ca intake would have been ideal, and greater attention should have been paid to other sources of Ca that may have been more applicable to this population.
The present study is the first to assess lifetime physical activity in relation to BMD in non-Caucasian women. As most of the women were of mixed racial descent from largely socioeconomically disadvantaged backgrounds, the results are most applicable to that ethnic group. Our results add to the literature that indicates that physical activity during childhood and adolescence is related to BMD in later life. Our results also suggest that activities such as walking, provide significant impact loading, which positively influences BMD. In addition, some aspects of physical activity early in life tracked over time. Further work is needed to define the separate contributions of physical activity at a young age and later on in life to adult BMD.
References
S Suleiman M Nelson F Li M Buxton-Thomas C Moniz (1997) ArticleTitleEffect of calcium intake and physical activity level on bone mass and turnover in healthy, white, postmenopausal women. Am J Clin Nutr 66 937–943 Occurrence Handle1:STN:280:ByiH28zoslQ%3D Occurrence Handle9322571
JF Aloia AN Vaswani JK Yeh SH Cohn (1988) ArticleTitlePremenopausal bone mass is related to physical activity. Arch Intern Med 148 121–123 Occurrence Handle10.1001/archinte.148.1.121 Occurrence Handle1:STN:280:BieC3crnsFU%3D Occurrence Handle3337589
B Kanders DW Dempster R Lindsay (1988) ArticleTitleInteraction of calcium nutrition and physical activity on bone mass in young women. J Bone Miner Res 3 145–149 Occurrence Handle1:STN:280:BiaC3c3mvVM%3D Occurrence Handle3213609
V Brewer BM Meyer MS Keele SJ Upton RD Hagan (1983) ArticleTitleRole of exercise in prevention of involutional bone loss. Med Sci Sports Exerc 15 445–449 Occurrence Handle1:STN:280:BiuD1M3lsVU%3D Occurrence Handle6656552
KM Khan KL Bennell JL Hopper L Flicker CA Nowson AJ Sherwin KJ Crichton PR Harcourt JD Wark (1998) ArticleTitleSelf-reported ballet classes undertaken at age 10-12 years and hip bone mineral density in later life. Osteoporos Int 8 165–173 Occurrence Handle1:STN:280:DyaK1czjtFGltA%3D%3D Occurrence Handle9666941
J Damilakis K Perisinakis G Kontakis E Vagios N Gourtsoyiannis (1999) ArticleTitleEffect of lifetime occupational physical activity on indices of bone mineral status in healthy postmenopausal women. Calcif Tissue Int 64 112–116 Occurrence Handle10.1007/s002239900588 Occurrence Handle1:CAS:528:DyaK1MXnvVagsw%3D%3D Occurrence Handle9914317
AM Kriska RB Sandier JA Cauley RE LaPorte DL Horn G Pambianco (1988) ArticleTitleThe assessment of historical physical activity and its relations to adult bone parameters. Am J Epidemiol 127 1053–1063 Occurrence Handle1:STN:280:BieC1M7os1Y%3D Occurrence Handle3358406
H Brahm H Mallmin K Michaelsson H Strom S Ljunghall (1998) ArticleTitleRelationships between bone mass measurements and lifetime physical activity in a Swedish population. Calcif Tissue Int 62 400–412 Occurrence Handle10.1007/s002239900452 Occurrence Handle1:CAS:528:DyaK1cXislSkt7c%3D Occurrence Handle9541517
HCG Kemper J Twisk W van Mechelen GB Post JC Roos P Lips (2000) ArticleTitleA fifteen-year longitudinal study in young adults on the relation of physical activity and fitness with the development of the bone mass: the Amsterdam Growth and Health Longitudinal Study. Bone 27 IssueID6 847–853 Occurrence Handle10.1016/S8756-3282(00)00397-5 Occurrence Handle1:STN:280:DC%2BD3M%2Fos1Cksw%3D%3D Occurrence Handle11113397
J Twisk HCG Kemper W van Mechelen (2000) ArticleTitleTracking of activity and fitness and the relationship with cardiovascular disease risk factors. Med Sci Sports Exerc 32 IssueID8 1455–1461 Occurrence Handle10.1097/00005768-200008000-00014 Occurrence Handle1:STN:280:DC%2BD3M%2FkvFSjsQ%3D%3D Occurrence Handle10949012
EJ Bassey SJ Ramsdale (1995) ArticleTitleWeight-bearing exercise and ground reaction forces: a 12-month randomized controlled trial of effects on bone mineral density in healthy postmenopausal women. Bone 16 469–476 Occurrence Handle1:STN:280:ByqA38bjvVU%3D Occurrence Handle7605708
WI Risser EJ Lee A Leblanc HBW Poindexter JMH Risser (1990) ArticleTitleBone density in eumenorrheic female college athletes. Med Sci Sports Exerc 22 570–574 Occurrence Handle1:STN:280:By6D2c7msVM%3D Occurrence Handle2233193
JE Dook C James NK Henderson RI Price (1997) ArticleTitleExercise and bone mineral density in mature female athletes. Med Sci Sports Exerc 29 291–296 Occurrence Handle1:STN:280:ByiB38jks1c%3D Occurrence Handle9139166
N Young C Formica G Szmukler E Seeman (1994) ArticleTitleBone density at weight-bearing and nonweight-bearing sites in ballet dancers: the effects of exercise, hypogonadism, and body weight. J Clin Endocrinol Metab 78 449–454 Occurrence Handle1:STN:280:ByuC2cbhsFU%3D Occurrence Handle8106634
GP Dalsky KS Stocke AA Ehsani E Slatopolsky WL Lee SJ Birge (1988) ArticleTitleWeight-bearing exercise training and lumbar bone mineral content in postmenopausal women. Arch Intern Med 108 824–828 Occurrence Handle1:STN:280:BieB38%2Foslc%3D
J Groothausen H Siemer HCG Kemper J Twisk DC Welten (1997) ArticleTitleInfluence of peak strain on lumbar bone mineral density: an analysis of 15-year physical activity in young males and females. Pediatric Exerc Sci 9 159–173
BE Ainsworth WL Haskell AS Leon HJ Montoye JF Sallis JRS Paffenbarger (1993) ArticleTitleCompendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 25 71–80 Occurrence Handle1:STN:280:ByyC2c%2FmtFU%3D Occurrence Handle8292105
RB Sandler CW Slemenda RE LaPorte JA Cauley MM Schramm ML Barresi AM Kriska (1985) ArticleTitlePostmenopausal bone density and milk consumption in childhood and adolescence. Am J Clin Nutr 42 270–274 Occurrence Handle1:STN:280:BiqB2sngsFM%3D Occurrence Handle3839625
World Health Organisation (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. World Health Organ Tech Rep Ser 843
CA Ulrich CC Georgiou DE Gillis CM Snow (1999) ArticleTitleLifetime physical activity is associated with bone mineral density in premenopausal women. J Women’s Health 8 365–375 Occurrence Handle1:STN:280:DyaK1M3ls12hsQ%3D%3D
AM Davee CJ Rosen RA Adler (1990) ArticleTitleExercise patterns and trabecular bone density in college women. J Bone Miner Res 5 245–250 Occurrence Handle1:STN:280:By%2BB2c%2FgvVI%3D Occurrence Handle2333783
Z Chen TG Lohman WA Stini C Ritenbaugh M Aickin (1997) ArticleTitleFat or lean tissue mass: Which one is the major determinant of bone mineral mass in healthy postmenopausal women? J Bone Miner Res 12 144–151 Occurrence Handle1:STN:280:ByiA1c3nsFA%3D Occurrence Handle9240737
DA Galuska MR Sowers (1999) ArticleTitleMenstrual history and bone density in young women. J Womens Health Gend Based Med 8 647–656 Occurrence Handle1:STN:280:DC%2BD3c3ps1Clsw%3D%3D Occurrence Handle10839651
L Chasan-Taber JB Erickson JW McBride PC Nasca S Chasan-Taber PS Freedson (2002) ArticleTitleReproducibility of a self-administered lifetime physical activity questionnaire among female college alumnae. Am J Epidemiol 155 282–289 Occurrence Handle10.1093/aje/155.3.282 Occurrence Handle11821254
Acknowledgements
The data collection for this study was supported by grant R01CA60954 from the U.S. National Cancer Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Micklesfield, L., Rosenberg, L., Cooper, D. et al. Bone Mineral Density and Lifetime Physical Activity in South African Women. Calcif Tissue Int 73, 463–469 (2003). https://doi.org/10.1007/s00223-002-2129-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-002-2129-8