Abstract
People with Parkinson’s disease (PD) have increased visual dependency for balance and suspected vestibular dysfunction. Immersive virtual reality (VR) allows graded manipulation of visual sensory inputs during balance tasks, and hence VR coupled with portable force platforms have emerged as feasible, affordable, and validated tools for assessing sensory-motor integration of balance. This study aims to determine (i) how people with PD perform on a VR-based visual perturbation standing balance task compared to healthy controls (HC), and (ii) whether balance performance is influenced by vestibular function, when other known factors are controlled for. This prospective observational study compared the balance performance under varying sensory conditions in 40 people with mild to moderate PD with 40 age-matched HC. Vestibular function was assessed via Head Impulse Test (HIMP), cervical and ocular vestibular evoked myogenic potentials (cVEMPs and oVEMPs) and subjective visual vertical (SVV). Regression analyses were used to determine associations between VR balance performance on firm and foam surfaces with age, group, vestibular function, and lower limb proprioception. PD failed at significantly lower levels of visual perturbation than HC on both surfaces. In PD, greater disease severity was significantly associated with lower fall thresholds on both surfaces. Multiple PD participants failed prior to visual perturbation on foam. On firm, PD had a greater visual dependency. Increasing age, impaired proprioception, impaired SVV, abnormal HIMP and cVEMP scores were associated with worse balance performance. The multivariate model containing these factors explained 29% of the variability in balance performance on both surfaces. Quantitative VR-based balance assessment is safe and feasible in PD. Balance performance on both surfaces was associated with age, HIMP abnormality and proprioception.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the past decade, there has been rapid advancement in the development and use of virtual reality (VR)-based balance assessment tools and rehabilitation paradigms in the vestibular and balance clinical arenas (Alahmari et al. 2014; Canning et al. 2020; Chiarovano et al. 2017; Wittstein et al. 2020). Like the historically ‘gold standard’ EquiTest (Neurocom®) sensory organisation test (SOT), as measured with computerised dynamic posturography, these VR-based assessment tools can manipulate the immersive sensory environment to assess the integration of visual, vestibular and somatosensory inputs. They can also assess participants’ ability to shift between reliance on a particular sensory modality and objectively measure the resultant motor response (via sway or task failure/fall) (Chiarovano et al. 2015, 2017; Dona et al. 2016; Wittstein et al. 2020). These tests aim to improve our understanding of the neural circuity involved in sensory-motor integration and the effects of aging and neurodegenerative diseases on balance performance to better inform focused therapeutic interventions. These tests also allow for objective measurement of balance performance parameters to monitor and track individuals’ response to balance specific rehabilitation programmes.
In contrast to the typical computerised posturography found in tertiary balance laboratories, the newly developed VR-based systems are portable, relatively low cost, and can measure multidirectional sway. The VR-based systems also benefit from having a wide range of visual sensory perturbation conditions dependent on the specific software programme. As such, many VR-based balance assessment systems have become widely used in general, non-specialist balance and falls clinics.
One such portable, feasible and cost-effective VR-based balance assessment tool was developed and validated to the SOT EquiTest (Chiarovano et al. 2015, 2017) and is currently used in over 50 clinics worldwide (personal data from Chiarovano). The protocol assesses quiet standing balance performance under differing sensory conditions via the threshold for failure/fall and the quantitative centre of pressure sway. Like the SOT, VR test conditions include altering proprioceptive input, i.e. standing on the firm, stable surface of the force platform versus standing on a high-density foam cushion; and altering visual inputs, i.e. eyes open versus eyes closed versus visual perturbation. However, the VR-based protocol has the benefit of delivering progressively complex visual sensory perturbation over subsequent trials to determine the exact threshold of visual perturbation required to induce failure/falls. Balance performance data from the study by Chiarovano et al. 2017 (Chiarovano et al. 2017) in healthy controls (HC) of varying ages showed that standing balance performance with visual perturbation deteriorated with increasing age, thus re-enforcing prior findings of increased visual dependence for postural stability with aging (Berard et al. 2012; Borger et al. 1999). Their data also showed reduced balance performance with a visual perturbation in participants with bilateral vestibular loss.
The Chiarovano VR-balance protocol has not yet been reported in people with the common neurodegenerative disorder Parkinson’s disease (PD). PD is known to affect postural stability, and people with PD fall significantly more often with more significant injuries sustained than their age-matched peers (Alfonso et al. 2017; Allen et al. 2013). Whilst PD-related postural instability and falls are complex and heterogeneous in their aetiology, impaired sensory-motor integrative circuitry has long been suspected. The basal ganglia and vestibular nuclei complex are critical central nervous system regions involved in the sensory-motor integration of vestibular, visual and proprioceptive inputs vital for balance (Abbruzzese and Berardelli 2003; Cullen 2012, 2016), and both regions are known to be affected explicitly by PD neurodegeneration (Seidel et al. 2015; Wellings et al. 2017). People with PD are also known to have increased visual dependency for balance (Yakubovich et al. 2020), reduced proprioceptive integration (Konczak et al. 2009; Tan et al. 2011; Teasdale et al. 2017), impaired postural responses and motor output (Kim et al. 2009; Horak et al. 1992) and suspected vestibular dysfunction (Hawkins et al. 2020; Smith 2018). People with moderate severity PD have also been reported to have excessive sway on SOT, to the point of failure/fall, on visually compromised and unstable surface conditions (Colnat-Coulbois et al. 2011; Frenklach et al. 2009; Rossi et al. 2009).
As such, this study aims to report and compare balance performance using the VR-based testing protocol under varying sensory conditions in people with PD compared to age-matched HC. Additionally, we aim to assess whether vestibular function affects performance on the VR-based sensory integration assessment tool when other factors known to affect balance performance are controlled for, specifically lower limb proprioception, age and group.
Methods
All participants provided written informed consent before data collection. The study was conducted following the ethical standards of the Helsinki Declaration of 1975, as revised in 1983. Ethical approval for this prospective observational study was obtained through the relevant Human Ethics Committee. Data were collected between February 2018 and August 2019 from 40 participants with neurologist diagnosed idiopathic PD during their ‘on’ parkinsonian medication state and 40 age-matched HC. Participants were included in the study if they were community-dwelling, aged 50–80 years and able to walk independently with or without an aid. Exclusion criteria were: a known history of previously diagnosed vestibular disorders; known dementia; severe visual impairment affecting the ability to visualise the standardised vHIT targets without usual spectacles; any known neurological conditions (apart from PD); cognitive impairment defined as ≤ 24 on the Mini-Mental State Examination (MMSE) (Folstein et al. 1975); severely reduced neck range of motion or pain during head-turning, or a diagnosis of atypical PD. One PD participant was excluded from the study after recruitment due to an MMSE score < 24.
Demographic and clinical information was obtained from all participants and included age, fall history in the previous 12 months; fear of falling as quantified by the Falls Efficacy Scale–International Questionnaire (Dewan and MacDermid 2014); and cognitive status as quantified by the MMSE. PD motor symptom severity was assessed with the motor section of the Movement Disorders Society sponsored version of the Unified Parkinson Disease Rating Scale (MDS-UPDRS Part 3) and the Hoehn and Yahr Scale; freezing of gait (FOG) status was determined by Part 1 of the New Freezing of Gait Questionnaire) (Nieuwboer et al. 2009). The levodopa equivalent daily dose (LEDD) was calculated to quantify parkinsonian medication use (Tomlinson et al. 2010). See supplementary file 1A for details of antiparkinsonian medications. Participants in the PD group were grouped into tremor dominant (TD) or postural instability/gait disorder (PIGD) subtypes based on Part 3 of the MDS-UPDRS. Participants were classified as indeterminate (IND) if subtyping was ambiguous (Stebbins et al. 2013). Lower limb proprioception was recorded seated with eyes closed with a lower limb joint position matching task (Lord et al. 2003).
The Head Impulse test (HIMP) measured with video oculography (vHIT) is used as a measure of semicircular canal mediated vestibulo-ocular reflex (VOR) function, and the testing methodology has been described and reviewed extensively elsewhere (Halmagyi et al. 2017; Macdougall et al. 2013). We used lightweight ICS Impulse video goggles and software (GN Otometrics, Taastrup, Denmark), which recorded motion of the right eye. Oculomotor screening was completed with ICS Impulse video goggles in room light before vestibular assessment and included checks for spontaneous nystagmus, gaze-evoked nystagmus, skew deviation, saccades, VOR suppression and visual VOR; these findings are not reported in this study. Lateral canal (LC) HIMP was considered abnormal if the VOR gain value was below the software-defined normal range for age and mean head velocity, and corrective saccades (either overt or covert) were present. One set of PD vHIT LC HIMP data was excluded from analysis due to congenital nystagmus affecting vHIT calibration.
Cervical and ocular vestibular evoked myogenic potentials (cVEMP and oVEMPs, respectively) reflect otolithic reflex pathway function, and the methodology for obtaining them has been described in our paper reporting on otolithic function in PD (Hawkins et al. 2020). As bone-conducted vibrations to the forehead with tendon hammer taps produce robust oVEMPs and cVEMPs in older-aged cohorts, we have reported on tap-induced oVEMP and cVEMPs in this study. The VEMP score was calculated for each participant using the method first described and validated to clinical presentation in multiple sclerosis (Gabelic et al. 2015) and in PD (Carpinelli et al. 2021). The total VEMP score represents the sum of left and right tendon hammer tap o and cVEMP scores (0 = normal, 1 = increased latency with normal reflex morphology and amplitude of major potentials, 2 = decrease in amplitudes or altered morphology of major potentials, 3 = absence of major potentials. For example, a score of 0 indicates normal bilateral oVEMPs and cVEMPs, whilst 12 indicates bilaterally absent oVEMPs and cVEMPs.
Subjective Visual Vertical (SVV) reflects a sensory integration task in terms of ‘verticality perception’ as well as the otoliths’ static function. The perceived visual vertical angle is compared to true gravitational vertical. In HC, a mean angle error of less than 2.5° (in absolute value) from the actual gravitational angle is considered normal (Schonfeld and Clarke 2011). We used the validated VR-based SVV assessment method (Chiarovano et al. 2018; Hawkins et al. 2020).
Standing balance assessment with VR-induced visual perturbation protocol reflects a sensory integration test; specifically, the ability to maintain upright standing for a 20-s testing epoch with progressively increasing complexity (in velocity and amplitude) of VR-delivered visual perturbation. The testing equipment specifics and validation to EquiTest were previously described in detail (Chiarovano et al. 2015, 2017).
In this study, all participants were tested whilst standing with feet together (or as close together as able, in case of orthopaedic limitations) and without shoes, in both proprioceptive conditions, i.e. on the firm surface of the force platform (Wii Balance board) and then on a high-density foam cushion (Airex Balance Pad, Airex AG, Sins, Switzerland, 41 cm × 50 cm x 6 cm thick) placed on the force platform. Successful completion required that the participant remained standing for the entire 20 s test period with feet together. The test was stopped if the person lost balance or had to uncross arms to support themselves on the wall behind; if the researcher had to support the person to prevent a fall; if the person took a step or opened their eyes during the eyes-closed condition. The entire test was ceased if the person consecutively failed twice on a particular testing condition (proprioceptive and visual condition). No participant fell to the ground, and there were no adverse events during testing. See Supplementary file 1B for test set up.
The BalanceRite App and Wii Balance board objectively measure multiplanar sway via a centre of pressure trace (sway analysis is not within the scope of this study) and time to ‘failure/fall’ per condition within each 20-s testing epoch. For operational ease, participants were first tested standing with eyes open and then closed before the VR headset was placed on their head. With customised, freely available software, the VR headset delivered first, at VR0.0, an immersive garden and house scene, replicating a ‘real-life visual world’ as if the eyes were open. During the subsequent visual conditions, the VR scene moves unpredictably in the x, y and z planes. The complexity of visual perturbation increases (i.e. increasing amplitude and velocity of visual motion) with the successful completion of each trial, i.e. beginning from the lowest complexity of VR0.1 up to the maximum complexity of VR0.5.
Participants were first tested on the firm surface under all visual conditions until two consecutive failures, then on the foam. The order for visual testing conditions was eyes open; eyes closed; VR0.0; then each VR level up to the maximum stimulation of VR0.5. If the participant failed with eyes closed, VR0.0 was still tested. Balance performance was coded based on the highest successful trial level achieved. See Supplementary file 1B for test scoring. Coding VR0.0 as easier than eyes closed is based on prior findings that the real-life visual world is replicated in immersive VR environments, and static standing balance measures are not significantly different to eyes open conditions (Robert et al. 2016).
Statistical analysis
All data were analysed using SPSS version 24 (IBM Corp, Armonk NY). Independent samples t-test (two-tailed) was used to compare group differences, including age, FES-I score, SVV and proprioception error angles, and mean VR balance performance. Fisher’s Exact (two-tailed) test was used to compare groups on categorical variables including ‘normal’ vs ‘abnormal’ VEMP, vHIT HIMP, SVV response and history of recurrent (i.e., 2 +) falls. ANOVA was used to compare balance performance on both surfaces between the PD subtypes. Significance was set at p < 0.05.
Univariate linear regression models were used to analyse the relationships between balance performance, on firm and foam surfaces, with predictor variables of the group, age, VEMP scores (cVEMP, oVEMP and combined VEMP score), proprioceptive error angle, SVV absolute error angle and for the PD group, MDS-UPDRS part 3 score and LEDD. Multivariate linear regression models (enter method) included vestibular predictors which were significant from univariate regression models; adjusted R2 values were reported for these multivariate models. Predictors entered into the multivariate models were not collinear (r < 0.7). For details see Supplementary file 4.
This study's power calculation was based on data reported by Chiarovano et al. (Chiarovano et al. 2017). They reported that 23% of healthy 60–69-year-old subjects (n = 17), i.e. the target age group for the present study, ‘failed’ on the foam when the lowest virtual reality visual perturbation of VR 0.1. was applied. It is hypothesised that double the percentage of people with PD (alpha 0.05, beta 0.2) will ‘fall/fail’ at this same visual stimulus compared to HC. Given the variability in the Chiarovano et al. data (Chiarovano et al. 2017) and previous evidence concerning people with PD’s postural stability, it was anticipated that this number would give adequate power to establish whether there are significant effects of PD on the performance of this standing balance task with the increasing complexity of VR-delivered visual perturbation.
Results
The demographic and clinical characteristics of both groups are presented in Table 1. Data are reported as mean (SD) or n (%). Both PD and HC were well matched in age and gender (p > 0.05), and there was no significant between-group difference on cognitive status as measured by MMSE (p = 0.21). The mean age of both groups was 69.73 SD 5.82 years. The PD participants had predominantly mild to moderate disease severity (78% recording a Hoehn and Yahr stage of 1 or 2), and no participant exhibited significant dyskinesias to affect balance performance. The PD group had a significantly worse (higher) cVEMP and combined VEMP score compared to HC (respectively, 1.53 SD 2.17 versus 0.65 SD 1.19, p = 0.03 and 2.68 SD 3.32 versus 1.28 SD 0.85, p = 0.02) though oVEMP scores were not significantly different, p = 0.25. They also had significantly more abnormal SVV findings than HC (57.5% versus 30%, p = 0.02) and greater absolute mean SVV error (3.54º SD 2.31 versus 1.98º SD 0.15, p = < 0.001). Findings of abnormal LC HIMP were not significantly different between PD and HC (18% versus 10% p = 0.35). In the PD group, 7 participants (18%) recorded abnormal LC HIMP (LC VOR gains between 0.50 and 0.75); of these, two recorded bilaterally abnormal HIMP, both of whom had higher disease severity (Hoehn and Yahr stage 3, MDS-UPDRS Part 3 scores > 52). In the HC group, 4 participants (10%) recorded abnormal HIMP (LC VOR gains between 0.63 and 0.78). Oculomotor screening with vHIT goggles (in room-light) did not indicate uncompensated peripheral vestibular asymmetry (direction fixed horizontal nystagmus) nor central oculomotor dysfunction (gaze-evoked nystagmus or vertical skew) in any participant at the time of testing.
Coding the successful completion of standing with VR0.0 as easier than standing with eyes closed was verified by balance performance in both groups. Of the 7 participants (one HC) who failed on foam with eyes closed, 6 could remain standing with VR0.0 and subsequently failed at VR0.1.
FIRM surface VR balance performance
The PD group had a significantly lower fall threshold with visual perturbation on the firm surface than HC, with a mean balance level of 7.70 SD 0.85 and 6.83 SD 1.96 respectively, p = 0.01, Fig. 1A and B. One PD participant could not maintain standing with feet together and eyes open, hence scoring 0. Removal of this participant’s data from the analysis did not alter significance levels; thus, their data were included in the overall analysis. Supplementary file 2 shows histogram data in table format.
A-D Histograms of balance performance of HC and PD on firm and foam. Y- axis indicates frequency, X-axis indicates best balance level achieved: 0 = fail all; 1 = achieved eyes open, fail at VR0.0; 2 = achieved VR0.0, fail eyes closed; 3 = achieved eyes closed, failed VR0.1; 4 = achieved VR0.1, failed at VR0.2; 5 = achieved VR0.2, failed VR0.3; 6 = achieved VR0.3, failed VR0.4; 7 = achieved VR0.5, failed at VR0.5; 8 = achieved at maximum visual perturbation at VR0.5
All HC participants maintained standing on the firm surface up to and including the second level of VR visual stimulation, i.e., VR0.2. Only people in the PD group failed at or below a visual stimulation level of VR0.2. Secondary analysis of the lowest-performing PD participants on the firm surface compared to the rest of the PD group showed that all 5 participants recorded an abnormal SVV, p = 0.03 (Fisher’s Exact). However, no association was found with abnormal HIMP or VEMP (p = 0.82) nor abnormal proprioception (p = 0.12). They also had higher disease severity (UPDRS part 3 score 41.40 SD 7.4 versus 23.71 SD 11.28 p = 0.003) and were significantly older (74.34 SD 4.5 years vs 69.35 SD 5.80 years, p = 0.05) than the rest of the PD group.
Univariate analysis revealed that age, group, proprioception angle, absolute SVV error angle and abnormal HIMP findings significantly impacted balance performance on the firm surface (Table 2). cVEMP score, oVEMP score and combined VEMP score were not significantly associated with firm surface balance performance. See supplementary file 3 for linear regression plots.
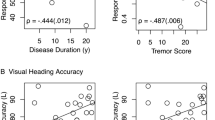
For the PD group, increasing PD motor disease severity (UPDRS score) had a statistically significant inverse relationship with balance performance, p = < 0.0001; as did LEDD, p = 0.04. See Fig. 2 for regression plots. There was no association with reported FOG (p = 0.10); nor with PD phenotype (F (2, 37) = 1.29, p = 0.29).
The multivariate analysis of the variables significantly associated with firm balance performance revealed that 29% of the variance of the balance score was explained by age, group, proprioception error angle, SVV absolute error angle, HIMP abnormality and cVEMP score (see Table 3). Within that model, age, proprioception angle and HIMP abnormality made independent contributions to balance performance; PD did not.
FOAM balance performance
Again, the PD group had a significantly lower fall threshold than HC, with a mean balance level of 3.65 SD 2.01 and 5.08 SD 1.62 respectively, p = 0.001. In the PD group, 11/40 (27.5%) participants failed before visual stimulation commenced. See Fig. 1B and D. Across both groups, 7/80 (1 in HC) failed with eyes closed; of these, 6 succeeded at VR0.0.
Of those who maintained standing on foam with eyes closed, 7/40 (17.5%) in the PD group and 6/40 (15%) in the HC group failed at the lowest level of visual perturbation, VR0.1.
Univariate analyses revealed that the same variables associated with firm balance performance, namely age, group, proprioception, SVV error angles, and abnormal HIMP findings, were associated with foam balance performance, though additionally, cVEMP score was associated with performance on foam (Table 2). Both groups’ balance performance deteriorated with age to a similar degree (see supplementary regression plots) on foam.
For the PD group, increasing PD motor disease severity (UPDRS score) had a statistically significant inverse relationship with balance performance, p = < 0.04; as did LEDD, p = 0.04. There was no association with reported FOG (p = 0.48); nor with PD phenotype (F (2, 37) = 0.03, p = 0.97).
Multivariate analysis (Table 3) of the variables significantly associated with foam balance performance revealed that 29% of the variance in balance performance score on the foam was explained by age, group, proprioception error angle, SVV error angle, HIMP abnormality and cVEMP score. Within that, age, proprioception angle and HIMP abnormality made independent contributions to balance performance, but PD did not.
Discussion
This study reports and compares standing balance performance, specifically the ‘fail threshold’ under varying sensory conditions, using the portable VR-based testing protocol (Chiarovano et al. 2017) in people with PD (78% had mild to moderate disease severity) and age-matched HC; we found that unsurprisingly people with PD performed worse than HC using this protocol. We also assessed whether vestibular function affected performance on this VR-based task when other known factors on balance performance are controlled for, specifically lower limb proprioception, age, and group, and found that an abnormal LC HIMP was significantly associated with worse balance performance on both surfaces.
The VR balance testing protocol was safe for all study participants, and there were no adverse events during testing. A few participants from both groups reported brief mild nausea after visual stimulation, and some required a few minutes break between subsequent tests, though no testing session was ceased due to symptoms. Our study also shows that immersive VR, without visual perturbation, i.e., VR0.0, adequately replicates the ‘real world’ visual environment during this static standing balance task. Across both groups and both proprioceptive conditions, in all but one case (PD, on foam), participants who failed with eyes closed could maintain standing with eyes open and with VR0.0. This finding is consistent with other immersive VR studies reporting that static standing balance measures are not significantly different to the ‘real world’ with eyes open (Robert et al. 2016).
In line with the findings in HC from Chiarovano et al. (Chiarovano et al. 2017), we found that increasing age had a significant inverse effect on balance performance for both groups, on both surfaces, though affecting PD more than HC on the firm surface (see supplementary file 3 for linear regression plots). Thus, supporting the notion of age-related increase in visual dependence for balance (Borger et al. 1999). On multivariate analysis, age made a significant individual contribution to balance performance on both firm and foam surfaces.
As expected, the PD group had a significantly lower fall threshold on this VR-balance task on both the firm and foam conditions than age-matched HC. On the firm surface, all participants in the HC group succeeded up to a visual stimulation VR0.2. Whereas in the PD group, 5/40 (12.5%) failed at the lowest levels of visual perturbation, VR0.1 or VR0.2. At the highest level of visual perturbation, VR0.5, 65% of the PD group versus 87.5% of the HC succeeded. Hence, our study’s VR-based protocol findings further support the suggestion of increased visual dependence with PD (Azulay et al. 2002; Bronstein et al. 1990; Yakubovich et al. 2020), even when somatosensory feedback is accurate (firm surface). From a practical standpoint, identifying participants with significant visual dependence, even when accurate somatosensory feedback is available, would have important implications for balance and falls prevention rehabilitation. These more visually dependent participants may respond particularly well to increased accurate visual cuing for balance or could be taught strategies to overcome or ignore ambiguous visual inputs in real-world environments. In our study, all of the participants in the PD group who failed at low levels of visual perturbation on the firm surface also had verticality perception dysfunction evident with abnormal SVV recordings. Further supporting the theory of sensory integration dysfunction in PD are our prior findings of increased absolute SVV error angle and increased variability in SVV response angle in PD versus HC (Hawkins et al. 2020), consistent with (Schindlbeck et al. 2018; Scocco et al. 2014), and this variable’s significant association with both firm and foam balance performance with the VR-based protocol.
A further finding was that LC HIMP abnormality was significantly associated with, and made an independent contribution to, balance performance on both surfaces. One of our study’s exclusion criteria was a known history of previously diagnosed vestibular dysfunction; though as ‘dizziness’ is a common symptom in the general older population (Zalewski 2015), particularly in PD (Berliner et al. 2020), we were expecting to find incidental vestibular dysfunction in our study cohort. We found abnormal LC HIMP on vHIT in 7 PD participants (2 with bilaterally reduced VOR gains) and 4 HC (one with mildly reduced VOR gains bilaterally). Oculomotor screening did not indicate acute uncompensated vestibular asymmetry in any participant; however, screening was done in room light, and future studies could address this by performing oculomotor screening without visual fixation. The VOR gains and HIMP abnormalities were not statistically significantly different between PD and HC in our study (see (Hawkins et al. 2021) for a more detailed description); however, HIMP abnormality played an independent role in balance performance on both surfaces, consistent with other recent studies (Anson et al. 2019, 2017).
The cVEMP score (representative of saccular function and the descending vestibulo-collic reflex pathways) was also associated with balance performance on foam, though with multivariate analysis, it did not make an independent contribution. cVEMP dysfunction is well documented in PD (de Natale et al. 2015a, b; de Natale et al. 2015a; Deriu et al. 2016; Hawkins et al. 2020). However, previous reports of balance performance in non-PD participants with computerised posturography have not closely correlated with cVEMP findings (Mallinson et al. 2019). Recent brain stem morphology studies report that the vestibular nuclei involved in the vestibulo-spinal reflex pathways are affected by PD neurodegeneration (Seidel et al. 2015; Wellings et al. 2017). The potential effects of PD, on vestibulo-collic and vestibulo-spinal reflex responses, across a range of disease severity could be further explored with well-powered studies using other known vestibular evoked motor output regions such as splenius, gastrocnemius, tibialis anterior, trapezius or triceps.
A limitation of our study is that, unlike previous VEMP score studies in neurodegenerative disease (Carpinelli et al. 2021; Gabelic et al. 2015), we calculated our VEMP scores using tap-induced VEMPs, rather than with air-conducted sound, as we were unable to screen for conductive hearing loss. Tendon hammer taps are known to produce robust VEMPs (Iwasaki et al. 2008; Taylor et al. 2020); however, the VEMP scoring method previously described may not directly translate to tap-induced VEMP scores. Future studies could compare tap and air-conducted sound VEMP scores to determine whether the VEMP stimulus mode yields similar VEMP scores.
Our study also found that, unsurprisingly, the PD group had significantly more abnormal proprioception findings and increased mean proprioception error angles than HC. Proprioceptive sensory integration impairment has been well documented in PD (Maschke et al. 2003; Tan et al. 2011; Teasdale et al. 2017). Across both groups, proprioceptive impairment was inversely associated with balance performance on both firm and foam surfaces, consistent with (Anson et al. 2017). On multivariate analyses, proprioception error angle made an independent contribution to balance performance on both surfaces. A limitation of our study, however, was that proprioception was assessed via the lower limb (knee joint) matching task described by (Lord et al. 2003), which does not explicitly assess ankle or hip proprioception, the joint segments predominantly involved in postural responses during this standing balance task. Future studies could measure the proprioceptive ability of the ankle (Ko et al. 2015).
Also unsurprising was the finding that on the unstable foam surface, there was a subset of participants in the PD group (11/40, 27.5%), consistent with previous studies (Feller et al. 2019; Frenklach et al. 2009; Horak et al. 1992), who failed even before visual stimulation commenced. Frenklach et al. (Frenklach et al. 2009) assessed 102 participants with varying stages of PD severity on the SOT. Part of their study reported on the presence of ‘falls’ over three trials per SOT sensory condition. They reported that very early-stage PD participants had postural sway similar to controls. However, participants with UPDRS part 3 score > 20 had excessive postural sway and were significantly more likely to ‘fall’ on the unstable support surface with eyes open, eyes closed, and with sway referenced vision than HC and very early-stage PD. Similar to our findings, this finding further emphasises that some people with PD rely heavily on accurate proprioceptive feedback for balance, despite being more likely to have proprioceptive impairments or integration deficits (Teasdale et al. 2017). Additionally, people with PD have also been shown to have impaired scaling, timing and amplitude of reactive and anticipatory motor output responses and impaired reaction time (Kim et al. 2009; Colnat-Coulbois et al. 2011; Horak et al. 1992). The unstable foam surface may accentuate these motor output deficits, which when combined with sensory impairment or sensory integration dysfunction, renders the testing condition too difficult for a subset of participants. Thus, in the PD cohort, vestibulo-visual sensory integration assessment using the VR-based assessment is perhaps most useful just utilising the firm surface when accurate proprioceptive input is available.
Consistent with other studies (Colnat-Coulbois et al. 2011; Frenklach et al. 2009), increased disease severity in PD (as determined by higher MDS-UPDRS part 3 score and LEDD) was associated with worse balance performance on both surfaces. Interestingly, the PD subtype did not significantly affect balance performance. The higher than expected proportion of participants in the TD group compared to PIGD group may have been related to only part 3 of the MDS-UPDRS being used to determine disease subtype, resulting in over-representation of TD specific over PIGD items. Future studies should aim to include both part 2 and 3 of the MDS-UPDRS to subtype participants accurately.
A further limitation of this study protocol is the non-randomisation of the order of the VR visual perturbation. The visual condition increased in complexity incrementally with each subsequent trial condition. In posturography studies in HC, increasing amplitude and velocity of visual perturbation is not necessarily linear with increased sway (Holten et al. 2016); this may also apply to the ‘fall’ threshold. Future studies could randomise VR perturbation level and test over repeated testing days to analyse whether there is an effect of test order on performance or a potential practice effect.
In conclusion, the VR-based balance assessment protocol is safe and feasible for use in PD and provides a portable objective measure of balance performance with visual perturbation. The PD group failed at lower levels of visual perturbation on both surfaces. In the PD cohort, vestibulo-visual sensory integration assessment using VR-based assessment is most useful just utilising the firm surface when accurate proprioceptive input is available. Age had the most significant effect on balance performance regardless of group. Our study findings suggest that in older cohorts, sensory impairment, whether vestibular (specifically HIMP, SVV or cVEMP score) or proprioceptive, significantly affects performance on this VR-balance protocol.
Data availability
The data that support the findings of this study are available from the corresponding author, KH, upon reasonable request.
Abbreviations
- cVEMP:
-
Cervical vestibular evoked myogenic potential
- FOG:
-
Freezing of gait
- HC:
-
Healthy controls
- HIMP:
-
Head impulse test
- IND:
-
Indeterminate phenotype
- LC:
-
Lateral canal
- LEDD:
-
Levodopa daily equivalent dose
- MMSE:
-
Mini-mental state examination
- oVEMP:
-
Ocular vestibular evoked myogenic potential
- PD:
-
Parkinson’s disease
- PIGD:
-
Postural instability/gait dysfunction phenotype
- SOT:
-
Sensory organisation test
- SVV:
-
Subjective visual vertical
- TD:
-
Tremor dominant phenotype
- vHIT:
-
Video head impulse test
- VOR:
-
Vestibulo-ocular reflex
- VR:
-
Virtual reality
References
Abbruzzese G, Berardelli A (2003) Sensorimotor integration in movement disorders. Mov Disord 18:231–240
Alahmari KA, Sparto PJ, Marchetti GF, Redfern MS, Furman JM, Whitney SL (2014) Comparison of virtual reality based therapy with customised vestibular physical therapy for the treatment of vestibular disorders. IEEE Trans Neural Syst Rehabil Eng 22:389–399
Allen N, Schwartzel A, Canning C (2013) Recurrent falls in Parkinson’s disease a systematic review. Parkinson’s Dis. https://doi.org/10.1155/2013/906274
Anson E, Bigelow RT, Swenor B, Deshpande N, Studenski S, Jeka JJ, Agrawal Y (2017) Loss of peripheral sensory function explains much of the increase in postural sway in healthy older adults. Front Aging Neurosci 9:202
Anson E, Bigelow RT, Studenski S, Deshpande N, Agrawal Y (2019) Failure on the foam eyes closed test of standing balance associated with reduced semicircular canal function in healthy older adults. Ear Hear 40:340–344
Azulay JP, Mesure S, Amblard B, Pouget J (2002) Increased visual dependence in Parkinson’s disease. Percept Mot Skills 95:1106–1114
Berard J, Fung J, Lamontagne A (2012) Impact of aging on visual reweighting during locomotion. Clin Neurophysiol 123:1422–1428
Berliner JM et al (2020) Patient perceptions of visual, vestibular, and oculomotor deficits in people with Parkinson’s disease. Physiother Theory Pract 36:701–708
Borger LL, Whitney SL, Redfern MS, Furman JM (1999) The influence of dynamic visual environments on postural sway in the elderly. J Vestib Res 9:197–205
Bronstein AM, Hood JD, Gresty MA, Panagi C (1990) Visual control of balance in cerebellar and parkinsonian syndromes. Brain 113:767–779
Canning C, Fasano A, Hausdorff JM, Lord S, Rochester L (2017) Falls in Parkinson’s disease: a complex and evolving picture. Mov Disord 32:1524–1536
Canning CG, Allen NE, Nackaerts E, Paul SS, Nieuwboer A, Gilat M (2020) Virtual reality in research and rehabilitation of gait and balance in Parkinson disease. Nat Rev Neurol 16:409–425
Carpinelli S et al (2021) Distinct vestibular evoked myogenic potentials in patients with Parkinson disease and progressive supranuclear palsy. Front Neurol. https://doi.org/10.3389/fneur.2020.598763
Chiarovano E, de Waele C, MacDougall HG, Rogers SJ, Burgess AM, IS. C, (2015) Maintaining balance when looking at a virtual reality three-dimensional display of a field of moving dots or at a virtual reality scene. Front Neurol. https://doi.org/10.3389/fneur.2015.00164
Chiarovano E, Wang W, Rogers SJ, MacDougall HG, Curthoys IS, De Waele C (2017) Balance in virtual reality: effect of age and bilateral vestibular loss. Front Neurol 20(8):5
Chiarovano E, McGarvie LA, Szmulewicz D, MacDougall HG (2018) Subjective visual vertical in virtual reality (Curator SVV): validation and normative data. Virtual Reality. https://doi.org/10.1007/s10055-018-0336-5
Colnat-Coulbois S, Gauchard GC, Maillard L, Barroche G, Vespignani H, Auque J, Perrin PP (2011) Management of postural sensory conflict and dynamic balance control in late-stage Parkinson’s disease. Neuroscience 193:363–369
Cullen KE (2012) The vestibular system: multimodal integration and encoding of self-motion for motor control. Trends Neurosci 35:185–196. https://doi.org/10.1016/j.tins.2011.12.001
Cullen KE (2016) Physiology of central pathways. Handb Clin Neurol 137:17–40. https://doi.org/10.1016/B978-0-444-63437-5.00002-9
de Natale E et al (2015a) Paired neurophysiological and clinical study of the brainstem at different stages of Parkinson’s disease. Clin Neurophysiol 126:1871–1878
de Natale ER et al (2015b) Abnormalities of vestibular-evoked myogenic potentials in idiopathic Parkinson’s disease are associated with clinical evidence of brainstem involvement. Neurol Sci 36:995–1001
Deriu F, de Natale ER, Magnano I, Ginatempo F (2016) Vemps in central neurological disorders. Clin Neurophysiol 127:2020–2021
Dewan N, MacDermid JC (2014) Fall efficacy scale-international (FES-I). J Physiother 60:60
Dona F et al (2016) Changes in postural control in patients with Parkinson’s disease: a posturographic study. Physiotherapy 102:272–279
Feller KJ, Peterka RJ, Horak FB (2019) Sensory re-weighting for postural control in Parkinson’s disease. Front Hum Neurosci 13:126
Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Frenklach A, Louie S, Koop MM, Bronte-Stewart H (2009) Excessive postural sway and the risk of falls at different stages of Parkinson’s disease. Mov Disord 24:377–385
Gabelic T, Krbot Skoric M, Adamec I, Barun B, Zadro I, Habek M (2015) The vestibular evoked myogenic potentials (VEMP) score: a promising tool for evaluation of brainstem involvement in multiple sclerosis. Eur J Neurol 22:261-e221
Halmagyi GM, Chen L, MacDougall HG, Weber KP, McGarvie LA, Curthoys IS (2017) The video head impulse test. Front Neurol. https://doi.org/10.3389/fneur.2017.00258
Hawkins KE, Chiarovano E, Paul SS, MacDougall HG, Curthoys IS (2020) Static and dynamic otolith reflex function in people with Parkinson’s disease. Eur Arch Otorhinolaryngol. https://doi.org/10.1007/s00405-020-06446-1
Hawkins KE, Chiarovano E, Paul SS, Burgess AM, MacDougall HG, Curthoys IS (2021) Vestibular semicircular canal function as detected by video head impulse test (vHIT) is essentially unchanged in people with Parkinson’s disease compared to healthy controls. J Vest Res. https://doi.org/10.3233/VES-201626
Holten V, van der Smagt MJ, Verstraten FAJ, Donker SF (2016) Interaction effects of visual stimulus speed and contrast on postural sway. Exp Brain Res 234:113–124
Horak FB, Nutt JG, Nashner LM (1992) Postural inflexibility in parkinsonian subjects. J Neurol Sci 111:46–58
Iwasaki S, Smulders YE, Burgess AM, McGarvie LA, Macdougall HG, Halmagyi GM, Curthoys IS (2008) Ocular vestibular evoked myogenic potentials to bone conducted vibration of the midline forehead at Fz in healthy subjects. Clin Neurophysiol 119:2135–2147
Kim S, Horak FB, Carlson-Kuhta P, Park S (2009) Postural feedback scaling deficits in Parkinson’s disease. J Neurophysiol 102(5):2910–2920
Ko SU, Simonsick E, Deshpande N, Ferrucci L (2015) Sex-specific age associations of ankle proprioception test performance in older adults: results from the Baltimore longitudinal study of aging. Age Ageing 44:485–490
Konczak J et al (2009) Proprioception and motor control in Parkinson’s disease. J Mot Behav 41:543–552
Lord SR, Menz HB, Tiedemann A (2003) A physiological profile approach to falls risk assessment and prevention. Phys Ther 83:237–252
Macdougall HG, McGarvie LA, Halmagyi GM, Curthoys IS, Weber KP (2013) The video head impulse test (vHIT) detects vertical semicircular canal dysfunction. PLoS ONE 8:e61488
Mallinson AI, Kuijpers ACM, Van Zwieten G, Kakal J, Mullings W, Longridge NS (2019) Computerized dynamic posturography does not detect measured CVEMP and OVEMP abnormalities. Gait Posture 67:248–250
Maschke M, Gomez CM, Tuite PJ, Konczak J (2003) Dysfunction of the basal ganglia, but not the cerebellum, impairs kinaesthesia. Brain 126:2312–2322
Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, Giladi N (2009) Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson’s disease and their carers. Gait Posture 30:459–463
Robert MT, Ballaz L, Lemay M (2016) The effect of viewing a virtual environment through a head-mounted display on balance. Gait Posture 48:261–266
Rossi M, Soto A, Santos S, Sesar A, Labella T (2009) A Prospective study of alterations in balance among patients with Parkinson’s disease. Eur Neurol 61:171–176
Schindlbeck KA, Naumann W, Maier A, Ehlen F, Marzinzik F, Klostermann F (2018) Disturbance of verticality perception and postural dysfunction in Parkinson’s disease. Acta Neurol Scand 137:212–217
Schonfeld U, Clarke AH (2011) A clinical study of the subjective visual vertical during unilateral centrifugation and static tilt. Acta Otolaryngol (stockh) 131:1040–1050
Scocco DH, Wagner JN, Racosta J, Chade A, Gershanik OS (2014) Subjective visual vertical in Pisa syndrome. Parkinsonism Relat Disord 20:878–883
Seidel K et al (2015) The brainstem pathologies of Parkinson’s disease and dementia with Lewy bodies. Brain Pathol 25:121–135
Smith PF (2018) Vestibular functions and Parkinson’s disease. Front Neurol 9:1085
Stebbins GT, Goetz CG, Burn DJ, Jankovic J, Khoo TK, Tilley BC (2013) How to identify tremor dominant and postural instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. Mov Disord 28:668–670
Tan T, Almeida QJ, Rahimi F (2011) Proprioceptive deficits in Parkinson’s disease patients with freezing of gait. Neuroscience 192:746–752
Taylor RL, Welgampola MS, Nham B, Rosengren SM (2020) Vestibular-evoked myogenic potential testing in vestibular localization and diagnosis. Semin Neurol 40:18–32
Teasdale H, Preston E, Waddington G (2017) Proprioception of the ankle is impaired in people with Parkinson’s disease. Mov Disord Clin Pract 4:524–528
Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 25:2649–2653
Wellings TP, Brichta AM, Lim R (2017) Altered neurofilament protein expression in the lateral vestibular nucleus in Parkinson’s disease. Exp Brain Res. https://doi.org/10.1007/s00221-017-5092-3
Wittstein MW, Crider A, Mastrocola S, Guerena Gonzalez M (2020) Use of virtual reality to assess dynamic posturography and sensory organization: instrument validation study. JMIR Serious Games 8:e19580
Yakubovich S, Israeli-Korn S, Halperin O, Yahalom G, Hassin-Baer S, Zaidel A (2020) Visual self-motion cues are impaired yet overweighted during visual-vestibular integration in Parkinson’s disease. Brain Commun 2:fcaa035
Zalewski CK (2015) Aging of the human vestibular system. Semin Hear 36:175–196
Acknowledgements
Dr Hamish G. MacDougall for resources and technical support
Funding
We gratefully acknowledge student research support for KEH from the Faculty of Science, University of Sydney.
Author information
Authors and Affiliations
Contributions
KH designed the study, performed the data collection and wrote the manuscript. EC and SP co-designed the study, supervised KH, assisted with data collection and reviewed the manuscript. IC supervised KH and reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no relevant financial or non-financial interests to disclose.
Ethical approval
All procedures performed in this study were in accordance with the 1964 Helsinki Declaration and its later amendments. Ethical approval was provided by the University of Sydney Human Ethics board (protocol 2017/925). Participants provided written informed consent before study enrolment.
Additional information
Communicated by Francesco Lacquaniti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.

221_2021_6219_MOESM2_ESM.tif
Supplementary file2 BalanceRite test setup with participant standing near a corner, on foam, feet together. The researcher stands in front for safety and controls VR input with VR hand controller and example of test recording sheet/ In this example the subject succeeds at all VR levels on the firm surface. On foam, the subject fails twice at VR0.3 (TIF 19929 KB)
221_2021_6219_MOESM3_ESM.docx
Supplementary file3 Showing balance performance in table format on firm and foam for each group. Ac %= accumulative percent (DOCX 15 KB)
221_2021_6219_MOESM4_ESM.tif
Supplementary file4 Showing regression plots of balance performance vs age, proprioception and SVV error angle. Blue dots are HC, green dots are PD (TIF 27106 KB)
Rights and permissions
About this article
Cite this article
Hawkins, K.E., Paul, S.S., Chiarovano, E. et al. Using virtual reality to assess vestibulo-visual interaction in people with Parkinson’s disease compared to healthy controls. Exp Brain Res 239, 3553–3564 (2021). https://doi.org/10.1007/s00221-021-06219-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-021-06219-0