Abstract
Affective touch plays an important role in human social bonding, affiliative behavior, and in general emotional well-being. A system of unmyelinated low-threshold mechanosensitive C-type afferents innervating hairy skin (C-tactile or CT system) is postulated to provide the neurophysiological background of affective touch perception. C-tactile afferents respond optimally to soft and slow strokes, and this response correlates positively with pleasure ratings of tactile stimuli. As gentle touch is consistently associated with oxytocin release further promoting prosocial behavior, it has been suggested that this effect is mediated by the response of C-tactile afferents. This study assesses a possible link between CT-optimal touch, its subjective pleasantness, EEG indices of cortical arousal, and peripheral oxytocin response. EEG was recorded in 28 healthy volunteers during resting state and tactile stimulation[gentle slow brush strokes on forearm (CT-targeted touch) and palm (non-CT-targeted touch)]. Saliva samples were collected before and after the touch stimulation. Oxytocin concentration increase was significantly associated with greater subjective ratings of CT-targeted touch but not of non-CT-targeted touch, and with lower peak alpha frequency values indicating decreased cortical arousal. The findings suggest that CT-targeted stimulation triggers oxytocin release but only when the touch is perceived at an individual level as having clearly positive affective salience. This corresponds to previous studies reporting that oxytocin response to touch can be related to different personality factors, and bears important implications for planning touch-based interventions in social and medical care.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social touch plays a vital role in human communication and is important for emotional well-being. The affective component of touch is perceived via C-tactile system—a system of unmyelinated low-threshold mechanosensitive C-type afferents (CT afferents) innervating hairy skin in human and other mammals and providing positive emotional response to slow gentle touch (Olausson et al. 2010; McGlone et al. 2014). CT afferents have been found only in hairy skin in human and other mammals, and they responds optimally to low force stroking touch delivered with velocities of 1–10 cm/s (Löken et al. 2009; McGlone et al. 2014). The information is transmitted via spinothalamic tract and processed in insular cortex in a labelled-line fashion (Björnsdotter et al. 2009), with further forward projections including social brain networks, particularly dorsal anterior cingulate cortex and orbitofrontal cortex (Craig 2009; Björnsdotter et al. 2010; Gordon et al. 2013). It is necessary to note that, generally, this system of emotional CT afferents is stimulated concurrently with the better-described system of myelinated Aβ nerve fibers providing discriminating, sensing tactile input and connected to somatosensory cortex via an anatomically distinct pathway (dorsal column and ventral postlateral nucleus of thalamus). Reciprocal connections between insular and secondary somatosensory cortex are suggested to allow mutual modulation of affective and sensory-related processing of touch (McGlone et al. 2014). It is hypothesized that C-tactile system serves as the neurobiological background providing rewarding and affiliative properties of social touch, promotes prosocial behavior, reduces physiological arousal, and acts as a mediator triggering response of several neuroendocrine systems, particularly, oxytocin release (Walker et al. 2017). Further development of this theory requires more studies establishing and verifying direct links between CT-optimal touch, cortical and peripheral biomarkers of arousal, and oxytocin response.
Electroencephalographic (EEG) oscillatory response to stimuli of different modalities provides a range of reliable indices of cortical arousal. Probably the most frequently used EEG marker of arousal is a decrease of amplitude of alpha rhythm, observed at frequencies of 8 to 14 Hz in response to cognitive or emotional activation and often called alpha desynchronization. It was first discovered and linked to vigilance and visual attention by Hans Berger (1929), who noticed that opening eyes leads to a decrease in alpha amplitude. Since then the decrease in alpha power in the EEG of conscious humans over the wide frequency range 8–12 Hz, is traditionally attributed to activation of the corresponding cortical zones. Alpha rhythm is highly heterogenous and, encompasses several rhythms with somewhat varying frequencies, different pacemakers and different functions (for an extensive review see (Başar 2012). Desynchronization in the low-frequency alpha range (8–10 Hz) reflects processes associated with general level of arousal and external attention (readiness for action, expectation, vigilance), while desynchronization of the high-frequency alpha rhythm (10–12 Hz) is associated with cognitive load, semantic analysis, and the operation of long-term memory (Klimesch 1999; Nenert et al. 2012), and since 1990s alpha range is often split into two sub-bands that are analyzed separately. Peak alpha frequency (PAF), defined as a particular frequency within 8–13 Hz range having the highest amplitude, is also an important activation marker. While at an inter-individual level resting state peak alpha frequency is believed to be a relatively stable neurophysiological trait marker (Grandy et al. 2013) positively correlating with cognitive performance (Klimesch et al. 1993; Klimesch 1999), it is also used as a reliable state indicator: PAF decreases in states of relaxation, meditation and fatigue, and increases with elevated emotional and cognitive arousal (for review see Mierau et al. 2017). Other EEG frequency bands are not as straightforwardly related to emotional arousal as alpha band. Delta and theta are traditionally linked to emotions (Başar-Eroglu and Demiralp 2001) but lower band responses are often task- and emotion-specific and increase can be observed both for states of elevated emotional arousal (Aftanas et al. 2002) and for states of relaxation and meditation (Aftanas and Golocheikine 2001). Beta and gamma bands are also often linked with emotional processing (Güntekin and Başar 2010, 2014); decrease of lower beta and increase of upper beta and gamma generally correspond to elevated emotional and cognitive arousal, though these effects are often not as robust and consistent as alpha response (see e.g., Aftanas et al. 2002; Knyazev et al. 2006).
There are relatively few studies reporting EEG changes in response to gentle touch. Two papers have reported event-related potentials (ERPs) to synchronized strokes. In a study by Ackerley and colleagues (2013), an ultra-late potential (ULP) was reported for slow CT-optimal strokes (3 cm/s) for prefrontal electrodes at about 2–3 s after stroke onset, accompanied by a transient amplitude increase in delta, theta, and lower beta bands over frontal electrodes and a pronounced alpha desynchronization over centroparietal cortical sites. Another study by Haggarty and colleagues (2020) has compared ERPs for slow CT-optimal strokes (3 cm/s) and fast non-CT-optimal strokes (30 cm/s) and has also reported an ULP for CT-optimal strokes peaking at a similar latency which amplitude correlated inversely with self-reported autistic traits of the participants. It is also worth noting that faster strokes elicited significantly more pronounced early P300-like positive component, presumably due to greater activation of Aβ-innervated receptors. Several more papers have assessed oscillatory responses to touch, and most of them reported alpha and lower beta rhythm desynchronization (Bauer et al. 2006; van Ede et al. 2010; Singh et al. 2014; Michail et al. 2016) and a transient increase of amplitude in delta and theta bands (van Ede et al. 2010; Michail et al. 2016). The study by von Mohr et al. (2018) bears the highest relevance to the current paper as it contrasts oscillatory responses to no touch (rest), CT-optimal touch (8 cm/s strokes), and non-CT-optimal touch (32 cm/s strokes); it reported an attenuation in alpha and beta activity to all touch conditions and a decrease in theta and parietal beta power specific for affective touch only.
Probably of the highest relevance to the present paper is a study by von Mohr et al. (von Mohr et al. 2018) contrasting oscillatory responses to no touch (rest), CT-optimal touch (8 cm/s strokes), and non-CT-optimal touch (32 cm/s strokes), and revealing attenuation in alpha and beta activity to all touch conditions and a decrease in theta and parietal beta power specific for affective touch only.
Oxytocin (OXT, OT) is a hypothalamic neuroactive hormone known for its various physiological effects and influence on social behavior. Oxytocin is implicated in functioning of mesocorticolimbic circuits and a social decision-making network (Stoop 2012), increases the bursting activity of dopaminergic neurons (Melis et al. 2009), participates in motivated behavior and positive social behavior like pair bonding and parenting (Insel and Young 2001). Experimental studies on animals and humans have both confirmed that oxytocin plays an important role in shaping human prosociality, and that touch is associated with the release of oxytocin and induces prosocial effects mimicking the impact of exogenous administration of oxytocin (Matthiesen et al. 2001; Grewen et al. 2005; Riem et al. 2017); for review see (Walker et al. 2017; Su and Su 2018). It has been also demonstrated that intranasal administration of oxytocin induced a significant increase in tactile sensitivity (Urtado Silva et al. 2019). A hypothesis that gentle touch perceived via C-tactile system reduces physiological arousal, modulates oxytocin response, and provides the neurobiological basis of social bonding and attachment has been first introduced more than 10 years ago (see for example McGlone et al. 2007), and has been recently elaborated by Walker and colleagues (Walker et al. 2017) who suggest that CT optimal stimulus appears sufficient to illicit the relaxing behavioral effects associated with oxytocin release triggered by cutaneous stimulation of the skin, though studies combining direct measurement of oxytocin release triggered by CT activating touch with behavioral and physiological indices of arousal are required.
Most studies examining peripheral oxytocin response to touch measured blood plasma oxytocin levels. Saliva as a biomaterial has several advantages over blood, as its collection is noninvasive, painless, and provides less emotional stress to the participants (Jong et al. 2015). Previously, doubts were expressed that oxytocin can be accumulated in saliva in measurable amounts and serve as a valid biomarker (Horvat-Gordon et al. 2005) because the blood–brain barrier restricts effectively oxytocin diffusion between the CNS and systemic circulation (Neumann and Landgraf 2012). Nonetheless, a range of thoroughly conducted studies has established that salivary oxytocin dynamically responds with quantifiable, rapid, and consistent increases to the stimuli that produce plasma oxytocin increase, like physical activity, sexual self-stimulation, breastfeeding (see e.g., Jong et al. 2015), and, particularly, massage and gentle social touch (Carter et al. 2007; White-Traut et al. 2009; Grewen et al. 2010; Feldman et al. 2010; Tsuji et al. 2015; Riem et al. 2017). The findings directly comparing plasma and salivary oxytocin levels are not always consistent, returning moderate to nonsignificant correlations (Grewen et al. 2010; Hoffman et al. 2012; Javor et al. 2014; Martin et al. 2018). As regards correlations between central and peripheral oxytocin, a systematic review and meta-analysis (Valstad et al. 2017) revealed that, while no association is typically observed under basal conditions, significant associations between central and plasma oxytocin were observed after intranasal oxytocin administration and after experimentally induced stress. This confirms an earlier hypothesis that hypothalamic oxytocin release into blood and into the CNS is uncoordinated under baseline conditions (Amico et al. 1990) but points out that for stimulation-induced or stress-induced changes there may be a coordinated axonal release of OT in specific brain areas and in the blood (see also (Lefevre et al. 2017)). Moreover, it was found that salivary oxytocin better correlates with cerebrospinal fluid oxytocin levels than plasma oxytocin (Martin et al. 2018), possibly providing a more reliable estimate of brain oxytocin levels. Considering these findings it is hardly surprising that salivary oxytocin is steadily gaining respect as a biomarker and has been successfully used in clinically anxious youth (Lebowitz et al. 2016), pregnant women (Takahata et al. 2018; Shishido et al. 2019), preschoolers (Fujii et al. 2016), and autistic children (Fujii et al. 2016; Bakker-Huvenaars et al. 2020), as advantages of saliva over blood collection are particularly important for dealing with emotionally vulnerable populations and for study designs targeting positive emotion induction.
As stated before, there is still a lack of studies linking direct measures of physiological arousal specifically with CT-optimal touch and peripheral oxytocin response. It has also been shown that peripheral oxytocin does not always increase to social touch and can be modulated by individual traits, mental condition (Holt-Lunstad et al. 2011; Riem et al. 2017), parental interaction styles (Feldman et al. 2010), and parent–child social synchrony (Feldman et al. 2014). For example, massage applied by a massage seat cover stimulates oxytocin release, which is manifested by increase in salivary oxytocin levels, but this effect was not observed for individuals with a history of emotional maltreatment (Riem et al. 2017). We hypothesize that one of more general factors mediating oxytocin response to touch is personal emotional attitude to the touch perceived during the experimental procedure. This attitude can vary due to many reasons that are not always easy to account for: individual traits, personal preferences, cultural issues, mood, attitude to the touch deliverer, subtle variations in the physical properties of touch delivered manually by different interaction partners, and above-mentioned social synchrony. The present study, therefore, aims to.
-
1.
Establish a direct link between salivary oxytocin response to gentle touch stimulation and the measures of cortical arousal assessed via EEG oscillatory response to CT-optimal and non-CT optimal touch;
-
2.
Assess a possible relation between subjective emotional responses to touch stimuli, salivary oxytocin, and cortical activation indices.
Our main research hypothesis is that greater oxytocin increase would correspond to attenuated cortical arousal, particularly to CT optimal touch. Our secondary research hypothesis is that higher subjective pleasure ratings (either to all the touch stimuli, or particularly to CT optimal touch) would correspond to both elevated oxytocin and decreased physiological arousal.
Materials and methods
Participants
A total of 28 healthy volunteers aged 16–39 years (Mean = 25.5, SD = 6.23) took part in the study. The participants included 11 men and 17 women (all the female participants were naturally cycling, between the fourths and the twelfth day of their menstrual cycle). Exclusion criteria were pregnancy, psychiatric or neurological disorders, and use of any interfering medications and recreational drugs. The participants reported not having any current or past neurological or psychological disorders.
The participants were informed about the experiment procedure. The study was conducted in accordance with the Helsinki Declaration, and the study protocol was approved by the Ethics Department of the Institute of Higher Nervous Activity and Neurophysiology of RAS. Written informed consent was obtained for all the participants.
Study procedure
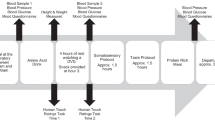
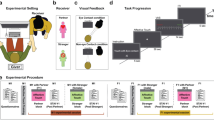
The participants were seated in a chair in front of a laptop, with their left hand resting comfortably on a cushion. After the EEG cap application the first saliva sample was collected. Two minutes resting state EEG with open eyes was recorded after that.
The tactile stimulation was performed manually for two body sites (left dorsal forearm, left palm) by a research assistant previously trained to deliver tactile stimulation with desired force and velocity using three types of brush, yielding six experimental conditions. The contrasting between palm and forearm allows discriminating between CT-targeted and non-CT-targeted touch, as CT afferents have never been reported in palms (glabrous skin). It was decided to keep the speed of the strokes constant, CT-optimal, to avoid a confounding stemming from greater activation of Aβ fibers by faster velocities and leading to more pronounced early sensory response to faster strokes (see Haggarty et al. 2020). Different kinds of brushes were selected based on pilot series and prior experience, to provide stimuli differing in emotional valence. Soft hair (pony) brush stimulation was selected as the most relaxing type of stimulus similar to brushes previously used for CT research (see also earlier psychophysical series on the pleasantness of different textures, e.g., Essick et al. 1999). A hard bristle brush provided somewhat harsher though not unpleasant feeling, and a peacock feather brush provided very low force stimulation inducing a tickle-like sensation with a distinctly arousing quality. For each experimental condition there were 12 strokes performed in smooth back-and-forth motion without taking the brush off the participant’s arm yielding 40 s of continuous tactile stimulation per condition. The stroke speed for all the stimuli was kept within 3–5 cm/s for all the stimuli. The indentation force was kept between 0.3 and 0.6 N for the soft and hard brushes, and was below 0.05 N for the peacock brush. The stimuli were presented using foue pseudorandom sequences counterbalanced across the participants. Similarly to earlier touch perception studies utilizing manual stimulus delivery (von Mohr et al. 2018; Haggarty et al. 2020), the stimulation was guided by a visual metronome cue providing information on stimulus type and setting the stroke tempo, and visible only to the research assistant (Presentation software by Neurobehavioral Systems). After each experimental condition, the participants rated their subjective experience using four Likert-type scales (0–10) assessing pleasantness, arousal, tickle, and pain ratings. The whole procedure took about 8 min, and after that the second saliva sample was collected.
EEG recording
Electroencephalogram data were acquired using a 19-channel EEG recording system “Encephalan” (Medicom MTD, Taganrog, Russian Federation) and 19-electrode caps with electrodes placed according to a modified international 10–20 system (Fp1, Fp2, F7, F3, Fz, F4, F8, T3, C3, Cz, C4, T4, T5, P3, Pz, P4, T6, O1, and O2). Averaged mastoids (A1 and A2) were used as the reference (Luck 2014). The bandpass filter during the acquisition was set to 1.6–30 Hz, the sampling rate was 250 Hz.
EEG data analysis
EEG data processing was performed using Matlab software with EEGlab software package (Delorme and Makeig 2004). Ocular movement artifacts were removed using Independent Component Analysis (ICA), and remaining movement-related and EMG-related artifacts were removed using amplitude criteria and visual inspection. For each participant a total of 60 s of artifact free EEG were selected for the rest condition (no touch), and 30 s of artifact free EEG were selected for each of the touch conditions. The data were transformed using FFT, and power spectral density (PSD) was calculated for the frequency bands of interest: delta (2–4 Hz), theta-1 (4–6 Hz), theta-2 (6–8 Hz), alpha-1 (8–10 Hz), alpha-2 (10–13 Hz), and beta (13–20 Hz). The PSD values were log-transformed for further analyses. Peak alpha frequency (PAF) was determined for each experimental condition as a frequency within 8–13 Hz range with highest PSD values, assessed by frequency discretization data (Aftanas et al. 2002).
Salivary oxytocin assay
A quantitative determination of oxytocin in saliva was performed using a competitive immunoassay using Oxytocin ELISA kit (Enzo, New York, NY, USA). After collecting and before analyzing, the samples had been stored at − 20 °C. The saliva samples were further processed without preliminary extraction, similarly to (Markova 2019; Tsai et al. 2019). The samples were centrifuged at 13,400 rpm for 5 min using an Eppendorf MiniSpin (Eppendorf AG, Hamburg, Germany) to sediment the suspension. If necessary, the samples were diluted in proportion of 1:1, 1:3, and 1:9 with the kit Assay Buffer to obtain the required final volume of 100 μL. When calculating the final concentrations of oxytocin, these dilutions were taken into account. All the samples run duplicates according to “Oxytocin ELISA kit” specifications. Added to the 96-well plate samples and diluted standards together with polyclonal oxytocin antibodies and an oxytocin conjugate (alkaline phosphatase) were incubated for 24 h at 4 °C. Then, the excess reagents were washed away (washer Termo Scientific WellWash Versa). After the incubation with a substrate for 1 h at room temperature, the enzyme reaction was stopped and the absorbance A was measured immediately (plate reader Multiscan GO; software ScanIt) at 405 nm with correction between 570 and 590 nm. The absorbance value used to quantify oxytocin concentration was calculated by the formula [(A405 − A570) + (A405 − A590)]/2. The resulting absorbance values were analyzed with ElisaAnalysis software (elisaanalysis.com) using a four-parameter logistic algorithm (4PL) to calculate obtain the oxytocin concentration. For the final calculations of the oxytocin concentration in saliva, the dilution factor and calibration of the kit according to international standards NIBSC/WHO were taken into account. The results are presented in pg/mL.
Statistical analysis
To test the main experimental hypothesis, the participants were split into two groups according to their salivary oxytocin response. The subjective emotional response was analyzed using a 3-way repeated measures ANOVA (OXI Group 2 × Body Site 2 (forearm, palm) × Brush Type 3) for pleasure ratings. The PSD data were analyzed using 5-way repeated measures ANOVAs (OXI Group 2 × Body Site 2 (forearm, palm) × Brush Type 3 × Electrode 4 (F3-F4, C3-C4, P3-P4, O1-O2) × Hemisphere 2) were planned and performed separately for each frequency band followed with Tukey post hoc comparisons. Similar 5-way repeated measures ANOVA was performed for PAF. The Bonferroni correction and the Greenhouse–Geisser correction were performed where appropriate; only the effects that survived the corrections are reported below. Only the main effects and the interactions involving Group, Body Site, and Brush Type were considered of interest. For the frequency bands where the effects of interest were observed, additional comparisons with the rest condition were performed (OXI Group 2 × Condition 7 × Electrode 4 (F3–F4, C3–C4, P3–P4, O1–O2) × Hemisphere 2) to provide the basis for a more accurate interpretation of the nature of the effects. When deemed reasonable, direct correlations between the EEG metrics and the subjective ratings were performed to further explore the ANOVA effects using Spearman correlation analysis corrected for multiple comparisons by cluster-based permutation test (Maris and Oostenveld 2007) using clustering method with 500 permutations at each node (Matlab toolbox for BCI) and Bonferroni correction.
Results
Changes in salivary oxytocin after the tactile stimulation
According to Shapiro–Wilk test, the salivary oxytocin concentrations determined both before and after the touch stimulation do not follow a normal distribution (p < 0.0001), having a very prominent right skew. Hence, we used non-parametric statistics to describe and compare the data. Though the mean group values were greater for the second OT sample (236.8 vs. 185.8), Wilcoxon matched pairs test revealed no significant effect of experimental procedure (p = 0.145) for the general sample of participants. To test our hypothesis that oxytocin response may be linked to the perceived affective valence of touch we have split the cohort into two subgroups according to the perceived pleasantness of the CT-optimal condition (soft brush strokes to forearm), using median split (Low valence group, n = 17, Mean = 5.94 (4–7 points), SD = 1.24; High valence group, n = 11, Mean = 8.45 (8–9 points), SD = 0.52), and separate Wilcoxon matched pairs tests were performed for the subgroups. As predicted, the touch task significantly increased oxytocin for the high valence group (p = 0.003), but not for the low valence group (p = 0.722).
For each participant, the coefficient K (relative increase) was calculated (K = (OTafter − OTbefore)/OTbefore), where OTafter and OTbefore are salivary oxytocin concentrations before and after the test. The total sample was divided into two subgroups for further analyses according to the K values: (1) OT−, in which oxytocin decreased (n = 11, mean K = − 0.2, SD = 0.14), and (2) OT + , which oxytocin increased (n = 17, mean K = 1.21, SD = 1.65). Mann–Whitney test revealed no significant difference between the groups in baseline oxytocin level (p = 0.466).
Subjective emotional response ratings
The ANOVA for for pleasure ratings revealed a robust main effect for Brush Type [F (2, 52) = 8.78, puncorr = 0.0005] indicating that the soft brush strokes were rated significantly more pleasant than the other strokes (for all post hocs p < 0.01) (Fig. 1a). Mean pleasure rating for all the brush types were above five (the mid-scale value) indicating that peacock and bristle brush strokes were also generally perceived as neutral or slightly pleasant. There was also a significant Group × Body Site interaction [F (1, 26) = 10.47, puncorr = 0.0033] revealing that the participants from the OT + group liked being stroked on the forearm more than the participants from the OT− group (post hoc p < 0.001), irrespective of the stroke type (Fig. 1b).
EEG power spectral density
Significant interactions of interest were observed only for the alpha-1 frequency band (8–10 Hz). A significant Body Site × Electrode interaction [F (3, 78) = 5.79, puncorr = 0.0012] indicated that alpha-1 amplitude was lower for palm stimulation than for forearm stimulation for frontal and central electrodes (for all post hoc p < 0.001) (Fig. 2, see also maps in Fig. 4).
A significant Brush Type × Electrode interaction [F (6, 156) = 3,7073, puncorr = 0.0018] revealed that alpha-1 amplitude was higher for the soft brush strokes over the frontal, central, and parietal electrodes (for all post hoc p < 0.0001) compared to the peacock brush, and over the frontal (post hoc p < 0.0001) and central electrodes (post hoc p < 0.05) compared to the bristle brush (Fig. 3, see also maps in Fig. 4).
Additional comparisons of the touch conditions to the rest condition revealed that, according to highly significant main effect of Condition [F (6, 156) = 4.24, puncorr = 0.0006] and the Condition × Electrode interaction [F (18, 468) = 2.81, puncorr = 0.0001], alpha-1 amplitude was higher for all the touch conditions than for the rest condition, particularly for the central, parietal, and occipital electrodes (Fig. 4).
Peak alpha frequency
The repeated measures ANOVA has yielded a significant Group × Body Site interaction [F (1, 26) = 7,97, p = ,0090] revealing that the participants from the OT + group had lower PAF than the participants from the OT- group, when stroked on the forearm. The PAF maps for the two groups for forearm stimulation are shown in Fig. 5a. Neither the interaction Group × Body Site × Brush Type nor the interaction Group × Brush Type reached statistical significance but the assessment of means shows that the between-group difference was the strongest for the soft brush condition. Given that the nature of the observed between-group differences was very similar to the Group × Body Site interaction for the subjective ratings, direct correlations between PAF and subjective ratings were performed for each electrode for each experimental condition. The permutation test revealed that the correlations were significant only for the CT-optimal condition (soft brush forearm stimulation) for several clusters of electrodes, for pleasure ratings (negative correlation with PAF) and for tickle ratings (positive correlation with PAF) (Fig. 5b).
(a) Peak alpha frequency (PAF) values for forearm stimulation for each brush type and each group. (b) Correlations between PAF values and subjective pleasure and tickle ratings for the CT-optimal condition (soft brush forearm stimulation). The color scale indicates Spearman’s ρ values. Black circles denote electrode sites with significant correlations according to cluster-based permutation test (Bonferroni corrected p < .05)
Discussion
The results of this study reveal that salivary oxytocin increase to touch stimulation was observed only for participants who had higher pleasantness ratings for CT-targeted stimulation—i.e., for slow strokes of moderate force delivered with a soft brush to dorsal forearm. Moreover, the oxytocin response to touch was directly linked to both subjective emotional experience and CT-specific cortical activation reflected in state-induced changes in peak alpha frequency (PAF): the participants responding with oxytocin increase, compared to the participants with no oxytocin increase, during forearm stimulation (a CT-innervated body site) but not during palm stimulation (a body site having abundant Aβ innervation but no CT-innervated mechanoreceptors) were characterized with lower PAF indicating a more relaxed state. These findings confirm our primary and secondary research hypotheses and provide direct empirical support to the social touch hypothesis (Olausson et al. 2010), elaborated by Walker et al. (2017) in respect to oxytocin, who suggested that C-tactile system may serve as a mediator of oxytocin release in response to social touch. Speculating about the nature of the causal relation between the oxytocin levels, cortical arousal, and emotional response to CT-targeted stimulation we cannot entirely disregard the possibility that it is not just the CT-driven emotions that trigger oxytocin release but oxytocin, in turn, affects both cortical arousal and gentle touch perception. Oxytocin administration has been shown to increase prosocial behavior influencing EEG response in social tasks (Perry et al. 2010; Festante et al. 2020) and facilitating metabolic brain response and perceived valence of social touch. However in our case, the experimental groups did not differ in base oxytocin levels suggesting that it was the CT-targeted stimulation that triggered the oxytocin release.
Concurrent to several earlier studies (Holt-Lunstad et al. 2011; Riem et al. 2017), for almost a half of the participants in our study the tactile stimulation did not substantially affect salivary oxytocin concentration or even led to a noticeable drop in its concentration. The finding that CT-optimal touch elicits oxytocin increase not for all healthy participants but only for those who subjectively perceived it as pleasant has several implications important both for theoretical research of neural perception of affective touch and for planning touch-based interventions. It seems plausible that it is not the CT stimulation per se but the change in our emotional state elicited by CT-delivered sensations that triggers oxytocin increase and further promotes prosocial behavior. As we have noted before, there can be a plethora of reasons modifying subjective attitude and emotional response to touch and related to the traits, state, and prior experience of the recipient, and the touch interaction context: individual sensory preferences (see e.g., Croy et al. 2020), cross-modal interferences (Croy et al. 2014) mental and neurodevelopmental conditions (Cascio et al. 2016; Fukuyama et al. 2017; Triscoli et al. 2019), emotions and mood (Kelley and Schmeichel 2014), attitude to the toucher and previous personal experience (for review see Knapp et al. 2013). It should also be noted that the repertoire, use, and specific meaning of social touch gestures varies greatly in different cultures (Knapp et al. 2013; Varlamov et al. 2020) and in different social and communication context (Bezemer and Kress 2014; Jewitt 2017; Varlamov et al. 2020) which, in turn, affects an individual’s attitudes towards different kinds of touch (Trotter et al. 2018; Suvilehto et al. 2019). There is other evidence that individual reactions to gentle touch are not as uniform as they were believed: a recent study of Croy et al. (Croy et al. 2020) reveals that the inverted U curve linking pleasantness and stroke velocity and consistently observed at the group level is found at the individual level for less than 45% of the participants. Given that the perceived valence of touch is easy to assess in the experimental settings, we believe that the inter-individual variance in attitude to affective touch should be more extensively taken into account.
The analysis of EEG response to touch revealed that in our experimental design all the significant effects related to differences between CT-optimal and non-CT-optimal touch were related to alpha band response. Peak alpha frequency changes to CT-optimal touch were sensitive to inter-individual differences, particularly to oxytocin response and subjective pleasure and tickle ratings. It is consistent with the current understanding of PAF as of an EEG biomarker reflecting both inter-individual trait differences and state-induced changes related to cortical arousal (Mierau et al. 2017). The decrease in PAF was previously observed in states of reduced vigilance (Ng and Raveendran 2007) or during Qi Gong (Zhang et al. 1988) or meditation practice (Cahn and Polich 2006; Saggar et al. 2012) and could be associated with relaxation and stress reduction, while increase of PAF was frequently associated with greater sensory or cognitive load (Klimesch et al. 1993) reflecting, in our case, greater activation of Aβ-system during the stimulation of palm which is characterized by abundant Aβ innervation. The response in alpha-1 band revealed that statistically independent effects were found 1) for stimulation site, where forearm stimulation was characterized by higher alpha-1 amplitudes than palm stimulation, and two) for brush type, where the highest amplitudes were observed for CT-optimal soft brush stimulation while the lowest values were found for very low force peacock brush stimulation producing more arousing tickle-like sensations. This kind of sensation is called knismesis (derived from the Greek word κνησμός meaning ‘itching’) and was first described by Hall and Allin (1897) as the light, feather-like type of tickling which is somewhat similar to an itch sensation, is not related to laughter, and can be induced to oneself. Knismesis is opposed to gargalesis (from the Greek γαργαλίζω meaning ‘tickle’), the harder, laughter-inducing tickling involving the repeated application of high pressure to sensitive areas. In research literature, knismesis has been previously linked with negative emotions, high arousal and a strong orienting response and is believed to evolve as a defensive measure against skin parasites (Harris 1999; Harris and Christenfeld 1999; Selden 2004). In our study, however, knismesis-eliciting stimuli were perceived as slightly rather pleasant than unpleasant; this might to a degree be linked to a reporting bias but we would rather suggest that knismesis sensation, though consistently arousing, may result in emotional responses of different valence and character, depending on the context and particular stimulus features. This corresponds well to the fact that light tickle is often used in playful mother–child interactions and is often used as erotic foreplay (Cuskelly 2011). Concurrent to that, knismesis stimulation induced the highest cortical arousal in our experimental design. The subjective ratings of pleasure and tickle had opposing effects on PAF and, considering all the evidences, we can hypothesize that knismesis sensation may counter the calming effect of gentle CT-optimal touch, particularly for individuals prone to sensory hyperarousal, like people with autism spectrum disorder. Indeed, there is plenty of first-person anecdotal evidences that autistic people with sensory processing issues are particularly intolerant to very light touch, the most notable being the works by Temple Grandin (Grandin 1992), though, to our knowledge, there is still no experimental studies of knismesis in ASD. If that is the case and light touch aversion in ASD is related to knismesis intolerance and not to C-tactile system abnormalities, particular touch interventions targeting CT and avoiding knismesis may have positive effects.
The results of this study do not entirely correspond to the findings by von Mohr et al. (2018), who obtained the CT-specific EEG changes in theta and beta ranges. We believe that it can be accounted for by the difference in the study designs. While our study assessed relatively long periods of continuous touch stimulation (about 40 s), the study by von Mohr et al. used shorter periods of touch stimulation of about 6 s interspersed with rest periods, similar to the stimulus presentation in study designs assessing event-related oscillations (e.g., Aftanas et al. 2002). It should be noted that stimulus-induced oscillatory response in lower bands and in beta band is often quite transient (Başar-Eroglu and Demiralp 2001; Aftanas et al. 2002); this has already been demonstrated for touch stimuli as well (Ackerley et al. 2013). Further research linking event-related oscillatory response captured by ERP-like experimental designs can better assess the link between theta and beta oscillations, gentle touch, and oxytocin response.
Conclusions
In this study, the oxytocin release in response to touch was directly linked to both higher subjective valence ratings of touch delivered to CT-innervated skin (but not to palm) and CT-specific cortical activation reflected by a decrease in peak alpha frequency (PAF) and higher amplitude in alpha-1 frequency range. These findings provide empirical support to the social touch hypothesis (Olausson et al. 2010) and a suggestion by Walker et al. (2017) that C-tactile system may serve as a mediator of oxytocin release in response to social touch. The data also point out that CT-targeted touch evokes oxytocin release only when it is perceived at an individual level as having clear and positive affective salience. This corresponds to previous studies reporting that cortical and oxytocin response to touch can be related to different personality traits, social factors, and mental conditions, and bears important implications for planning touch-based interventions in social and medical care.
References
Ackerley R, Eriksson E, Wessberg J (2013) Ultra-late EEG potential evoked by preferential activation of unmyelinated tactile afferents in human hairy skin. Neurosci Lett 535:62–66. https://doi.org/10.1016/j.neulet.2013.01.004
Aftanas LI, Golocheikine SA (2001) Human anterior and frontal midline theta and lower alpha reflect emotionally positive state and internalized attention: high-resolution EEG investigation of meditation. Neurosci Lett 310:57–60. https://doi.org/10.1016/S0304-3940(01)02094-8
Aftanas LI, Varlamov AA, Pavlov SV et al (2002) Time-dependent cortical asymmetries induced by emotional arousal: EEG analysis of event-related synchronization and desynchronization in individually defined frequency bands. Int J Psychophysiol 44:67–82. https://doi.org/10.1016/S0167-8760(01)00194-5
Amico JA, Challinor SM, Cameron JL (1990) Pattern of oxytocin concentrations in the plasma and cerebrospinal fluid of lactating rhesus monkeys (macaca mulatto,): Evidence for functionally independent oxytocinergic pathways in primates. J Clin Endocrinol Metab. https://doi.org/10.1210/jcem-71-6-1531
Bakker-Huvenaars MJ, Greven CU, Herpers P et al (2020) Saliva oxytocin, cortisol, and testosterone levels in adolescent boys with autism spectrum disorder, oppositional defiant disorder/conduct disorder and typically developing individuals. Eur Neuropsychopharmacol. https://doi.org/10.1016/j.euroneuro.2018.07.097
Başar E (2012) A review of alpha activity in integrative brain function: Fundamental physiology, sensory coding, cognition and pathology. Int J Psychophysiol 86:1–24. https://doi.org/10.1016/j.ijpsycho.2012.07.002
Başar-Eroglu C, Demiralp T (2001) Event-related theta oscillations: an integrative and comparative approach in the human and animal brain. Int J Psychophysiol 39:167–195. https://doi.org/10.1016/S0167-8760(00)00140-9
Bauer M, Oostenveld R, Peeters M, Fries P (2006) Tactile spatial attention enhances gamma-band activity in somatosensory cortex and reduces low-frequency activity in parieto-occipital areas. J Neurosci 26:490–501. https://doi.org/10.1523/JNEUROSCI.5228-04.2006
Berger H (1929) Über das Elektrenkephalogramm des Menschen. Arch Psychiatr Nervenkr 87:527–570. https://doi.org/10.1007/BF01797193
Bezemer J, Kress G (2014) Touch: a resource for making meaning. Aust J Lang Lit 37:77–85
Björnsdotter M, Loken L, Olausson H et al (2009) Somatotopic organization of gentle touch processing in the posterior insular Cortex. J Neurosci 29:9314–9320. https://doi.org/10.1523/JNEUROSCI.0400-09.2009
Björnsdotter M, Morrison I, Olausson H (2010) Feeling good: on the role of C fiber mediated touch in interoception. Exp Brain Res 207:149–155. https://doi.org/10.1007/s00221-010-2408-y
Cahn BR, Polich J (2006) Meditation states and traits: EEG, ERP, and neuroimaging studies. Psychol Bull 132:180–211. https://doi.org/10.1037/0033-2909.132.2.180
Carter CS, Pournajafi-Nazarloo H, Kramer KM et al (2007) Oxytocin: behavioral associations and potential as a salivary biomarker. Ann N Y Acad Sci 1098:312–322. https://doi.org/10.1196/annals.1384.006
Cascio CJ, Lorenzi J, Baranek GT (2016) Self-reported pleasantness ratings and examiner-coded defensiveness in response to touch in children with ASD: effects of stimulus material and Bodily location. J Autism Dev Disord 46:1528–1537. https://doi.org/10.1007/s10803-013-1961-1
Craig AD (2009) How do you feel — now? The anterior insula and human awareness. Nat Rev Neurosci 10:59–70. https://doi.org/10.1038/nrn2555
Croy I, Angelo S (2014) Reduced pleasant touch appraisal in the presence of a disgusting odor. PLoS ONE 9:e92975. https://doi.org/10.1371/journal.pone.0092975
Croy I, Bierling A, Sailer U, Ackerley R (2020) Individual variability of pleasantness ratings to stroking touch over different velocities. Neuroscience. https://doi.org/10.1016/j.neuroscience.2020.03.030
Cuskelly M (2011) Original Skin: exploring the marvels of the human hide. Counterpoint Press, Berkeley
de Jong TR, Menon R, Bludau A et al (2015) Salivary oxytocin concentrations in response to running, sexual self-stimulation, breastfeeding and the TSST: the regensburg oxytocin challenge (ROC) study. Psychoneuroendocrinology 62:381–388. https://doi.org/10.1016/j.psyneuen.2015.08.027
Delorme A, Makeig S (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134:9–21. https://doi.org/10.1016/j.jneumeth.2003.10.009
Essick GK, James A, McGlone FP (1999) Psychophysical assessment of the affective components of non-painful touch. NeuroReport 10:2083–2087. https://doi.org/10.1097/00001756-199907130-00017
Feldman R, Golan O, Hirschler-Guttenberg Y et al (2014) Parent–child interaction and oxytocin production in pre-schoolers with autism spectrum disorder. Br J Psychiatry 205:107–112. https://doi.org/10.1192/bjp.bp.113.137513
Feldman R, Gordon I, Schneiderman I et al (2010) Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent–infant contact. Psychoneuroendocrinology 35:1133–1141. https://doi.org/10.1016/j.psyneuen.2010.01.013
Festante F, Ferrari PF, Thorpe SG et al (2020) Intranasal oxytocin enhances EEG mu rhythm desynchronization during execution and observation of social action: an exploratory study. Psychoneuroendocrinology 111:104467. https://doi.org/10.1016/j.psyneuen.2019.104467
Fujii T, Schug J, Nishina K et al (2016) Relationship between salivary oxytocin levels and generosity in preschoolers. Sci Rep. https://doi.org/10.1038/srep38662
Fukuyama H, Kumagaya S, Asada K et al (2017) Autonomic versus perceptual accounts for tactile hypersensitivity in autism spectrum disorder. Sci Rep 7:8259. https://doi.org/10.1038/s41598-017-08730-3
Gordon I, Voos AC, Bennett RH et al (2013) Brain mechanisms for processing affective touch. Hum Brain Mapp 34:914–922. https://doi.org/10.1002/hbm.21480
Grandin T (1992) Calming Effects of Deep Touch Pressure in Patients with Autistic Disorder, College Students, and Animals. J Child Adolesc Psychopharmacol 2:63–72. https://doi.org/10.1089/cap.1992.2.63
Grandy TH, Werkle-Bergner M, Chicherio C et al (2013) Individual alpha peak frequency is related to latent factors of general cognitive abilities. Neuroimage 79:10–18. https://doi.org/10.1016/j.neuroimage.2013.04.059
Grewen KM, Davenport RE, Light KC (2010) An investigation of plasma and salivary oxytocin responses in breast- and formula-feeding mothers of infants. Psychophysiology 47:625–632. https://doi.org/10.1111/j.1469-8986.2009.00968.x
Grewen KM, Girdler SS, Amico J, Light KC (2005) Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom Med 67:531–538. https://doi.org/10.1097/01.psy.0000170341.88395.47
Güntekin B, Başar E (2010) Event-related beta oscillations are affected by emotional eliciting stimuli. Neurosci Lett 483:173–178. https://doi.org/10.1016/j.neulet.2010.08.002
Güntekin B, Başar E (2014) A review of brain oscillations in perception of faces and emotional pictures. Neuropsychologia 58:33–51. https://doi.org/10.1016/j.neuropsychologia.2014.03.014
Haggarty CJ, Malinowski P, McGlone FP, Walker SC (2020) Autistic traits modulate cortical responses to affective but not discriminative touch. Eur J Neurosci 51:1844–1855. https://doi.org/10.1111/ejn.14637
Hall GS, Allin A (1897) The Psychology of tickling, laughing, and the comic. Am J Psychol 9:1. https://doi.org/10.2307/1411471
Harris CR (1999) The mystery of ticklish laughter 344:1–7
Harris CR, Christenfeld N (1999) Can a machine tickle? Psychon Bull Rev 6:504–510. https://doi.org/10.3758/BF03210841
Hoffman ER, Brownley KA, Hamer RM, Bulik CM (2012) Plasma, salivary, and urinary oxytocin in anorexia nervosa: a pilot study. Eat Behav 13:256–259. https://doi.org/10.1016/j.eatbeh.2012.02.004
Holt-Lunstad J, Birmingham W, Light KC (2011) The influence of depressive symptomatology and perceived stress on plasma and salivary oxytocin before, during and after a support enhancement intervention. Psychoneuroendocrinology 36:1249–1256. https://doi.org/10.1016/j.psyneuen.2011.03.007
Horvat-Gordon M, Granger DA, Schwartz EB et al (2005) Oxytocin is not a valid biomarker when measured in saliva by immunoassay. Physiol Behav. https://doi.org/10.1016/j.physbeh.2005.01.007
Insel TR, Young LJ (2001) The neurobiology of attachment. Nat Rev Neurosci. https://doi.org/10.1038/35053579
Javor A, Riedl R, Kindermann H et al (2014) Correlation of plasma and salivary oxytocin in healthy young men - experimental evidence. Neuro Endocrinol Lett 35:470–473
Jewitt C (2017) Towards a multimodal social semiotic agenda for touch. In: Zhao S, Djonov E, Anders Björkvall MB (eds) Advancing multimodal and critical discourse studies: Interdisciplinary research inspired by Theo van Leeuwen’s social semiotics. Routledge, New York, NY
Kelley NJ, Schmeichel BJ (2014) The effects of negative emotions on sensory perception: fear but not anger decreases tactile sensitivity. Front Psychol 5:942. https://doi.org/10.3389/fpsyg.2014.00942
Klimesch W (1999) EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Brain Res Rev 29:169–195. https://doi.org/10.1016/s0165-0173(98)00056-3
Klimesch W, Schimke H, Pfurtscheller G (1993) Alpha frequency, cognitive load and memory performance. Brain Topogr 5:241–251. https://doi.org/10.1007/bf01128991
Knapp ML, Hall JA, Horgan TG (2013) Nonverbal Communication in Human Interaction, 8th edn. Cengage Learning, Andover
Knyazev GG, Savostyanov AN, Levin EA (2006) Alpha synchronization and anxiety: Implications for inhibition vs. alertness hypotheses. Int J Psychophysiol 59:151–158. https://doi.org/10.1016/j.ijpsycho.2005.03.025
Lebowitz ER, Leckman JF, Feldman R et al (2016) Salivary oxytocin in clinically anxious youth: associations with separation anxiety and family accommodation. Psychoneuroendocrinology. https://doi.org/10.1016/j.psyneuen.2015.12.007
Lefevre A, Mottolese R, Dirheimer M et al (2017) A comparison of methods to measure central and peripheral oxytocin concentrations in human and non-human primates. Sci Rep. https://doi.org/10.1038/s41598-017-17674-7
Löken LS, Wessberg J, Morrison I et al (2009) Coding of pleasant touch by unmyelinated afferents in humans. Nat Neurosci 12:547–548. https://doi.org/10.1038/nn.2312
Luck SJ (2014) An Introduction to the event-related potential technique, 2nd edn. MIT Press, Boston
Maris E, Oostenveld R (2007) Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. https://doi.org/10.1016/j.jneumeth.2007.03.024
Markova G (2019) The role of oxytocin in early mother-infant interactions: Variations in maternal affect attunement. Infant Behav Dev. https://doi.org/10.1016/j.infbeh.2019.03.003
Martin J, Kagerbauer SM, Gempt J et al (2018) Oxytocin levels in saliva correlate better than plasma levels with concentrations in the cerebrospinal fluid of patients in neurocritical care. J Neuroendocrinol 1:e12596. https://doi.org/10.1111/jne.12596
Matthiesen AS, Ransjo-Arvidson AB, Nissen E, Uvnas-Moberg K (2001) Postpartum maternal oxytocin release by newborns: effects of infant hand massage and sucking. Birth 28:13–19. https://doi.org/10.1046/j.1523-536x.2001.00013.x
McGlone F, Vallbo AB, Olausson H et al (2007) Discriminative touch and emotional touch. Can J Exp Psychol Can Psychol expérimentale 61:173–183. https://doi.org/10.1037/cjep2007019
McGlone F, Wessberg J, Olausson H (2014) Discriminative and affective touch: sensing and feeling. Neuron 82:737–755. https://doi.org/10.1016/j.neuron.2014.05.001
Melis MR, Succu S, Sanna F et al (2009) Oxytocin injected into the ventral subiculum or the posteromedial cortical nucleus of the amygdala induces penile erection and increases extracellular dopamine levels in the nucleus accumbens of male rats. Eur J Neurosci. https://doi.org/10.1111/j.1460-9568.2009.06912.x
Michail G, Dresel C, Witkovsky V et al (2016) Neuronal oscillations in various frequency bands differ between pain and touch. Front Hum Neurosci 10:182. https://doi.org/10.3389/fnhum.2016.00182
Mierau A, Klimesch W, Lefebvre J (2017) State-dependent alpha peak frequency shifts: experimental evidence, potential mechanisms and functional implications. Neuroscience 360:146–154. https://doi.org/10.1016/j.neuroscience.2017.07.037
Nenert R, Viswanathan S, Dubuc DM, Visscher KM (2012) Modulations of ongoing alpha oscillations predict successful short-term visual memory encoding. Front Hum Neurosci 6:127. https://doi.org/10.3389/fnhum.2012.00127
Neumann ID, Landgraf R (2012) Balance of brain oxytocin and vasopressin: Implications for anxiety, depression, and social behaviors. Trends Neurosci. 2:75
Ng SC, Raveendran P (2007) EEG Peak Alpha Frequency as an Indicator for Physical Fatigue. In: Jarm T, Kramar P, Zupanik A (eds) 11th Mediterranean Conference on Medical and Biomedical Engineering and Computing 2007. Springer, Berlin Heidelberg, Berlin, Heidelberg, pp 517–520
Olausson H, Wessberg J, Morrison I et al (2010) The neurophysiology of unmyelinated tactile afferents. Neurosci Biobehav Rev 34:185–191. https://doi.org/10.1016/j.neubiorev.2008.09.011
Perry A, Bentin S, Shalev I et al (2010) Intranasal oxytocin modulates EEG mu/alpha and beta rhythms during perception of biological motion. Psychoneuroendocrinology 35:1446–1453. https://doi.org/10.1016/j.psyneuen.2010.04.011
Riem MME, Carli P et al (2017) Emotional maltreatment is associated with atypical responding to stimulation of endogenous oxytocin release through mechanically-delivered massage in males. Psychoneuroendocrinology 85:115–122. https://doi.org/10.1016/j.psyneuen.2017.08.017
Saggar M, King BG, Zanesco AP et al (2012) Intensive training induces longitudinal changes in meditation state-related EEG oscillatory activity. Front Hum Neurosci 6:256. https://doi.org/10.3389/fnhum.2012.00256
Selden ST (2004) Tickle 3:93–97. https://doi.org/10.1016/S0190-9622(03)02737-3
Shishido E, Shuo T, Takahata K, Horiuchi S (2019) Changes in salivary oxytocin levels and bonding disorder in women from late pregnancy to early postpartum: a pilot study. PLoS ONE. https://doi.org/10.1371/journal.pone.0221821
Singh H, Bauer M, Chowanski W et al (2014) The brain’s response to pleasant touch: an EEG investigation of tactile caressing. Front Hum Neurosci 8:893. https://doi.org/10.3389/fnhum.2014.00893
Stoop R (2012) Neuromodulation by oxytocin and vasopressin. Neuron 76:142–159. https://doi.org/10.1016/j.neuron.2012.09.025
Su J, Su Y (2018) A touch-scaffolded model of human prosociality. Neurosci Biobehav Rev 92:453–463. https://doi.org/10.1016/j.neubiorev.2018.07.008
Suvilehto JT, Nummenmaa L, Harada T et al (2019) Cross-cultural similarity in relationship-specific social touching. Proc R Soc B Biol Sci 286:20190467. https://doi.org/10.1098/rspb.2019.0467
Takahata K, Horiuchi S, Tadokoro Y et al (2018) Effects of breast stimulation for spontaneous onset of labor on salivary oxytocin levels in low-risk pregnant women: a feasibility study. PLoS ONE. https://doi.org/10.1371/journal.pone.0192757
Triscoli C, Croy I, Sailer U (2019) Depression predicts interpersonal problems partially through the attitude towards social touch. J Affect Disord 246:234–240. https://doi.org/10.1016/j.jad.2018.12.054
Trotter P, Belovol E, McGlone F, Varlamov A (2018) Validation and psychometric properties of the Russian version of the touch experiences and attitudes questionnaire (TEAQ-37 Rus). PLoS ONE 13:e0206905. https://doi.org/10.1371/journal.pone.0206905
Tsai TY, Tseng HH, Chi MH et al (2019) The interaction of oxytocin and social support, loneliness, and cortisol level in major depression. Clin Psychopharmacol Neurosci. https://doi.org/10.9758/cpn.2019.17.4.487
Tsuji S, Yuhi T, Furuhara K et al (2015) Salivary oxytocin concentrations in seven boys with autism spectrum disorder received massage from their mothers: a pilot study. Front Psychiatry 6:58. https://doi.org/10.3389/fpsyt.2015.00058
Urtado Silva J, Galhardoni R, Ciampi de Andrade D, Brito I (2019) Effects of intranasal oxytocin on tactile perception. Neurosci Lett 698:64–68. https://doi.org/10.1016/j.neulet.2018.12.032
Valstad M, Alvares GA, Egknud M et al (2017) The correlation between central and peripheral oxytocin concentrations: a systematic review and meta-analysis. Neurosci Biobehav, Rev
van Ede F, Jensen O, Maris E (2010) Tactile expectation modulates pre-stimulus β-band oscillations in human sensorimotor cortex. Neuroimage 51:867–876. https://doi.org/10.1016/j.neuroimage.2010.02.053
Varlamov AA, Kravchenko AN, Gorbacheva AV, Osadchiy MA (2020) Language of touch: Biological aspects of social touch perception and the system of tactile communicative signals. Vopr Jazyk 4:75–92. https://doi.org/10.31857/S0373658X0008857-1
von Mohr M, Crowley MJ, Walthall J et al (2018) EEG captures affective touch: CT-optimal touch and neural oscillations. Cogn Affect Behav Neurosci 18:155–166. https://doi.org/10.3758/s13415-017-0560-6
Walker SC, Trotter PD, Swaney WT et al (2017) C-tactile afferents: Cutaneous mediators of oxytocin release during affiliative tactile interactions? Neuropeptides 64:27–38
White-Traut R, Watanabe K, Pournajafi-Nazarloo H et al (2009) Detection of salivary oxytocin levels in lactating women. Dev Psychobiol. https://doi.org/10.1002/dev.20376
Zhang J-Z, Zhao J, He Q (1988) EEG findings during special psychical state (Qi Gong state) by means of compressed spectral array and topographic mapping. Comput Biol Med 18:455–463. https://doi.org/10.1016/0010-4825(88)90063-7
Funding
The study was supported by RFBR, project No 18-00-01511-komfi (18-00-01511).
Author information
Authors and Affiliations
Contributions
GP: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, visualization, original draft, review and editing. EP: conceptualization, data curation, methodology, supervision, validation, original draft, review and editing. SS formal analysis, investigation, validation, visualization. IS: formal analysis, investigation, software, project administration, AV: conceptualization, funding acquisition, investigation, methodology, supervision, visualization, review and editing.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no conflicts of interest to disclose.
Additional information
Communicated by Francesco Lacquaniti.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Portnova, G.V., Proskurnina, E.V., Sokolova, S.V. et al. Perceived pleasantness of gentle touch in healthy individuals is related to salivary oxytocin response and EEG markers of arousal. Exp Brain Res 238, 2257–2268 (2020). https://doi.org/10.1007/s00221-020-05891-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-020-05891-y