Abstract
Patients with chronic pain conditions such as fibromyalgia often demonstrate hypervigilance—undue alertness for unpleasant or threatening bodily sensations—as well as enhancement of these sensations. The generalized hypervigilance hypothesis (GHH) of Rollman and colleagues asserts that hypervigilance leads to this perceptual amplification. However, cause-and-effect relationships are difficult to establish in studies using a quasi-experimental design. In the present study, we sought to address this issue by attempting to induce hypervigilance experimentally, in one of two groups to which young, healthy adults had been randomly assigned. Those in the experimental group wrote about the flu and practiced counting their own blinks, breaths, and heartbeats; those in the control group wrote about a neutral topic and counted innocuous lights and sounds. Next, both groups rated the intensity and unpleasantness of pressure sensations (ranging from mild to painful) caused by a series of applications of a weighted rod to the forearm. The intensity/unpleasantness ratio of these ratings was significantly greater in the experimental group, suggesting that induced hypervigilance had caused perceptual amplification that generalized to pressure sensations, which had not been part of the experimental manipulation. Psychometric measures of anxiety and catastrophizing were equivalent in the two groups, indicating that the experimental manipulation operated via attentional rather than emotional changes. The results support the GHH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The concept of hypervigilance in pain patients has evolved considerably since Chapman’s (1978) initial observation that “some individuals develop perceptual habits of vigilance for somatic distress signals, in particular, pain sensations.” Rollman and Lautenbacher (1993) subsequently proposed that hypervigilant attention, in patients with fibromyalgia and other idiopathic conditions, actually amplifies the sensations it is focused on and, furthermore, that the hypervigilance may generalize to other aversive sensations, such as loud sounds. These two ideas—perceptual amplification and generalization—are combined in the Rollman group’s generalized hypervigilance hypothesis (GHH) (McDermid et al. 1996; Rollman 2009; Rollman and Lautenbacher 1993). This hypothesis is consistent with, but goes beyond, the idea that attention to specific noxious stimuli increases their perceived intensity, an effect for which there is considerable experimental evidence (Buhle and Wager 2010; Miron et al. 1989; Villemure et al. 2003).
The question of how, and along which dimensions, hypervigilance generalizes is an unsettled one. Hollins et al. (2009) showed that individuals with fibromyalgia, who were indicated by questionnaire responses to be hypervigilant, showed perceptual amplification not just of painful pressure sensations, but of weak, innocuous ones that the participants themselves did not consider unpleasant. Loudness judgments of auditory stimuli were also elevated (consistent with McDermid et al. 1996), but much less than pressure sensations. The data suggest that hypervigilance in pain patients does generalize, but most robustly within the somesthetic modality.
The other main element of the GHH, namely that hypervigilance is a cause of perceptual amplification, is also plausible. However, the evidence favoring it (Geisser et al. 2008; Hollins et al. 2009; McDermid et al. 1996), while persuasive, is not compelling, mainly because comparisons of clinical and non-clinical populations do not have the advantage of random assignment: That is, the higher pain ratings of the patients may be due to factors other than their increased hypervigilance, such as a response bias, i.e., a tendency to assign higher than normal numbers to a given sensory experience. The hypothesis therefore remains controversial (Crombez et al. 2005; Tiemann et al. 2012; Van Damme et al. 2015).
In the present study, we evaluate this key component of the GHH—that hypervigilance leads to perceptual amplification—by attempting to create a mild, transient hypervigilance in healthy subjects. The subjects are randomly assigned either to this experimental condition or to a control condition. Subjects in the experimental group are induced, both directly and indirectly, to attend to somatic sensations. The direct component consists of asking them to count bodily events, such as their own breathing; the indirect component consists of asking them to write about a health topic, the flu, that involves unpleasant and threatening somatic sensations. After the manipulation, we determine whether perceptual amplification is present in the experimental group, by asking both groups to rate the sensations resulting from pressure stimuli ranging from gentle to painful.
If increases in pressure sensations do occur, how can it be established that they are a result of attentional amplification, rather than of some other change, such as a response bias? One answer to this challenge lies in the fact that sensations are multidimensional experiences, with both sensory and emotional components; in the case of pain, where these dimensions have been extensively studied, they are usually called intensity and unpleasantness. A number of experimental manipulations have been shown to selectively affect one dimension or the other: For example, fentanyl selectively reduces pain intensity (Gracely et al. 1979), whereas cannabis selectively reduces pain unpleasantness (Lee et al. 2013).
Importantly, directed attention to noxious stimuli has been found to affect primarily the intensity of the pain those stimuli cause, rather than its unpleasantness (Villemure et al. 2003; Villemure and Bushnell 2009). By extension, hypervigilance—heightened attention to a wide range of threatening sensations, according to the GHH—can therefore be expected to have a larger effect on their intensity than on their unpleasantness.
The most direct way to detect such a relative change is to calculate the ratio of intensity to unpleasantness ratings (or the reverse), an approach that has been found informative in examining experimental pain of different types (Rainville et al. 1992) and in different populations (Petzke et al. 2005). Hypervigilance, if induced by our experimental manipulation, would be expected to increase the intensity/unpleasantness ratio of the test stimuli. In contrast, other types of changes would more likely have different effects: Induced anxiety would presumably reduce the intensity/unpleasantness ratio (by boosting unpleasantness), and a response bias would be expected to raise both components, leaving their ratio unchanged.
Even if the experimental manipulation induces hypervigilance, it might also increase anxiety or lead to catastrophizing in the experimental group. To evaluate this possibility, we administer psychometric measures of these two psychological processes.
The results of the study should thus allow us to determine whether a transient hypervigilance can be induced by a brief experimental manipulation, and if so, whether this state is primarily the perceptual “habit” described by the GHH, or a more complex state comprising emotional as well as cognitive components.
Methods
Participants
Fifty subjects were recruited into the study. They were enrolled in an introductory psychology course and received research credits for their participation. Subjects gave written informed consent. All aspects of the study were approved in advance by the Institutional Review Board of the University of North Carolina at Chapel Hill. Subjects were run individually; participation consisted of one session lasting about an hour.
Exclusion criteria were that subjects could not: be less than 18 or more than 25 years of age; be in a course taught by the PI; have diabetes; have a neurological impairment; have a history of nerve damage or surgery, or a current injury such as a cut or bruise, on the right forearm; or have been diagnosed with a chronic pain disorder.
Although the exclusion criteria were explained to potential subjects and listed on the consent form, answers on a survey that was part of the study (described below) revealed that four currently had chronic pain, defined there as pain that had lasted for 3 months or more. The data of these four individuals were excluded from the analysis. The data of an additional three subjects were excluded because they did not follow instructions on the writing task, a critical part of the experimental manipulation (two wrote nothing and the third did not write on the assigned topic).
The final sample thus consisted of 43 individuals, of whom 26 were female. The mean age was 19.5 (SD = 1.4); all but two were right-handed by self-report.
Experimental design
Subjects were randomly assigned to either the experimental (N = 22) or the control (N = 21) condition. The experimental condition was designed to heighten subjects’ alertness to bodily sensations, especially aversive ones—in other words, to increase subjects’ hypervigilance. The manipulation had two parts. The first was a writing task in which subjects were asked to write for 10 min about the ways they prepare for flu season (the experiment was carried out in winter). Asking subjects to write about certain types of experiences is an established method for manipulating mood or inducing specific emotions (Algoe and Haidt 2009; Gasper and Clore 2000; Schwarz and Clore 1983).
The second part of the experimental manipulation was a set of three counting tasks, in which subjects attempted to count their blinks, breaths, and heartbeats, respectively, during periods of time (75, 105, and 90 s) initiated and terminated by the experimenter.
The control condition assigned to subjects activities comparable in form and duration to those in the experimental condition, but which were designed not to affect hypervigilance. For the writing task, subjects were asked to write about their daily routine—e.g., how they get up in the morning and get ready for class. For the counting task, subjects were asked to count three types of external stimulus events of low-to-moderate intensity, presented on a laptop computer: high-pitched pings, brightening and darkening of the computer screen, and low-pitched beeps. The durations of these three counting tasks were equated to those in the experimental condition.
Procedure
Writing task
After giving informed consent, subjects were randomly assigned to either the experimental or control group and proceeded directly to the writing task. This was administered using a Qualtrics program on a desktop computer. Different prompts were given in the two conditions. In the experimental condition, the prompt reminded subjects that “flu is a contagious respiratory illness caused by influenza viruses that infect the nose, throat, and lungs. The disease is quite contagious and can be spread through the air by coughing or sneezing… Fortunately, there are steps most people can take to greatly reduce their chances of contracting the flu, although no one measure provides complete protection. We would like you to think about the things you do on a regular basis to ward off the flu, especially as flu season approaches and throughout the winter months…” The control prompt, in contrast, asked subjects “to think about how you get ready for school on a daily basis—your typical morning routine starting from the time you wake up. It does not matter how big or small the action, just try to recall it in as much detail as you can. For example, think about whether you use a clock for an alarm or something else, what type of toothpaste you use, and what you eat for breakfast…” Both 277-word prompts ended by asking the subject to write on the topic for 10 min, by typing in a box. At the end of the writing period, the subject moved to a different table where the counting tasks were carried out.
Counting tasks
Each subject participated in three counting tasks. In the first of these, lasting 75 s, the subject was instructed to count his/her blinks (experimental group) or the number of clearly audible high-pitched pings presented through headphones (control group).
In the second task, the subject counted the number of his/her breaths (experimental group) or the number of bright–dark cycles of the fluctuating computer screen (control group) occurring in a 105-s period. In the third task, subjects were asked to try to count their heartbeats, without using a hand to take their pulse (experimental group), or to count the number of faint, low-pitched beeps presented through headphones (control group); this third period had a duration of 90 s.
The experimenter indicated to the subject, in each task, when to begin and when to stop counting. Different durations were used for the three tasks to prevent the subject from anticipating the end of the interval; the same three intervals were used for all subjects, to make the experimental and control tasks as formally similar as possible.
Weighted-rod test
All subjects next underwent the weighted-rod test, in which a contactor pressed down on the subject’s right forearm. Pressure stimuli were delivered using the same apparatus employed by Hollins et al. (2009). Briefly, a vertical rod ending in a Delrin contactor with a diameter of 5 mm was lowered onto the skin and left in place for 15 s on each trial. The rod was supported by scaffolding and supported interchangeable weights that enabled it to apply various levels of force to the dorsal surface of the subject’s pronated forearm, about midway between wrist and elbow. Baffles prevented the subject from seeing the apparatus and the stimulated region of the forearm.
After each trial, subjects rated the intensity and unpleasantness of the sensation produced by the stimulus, as well as indicating whether it was painful, unpleasant but not painful, or neutral (meaning neither pleasant nor unpleasant). Eleven levels of force, ranging in 100-g steps from 77 to 1077 g, were presented in a random order that was different for each subject. The stimulation and rating procedures are the same as those used by Hollins et al. (2009), although here, each force level was presented only once. Briefly, the experimenter lowered the weighted contactor onto the skin gently, over the course of about 1 s; it was left in place for 15 s and then lifted off over the course of an additional second. The subject’s forearm was moved slightly between trials so as to prevent the same spot from being repeatedly stimulated.
Questionnaires
Next, subjects completed a number of psychometric instruments: a short current pain questionnaire and demographic information form of our own design; the Pennebaker Inventory of Limbic Languidness (PILL), a symptom inventory sometimes used as a measure of hypervigilance (Pennebaker 1982); the Pain Catastrophizing Scale (PCS; Sullivan 2009); and the State-Trait Anxiety Inventory (STAI; Spielberger 1983). At the end of the session, subjects were debriefed and thanked for their participation.
Results
Writing and counting tasks
The purpose of the writing task was to direct the attention of subjects in the experimental group to unpleasant somatic sensations associated with the flu, while directing the attention of control subjects to a topic (their morning routine) that presumably was less associated with bodily sensations. To determine whether this manipulation was successful, we counted the number of subjects in each group who mentioned somatic sensations in their essays. Of the 22 subjects in the experimental group, 18 did so (mentioning mostly flu symptoms), while only 8 of the 21 subjects in the control group did so. Reported somatic sensations of control subjects were diverse, ranging from waiting for tap water to get hot to rubbing the eyes to wake up. The difference between the groups in proportion of subjects referring to bodily sensations was statistically significant, χ 2 = 6.86, df = 1, p < .01.
The number of subjects in each group whose essays contained negative affect was also tallied. In the experimental group, 11 of the 22 subjects expressed negative affect, mostly aversion to flu symptoms or fear of germs. In contrast, only 3 of the 21 control subjects expressed negative affect, e.g., annoyance over a dirty bathroom. The difference between groups in the proportion of subjects expressing negative affect was statistically significant, χ 2 = 4.72, df = 1, p < .05. Overall, these results show that the writing manipulation caused experimental subjects to focus more on somatic sensations and negative affect than control subjects, at least for the duration of this task.
The writing task was followed by the counting task, which was designed to again focus the attention of experimental, but not control, subjects on bodily sensations. Although the counting task was formally similar in the experimental and control groups, subjects in the two groups counted different things; thus, their results cannot be directly compared. We did not measure the physiological events (blinking, breathing, and pulse) in the experimental group so cannot say how accurate subjects were at counting these; however, the tight clustering of control-group responses about the correct values (pings, M = 21.0, SD = 0.0; light/dark cycles, M = 33.1, SD = 0.7; beeps, M = 90.7, SD = 0.7) indicates that subjects attended carefully to the sensory events they were instructed to count.
The critical question is whether the combination of these two tasks differently affected the performance of subjects on a subsequent task, the rating of pressure sensations.
Intensity and unpleasantness ratings
Consistent with standard practice in analysis of ratio-scale psychophysical data (Gescheider 1997), ratings of sensation intensity and unpleasantness were converted to logarithmic form. Since the logarithm of zero is undefined, occasional zero ratings were replaced with a small number (half the smallest nonzero rating given to any stimulus by any subject) prior to logarithmic conversion. Statistical analyses were carried out on the log values.
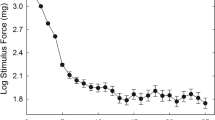
Mean log ratings of intensity (log I) and unpleasantness (log U) are shown in Fig. 1. The four sets of ratings are fairly similar and all increase steadily with increasing force. There is a suggestion in the data that intensity ratings for the experimental group were slightly higher than for the control group, but this was not supported by statistical analysis. A mixed-model ANOVA on the intensity ratings showed that the effect of force was highly significant [F(10,410) = 72.12, p < .001], but the effect of group was not [F(1,41) = 1.43, p = .24]; the interaction term was likewise not significant [F(10,410) = .78, p = .65], indicating that the difference in intensity ratings between groups was not consistently larger at some force levels than others.
Logarithm of intensity (filled symbols) and unpleasantness (open symbols) ratings of cutaneous pressure, at 11 levels of force, are shown for the experimental group (circles) and control group (triangles). Error bars represent 1 SEM; they were comparable for the two groups, but are omitted for the control group for clarity
Unpleasantness ratings similarly failed to distinguish between the groups [F(1,41) = .51, p = .48], and the interaction of force and group was again not significant [F(10,410) = .27, p = .99].
However, inspection of the figure shows that the control group’s ratings are sandwiched between the intensity (above) and unpleasantness (below) ratings of the experimental group, suggesting a difference in intensity/unpleasantness ratio between the groups. The logarithm of this ratio is plotted in Fig. 2, as a function of stimulus force. This quantity differed significantly between the experimental and control groups, F(1,41) = 7.24, p = .010. The effect of force was also significant [F(10,410) = 1.89, p = .045], but the interaction of force and group was not [F(10,410) = .84, p = .59], indicating that the I/U ratio was higher at some force levels than others, but to an equivalent degree for both groups.
Classification responses
Participants rated each pressure stimulus as (a) painful, (b) unpleasant but not painful, or (c) neutral, i.e., neither pleasant nor unpleasant. The weakest stimulus was called “neutral” by all but one of the participants; as force increased, responses first of unpleasant, and then of painful, became increasingly common: The modal response was “neutral” for forces below 500 g, “unpleasant” for forces between 500 and 1000 g, and “painful” for 1077 g. It was thus possible to calculate, for each subject, an unpleasantness threshold—the force at which responses changed from neutral to unpleasant. Operationally, this was defined as halfway between the highest force yielding a “neutral” response and the lowest to which the label “unpleasant” was given. The one subject who called the lowest force unpleasant was given an unpleasantness threshold half a step below it, and the four subjects who responded “neutral” to all stimuli were assigned an unpleasantness threshold half a step above the highest value employed. Mean pain thresholds were not calculated because only 26 subjects used this label to describe one or more of the stimuli.
Unpleasantness thresholds were closely comparable in the experimental (mean = 538 g, SD = 274 g) and control (mean = 542 g, SD = 268 g) groups, t(41) = −.045, p = .96. This result is consistent with the fact that unpleasantness ratings were likewise not statistically different in the two groups.
Anxiety and catastrophizing
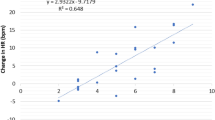
State Anxiety (STAI) scores in the experimental (mean = 33.0, SD = 9.0) and control (mean = 32.5, SD = 9.0) groups were statistically equivalent, t(41) = .191, p = .85. Combined across groups, these scores were significantly correlated with individual subjects’ mean values of log I, r(43) = .430, p = .004 (Fig. 3). A similar correlation existed between STAI scores and log U, r(43) = .398, p = .008. However, log (I/U) is not correlated with state anxiety [r(43) = −.150, p = .336] because calculating this ratio factors out the anxiety-related variance that log I and log U share (Fig. 3). Since state anxiety does not affect the I/U ratio, it cannot be responsible for the effect of the experimental manipulation on that ratio (Fig. 2).
Log of perceived intensity, log I, is significantly correlated with state anxiety (circles), but the log of the ratio of intensity to unpleasantness, log (I/U), is not (triangles). Each symbol shows the mean value of log I or log (I/U) for an individual participant, averaged across force levels. Filled symbols represent members of the experimental group, and open symbols represent members of the control group
Trait anxiety scores showed a generally similar pattern: They likewise did not distinguish between the experimental and control groups [t(41) = −.239, p = .81] and were not correlated with log (I/U). There was a tendency for them to be associated with log I and log U, but these low correlations (.2 < r ≤ .3) only approached significance (.05 < p < .10), suggesting that some, at least, of the association between state anxiety on the one hand, and ratings of sensation intensity and unpleasantness on the other, are the result of situational factors common to both groups, such as the experimental setting.
Catastrophizing (PCS) scores showed a similar pattern: Scores for the experimental (mean = 23.1, SD = 5.6) and control (mean = 24.2, SD = 8.6) groups were statistically indistinguishable, t(41) = −.479, p = .635. They were therefore combined across groups. Combined PCS scores were correlated both with log I [r(43) = .352, p = .021] and with log U [r(43) = .303, p = .048], but not with log (I/U), r(43) = −.087, p = .58. Despite the similar patterns of results for PCS and STAI-State scores, the correlation between these two sets of scores was not significant, r(43) = .236, p = .128.
Taken as a whole, these results show that the experimental manipulation raises neither state anxiety nor catastrophizing. These two characteristics are associated with a tendency (perhaps situational) to rate both pain intensity and unpleasantness highly. This tendency is factored out when the I/U ratio is determined, and therefore the ratio is not correlated with either anxiety or catastrophizing. The experimental manipulation is changing the I/U ratio, but is apparently doing this by modifying something other than emotional state.
Interestingly, PILL scores were not affected by the experimental manipulation: They were closely comparable in the experimental (mean = 14.5, SD = 8.4) and control (mean = 15.5, SD = 9.1) groups, with no statistical difference between the two, t(41) = −.348, p = .730. PILL scores were not correlated with either log I, log U, or log (I/U), r ≤ .1, p ≥ .5 in all cases. Although the PILL is often used as a measure of hypervigilance, it depends on memory for and attentiveness to symptoms over an extended period of time and thus would not be expected to capture brief changes in hypervigilance as in the present study.
Discussion
The generalized hypervigilance hypothesis of Rollman and colleagues (McDermid et al. 1996; Rollman 2009; Rollman and Lautenbacher 1993) is an attempt to explain the fact that in fibromyalgia and other idiopathic pain conditions, patients often show high responsivity not only to noxious stimuli (hyperalgesia) but to a variety of stimuli that are unpleasant or threatening but not painful. The key element of the GHH is that patients develop a “perceptual habit” of undue attention to such stimuli, and this ongoing hypervigilance causes perceptions to be amplified. The patient’s responses thus seem to him/her to be appropriate to the intensity of the sensation, but to a non-hypervigilant observer they seem excessive. If attention is directed to all stimuli sharing some abstract property (e.g., threatening stimuli), the perceptual amplification may generalize to a range of stimuli (threatening pressure, threatening sounds, etc.).
The idea that directing attention to a stimulus can cause enhancement of the sensory signal is one that has gained increasing acceptance, especially in vision research (Carrasco 2011). And it has been convincingly demonstrated that attending to a noxious stimulus can increase its painfulness (Buhle and Wager 2010; Miron et al. 1989; Villemure et al. 2003). The GHH adds to these findings by maintaining that hypervigilance generalizes to any threatening or aversive stimuli, even if they are not painful.
Support for the GHH has usually taken the form of comparisons of fibromyalgia patients and heathy controls, showing that the patients react more strongly to a variety of stimuli (Geisser et al. 2008; Hollins et al. 2009; McDermid et al. 1996). This evidence is consistent with the GHH, but the possibility cannot be ruled out that some difference between patients and controls, other than the hypervigilance of the former, explains the results. The hypothesis therefore remains controversial.
The present study is an attempt to test the GHH by inducing a transient state of hypervigilance in healthy subjects, by means of an experimental manipulation. Crucially, subjects were assigned randomly to the experimental and control groups. To induce a focus on bodily sensations in the experimental group, we used a two-step approach. In the first step, subjects in this group were asked to write for 10 min about the way they prepare for flu season, to make it likely that they would think about the aversive somatic sensations that accompany this illness. In contrast, subjects in the control group wrote for 10 min about their morning routine. Second, we required subjects in the experimental group to focus their attention on specific bodily events—breathing, blinking, and heartbeat—by asking them to count the number of occurrences of these events during fixed time periods. In contrast, subjects in the control group counted external stimuli: lights and sounds. A difference in cognitive load between the tasks assigned to the experimental and control groups is possible, but seems unlikely to have affected subsequent pressure ratings.
The fact that subjects focused on bodily sensations when they were induced or instructed to do so does not, by itself, demonstrate hypervigilance, or provide evidence for the GHH. A performance difference between groups on a subsequent task—rating somesthetic stimuli unlike those used during the writing and counting tasks—could, however, provide such evidence.
To determine whether perceptual amplification was occurring, we asked all subjects, during a subsequent testing phase, to rate the intensity and unpleasantness of pressure sensations caused by a weighted rod pressing on the forearm. It is known, from research on pain by Villemure et al. (2003) and Villemure and Bushnell (2009), that attention selectively amplifies sensation intensity relative to sensation unpleasantness. An increase in the I/U ratio for somatic stimuli—such as mild pressures that signal the possibility of stronger ones—would therefore constitute a signature of hypervigilance. Furthermore, such a result in the present study would imply the presence of generalized hypervigilance, since pressure sensations were never mentioned (by experimenter or subjects) as a flu symptom and did not figure in the counting tasks. Exactly this result was obtained.
It may seem puzzling that the I/U ratio differs substantially between groups, although sensation intensity ratings for the pressure stimuli showed only a nonsignificant trend toward elevation in the experimental group. Several factors may have contributed to this combination of results. First, the experimental manipulation may have induced different response biases in the two groups, causing a shift in both intensity and unpleasantness in one group relative to the other. Such a shift could mask a selective effect of hypervigilance on perceived intensity in the experimental group, but calculating the I/U ratio would unmask it. Second, because intensity and unpleasantness ratings were highly correlated, taking their ratio would factor out shared variance, revealing more clearly any evidence of hypervigilance.
A third possible factor is that the nonsignificant tendency for unpleasantness ratings to be lower in the experimental group (and thus for I/U ratios to be higher) may represent a small but genuine effect of the experimental manipulation. It would not be surprising if counting breaths, blinks, and heartbeats did reduce unpleasantness, for attention to somatic sensations (especially breathing) is a frequent component of relaxation training, which can improve mood (Smith 2005). Future studies should attempt to disentangle and separately evaluate these three potential factors, which are not mutually exclusive.
A limitation of the present study is that objective measurements of breathing rate, blinking rate, and heart rate were not made. Accuracy in counting breaths and blinks was probably high, but counting heartbeats is more difficult: Subjects vary widely in the accuracy with which they do this and in their confidence that they can perceive their heart beating (Garfinkel et al. 2015). A comparison of accuracy or confidence scores in the experimental and control groups would provide an additional test of the view that hypervigilance had been induced in the former.
Taken as a whole, the results of the present study indicate that a mild and presumably transient state of hypervigilance can be induced, in normal subjects, by a simple experimental manipulation administered within a single session and that this hypervigilance can cause an increase in the perceived intensity, relative to the perceived unpleasantness, of threatening (but not necessarily painful) stimuli. The difference in the I/U ratio between groups did not result from differences in anxiety or catastrophizing, a result consistent with the view that hypervigilance is a perceptual habit, rather than an emotional state. And since the test stimuli employed were not of a type to which subjects’ attention had been drawn during the experimental manipulation, the hypervigilance produced may be described as generalized. The study thus provides support for the GHH.
References
Algoe SB, Haidt J (2009) Witnessing excellence in action: the ‘other-praising’ emotions of elevation, gratitude, and admiration. J Posit Psychol 4(2):105–127
Buhle J, Wager TD (2010) Performance-dependent inhibition of pain by an executive working memory task. Pain 149(1):19–26
Carrasco M (2011) Visual attention: the past 25 years. Vis Res 51(13):1484–1525
Crombez G, Van Damme S, Eccleston C (2005) Hypervigilance to pain: an experimental and clinical analysis. Pain 116(1–2):4–7
Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD (2015) Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biol Psychol 104:65–74
Gasper K, Clore GL (2000) Do you have to pay attention to your feelings to be influenced by them? Pers Soc Psychol Bull 26(6):698–711
Geisser ME, Glass JM, Rajcevska LD, Clauw DJ, Williams DA, Kileny PR, Gracely RH (2008) A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain 9(5):417–422
Gescheider GA (1997) Psychophysics: the fundamentals, 3rd edn. Lawrence Erlbaum Assoc, Mahwah
Gracely RH, Dubner R, McGrath PA (1979) Narcotic analgesia: fentanyl reduces the intensity but not the unpleasantness of painful tooth pulp sensations. Science 203(4386):1261–1263
Hollins M, Harper D, Gallagher S, Owings EW, Lim PF, Miller V, Siddiqi MQ, Maixner W (2009) Perceived intensity and unpleasantness of cutaneous and auditory stimuli: an evaluation of the generalized hypervigilance hypothesis. Pain 141(3):215–221
Lee MC, Ploner M, Wiech K, Bingel U, Wanigasekera V, Brooks J, Menon DK, Tracey I (2013) Amygdala activity contributes to the dissociative effect of cannabis on pain perception. Pain 154(1):124–134
McDermid AJ, Rollman GB, McCain GA (1996) Generalized hypervigilance in fibromyalgia: evidence of perceptual amplification. Pain 66(2–3):133–144
Miron D, Duncan GH, Bushnell MC (1989) Effects of attention on the intensity and unpleasantness of thermal pain. Pain 39(3):345–352
Pennebaker JW (1982) The psychology of physical symptoms. Springer, New York
Petzke F, Harris RE, Williams DA, Clauw DJ, Gracely RH (2005) Differences in unpleasantness induced by experimental pressure pain between patients with fibromyalgia and healthy controls. Eur J Pain 9(3):325–335
Rainville P, Feine JS, Bushnell MC, Duncan GH (1992) A psychophysical comparison of sensory and affective responses to four modalities of experimental pain. Somatosens Mot Res 9(4):265–277
Rollman GB (2009) Perspectives on hypervigilance. Pain 141(3):183–184
Rollman GB, Lautenbacher S (1993) Hypervigilance effects in fibromyalgia: pain experience and pain perception. In: Værøy H, Merskey H (eds) Progress in fibromyalgia and myofascial pain, vol 6. Elsevier, Amsterdam, pp 149–159
Schwarz N, Clore GL (1983) Mood, misattribution, and judgments of well-being: informative and directive functions of affective states. J Pers Soc Psychol 45(3):513–523
Smith JC (2005) Relaxation, meditation, and mindfulness: a mental health practitioner’s guide to new and traditional approaches. Springer, New York
Spielberger CD (1983) Manual for the state-trait anxiety inventory (STAI). Consulting Psychologists Press, Palo Alto
Sullivan MJL (2009) PCS: the pain catastrophizing scale, user manual. McGill Univ, Montreal
Tiemann L, Schulz E, Winkelmann A, Ronel J, Henningsen P, Ploner M (2012) Behavioral and neuronal investigations of hypervigilance in patients with fibromyalgia syndrome. PLoS One 74(4):e35068
Van Damme S, Van Hulle L, Spence C, Devulder J, Brusselmans G, Crombez G (2015) Hypervigilance for innocuous tactile stimuli in patients with fibromyalgia: an experimental approach. Eur J Pain 19(5):706–714
Villemure C, Bushnell MC (2009) Mood influences supraspinal pain processing separately from attention. J Neurosci 29(3):705–715
Villemure C, Slotnick BM, Bushnell MC (2003) Effects of odors on pain perception: deciphering the roles of emotion and attention. Pain 106(1–2):101–108
Acknowledgments
Thanks are due to Dillon Taylor for help in developing the counting tasks, to MC Whatley for assistance in running subjects, and to Page Sloan for statistical advice. We are also grateful to an anonymous reviewer for helpful comments on an earlier version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to report.
Rights and permissions
About this article
Cite this article
Hollins, M., Walters, S. Experimental hypervigilance changes the intensity/unpleasantness ratio of pressure sensations: evidence for the generalized hypervigilance hypothesis. Exp Brain Res 234, 1377–1384 (2016). https://doi.org/10.1007/s00221-015-4541-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-015-4541-0