Abstract
Chronic low back pain (CLBP) is often associated with impaired control of deep trunk muscles and reorganization of the primary motor areas (M1). Precisely, functional changes of the lumbar multifidus muscles (MF) involved in spine stability may be of special interest in rehabilitation. Therefore, we tested MF corticomotor control using double transcranial magnetic stimulation (TMS) paradigms for the first time in this muscle and examined its link with MF volitional activation. Eleven individuals with lateralized CLBP and 13 pain-free participants were recruited. Ultrasound imaging enabled measurement of MF volitional isometric contraction in prone lying. TMS of MF M1 area was used to test hemispheric excitability and mechanisms in relation to motor programming, i.e., active motor threshold (AMT), amplitude of motor-evoked potentials and short-interval intracortical inhibition (SICI) and facilitation (SICF). In CLBP, SICI level was lower in the left hemisphere and MF volitional contraction was not related to AMT (M1 excitability), conversely to what was observed in the pain-free group. No other between-group difference was detected. These original findings support a plasticity of cortical maps controlling paravertebral muscles and likely including a different motor strategy for the control of MF. Changes of M1 function may thus underlie impaired motor control of lumbopelvic spine and pain persistence in CLBP.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic low back pain (CLBP) is related to the impaired control of many lumbopelvic muscles (Hodges and Richardson 1996; Moseley et al. 2002; van Dieen et al. 2003) such as the transversus abdominis (TrA) and multifidus (MF), two deep trunk muscles that fine-tune and control the lumbar intervertebral movement. Electromyographic (EMG) evidence of altered control of these muscles has been shown in delayed anticipatory activation prior to rapid limb movements in standing (Hodges and Richardson 1996; Moseley et al. 2002; Masse-Alarie et al. 2012). Altered control has also been demonstrated using ultrasound imaging, with decreased activation evident during sustained postures and limb movements, and during both voluntary (Ferreira et al. 2004, 2010; Kiesel et al. 2007b; Wallwork et al. 2009; Hides et al. 2011) and automatic contractions (Teyhen et al. 2009). The reason for this impaired control, however, is not well understood. Therefore, testing the existence of supraspinal impairments and determining their link to changes in functional muscle activation in CLBP was an important step to better understanding, and treating, this condition.
Studies using transcranial magnetic stimulation (TMS) of the primary motor cortex (M1) have produced a growing body of evidence that supports a link between changes of brain function and impaired trunk motor control in CLBP. TMS of M1 activates the corticospinal cells and produces a measurable motor-evoked potential (MEP) in the contralateral muscle. In CLBP, it was shown that the M1 area controlling TrA was shifted posterolaterally, and that this shift was correlated with a delay of TrA activation in a rapid shoulder flexion task (Tsao et al. 2008). Specific motor training, used to help recover TrA anticipatory activation, was accompanied by the normalization of TrA M1 maps (Tsao et al. 2010). M1 areas of the superficial erector spinae (ES) and deep MF muscles, which are clearly distinct in pain-free individuals, have been shown to overlap in people with CLBP (Tsao et al. 2011b). Separation of these areas may allow cortical control to concurrently increase lumbar MF activity and decrease thoracolumbar ES for positioning the spine in lumbar lordosis (Hodges 2012). Overlap, or “smudging,” of these two M1 areas could potentially explain why individuals with CLBP cannot easily replicate lumbar lordosis. Previous TMS studies thus support a link between motor impairment in CLBP and reorganization of M1 (Tsao et al. 2008; Masse-Alarie et al. 2012).
TMS outcomes can also provide information related to the level of corticospinal excitability. It has been shown, for example, that corticospinal excitability of the paravertebral muscles (including MF) is decreased in CLBP (Strutton et al. 2005), thus suggesting an impaired corticomotor drive to these muscles. More specifically, the paradigms of double-pulse TMS (a combination of a conditioning and a test TMS pulses) are useful in motor control studies to probe the excitability of M1 circuits surrounding the corticospinal cells and regulating their excitability (Kujirai et al. 1993). The short-interval intracortical inhibition (SICI) paradigm probes the excitability GABAA circuits, and the short-interval intracortical facilitation (SICF) probes glutamatergic circuits (Ziemann et al. 2014). Double-pulse TMS studies have shown that SICI is present in resting or isometric contraction conditions (Reynolds and Ashby 1999; Gagné and Schneider 2008a, b) and is usually reduced (M1 area released from inhibition) prior to motor action (Reynolds and Ashby 1999), during synchronized but not syncopated movements (Byblow and Stinear 2006), and between M1 areas both activated for the recruitment of a proximodistal joint synergy (Gagné and Schneider 2008b). In CLBP, we provided the first evidence that SICI of the deep abdominal muscles was completely absent (total disinhibition of M1 area) when compared to pain-free subjects (Masse-Alarie et al. 2012), thus further supporting that changes of M1 function could underlie impairment of motor planning for trunk muscle control in CLBP (Strutton et al. 2003, 2005; Tsao et al. 2008, 2011b).
The present study tested SICI and SICF circuits involved in the volitional control of the MF muscle, using TMS, and isometric activation of the MF muscle, using ultrasound imaging, in individuals with CLBP and in pain-free subjects. It was hypothesized that MF isometric activation (ultrasound imaging) would be decreased in CLBP as compared to pain-free counterparts and that this would be paralleled by changes of M1 function as denoted by impaired TMS outcomes. Part of this work was already published in abstract form (Masse-Alarie et al. 2014).
Methods
Participants
Eleven (11) individuals with lateralized CLBP (pain ≥3 months, one side more painful) were recruited. Potential participants were excluded if they had non-mechanical LBP (e.g., fracture, malignancy, infection), more than 2 radicular signs, lumbar infiltration in the last 6 months, facet denervation, lumbar surgery, other chronic pain pathology, litigation, specific training of trunk muscles, any major circulatory, respiratory, neurological or cardiac disease, severe orthopedic troubles (e.g., scoliosis with gibbosity >8 mm), cognitive deficit or recent/current pregnancy. The visual analog scale was used to determine the intensity of spontaneous pain (perceived at the onset of experiment) and mean last-week pain (perceived during the week preceding the experiment). A body scheme was used to determine the more painful side. The Oswestry Disability Index (ODI) was used to assess pain-related disability. The Tampa Scale of Kinesiophobia was used to assess pain-related fear of movement. Thirteen (13) pain-free individuals with no history of low back pain in the past year also participated as a control group (CTL).
Exclusion criteria related to TMS testing, for both groups, are reported elsewhere (Rossi et al. 2009), but mainly concerned brain surgery, lesion or injury, any history of seizure or concussion, pacemaker/pump holder, change of medication in the 2 weeks preceding enrollment, medication influencing cortical excitability or metallic implants in the skull or jaw.
All participants were administered the Global Physical Activity Questionnaire (GPAQ). Group characteristics are presented in Table 1. There was no between-group difference for gender, age, height, weight, BMI or level of physical activity (GPAQ score). All participants provided informed written consent approved by the local ethics board.
Ultrasound imaging (USI) procedure
Participants lay prone on a therapeutic bed with a pillow under the pelvis to decrease lumbar lordosis (Stokes et al. 2007). A broadband multifrequency linear transducer (Terason t3200 MSK series15L4, Burlington, MA, USA) was used in B-mode to measure the thickness of both the left and right MF. The transducer was first positioned longitudinal to the lumbar spine and then moved laterally over MF muscle until the L4–L5 zygapophyseal joint was observed. If necessary, a small medial rotation of the transducer was used to help visualize the zygapophyseal joint (Stokes et al. 2007; Whittaker 2007). The L5 level was targeted because it has previously been shown to be most atrophied and/or affected by fatty infiltration (Kjaer et al. 2007; Wallwork et al. 2009; Hides et al. 2011; D’Hooge et al. 2012), which has been linked to poor motor control (Wallwork et al. 2009; Hides et al. 2011).
As multiple USI measures increase reliability (Hebert et al. 2009), three MF images were recorded at rest (participants completely relaxed) and during voluntary, isometric activation of MF (Richardson et al. 2004). For the latter, participants were first explained MF morphology and function by a physical therapist and practiced isometric contractions with the biceps brachii. They were asked to avoid moving the pelvis and thoracolumbar spine and to “swell” the MF in a slow, gentle contraction (Van et al. 2006; Whittaker 2007; Wallwork et al. 2009). Three practice trials were allowed prior to testing. The participants then had 5 s to activate the MF, and the image was recorded. Images (3 per condition, for a total of 6 per participant) were saved on a computer for post hoc analysis using ImageJ (public domain, National Institute of Health, Bethesda, MD, USA, see Fig. 1). This method of MF activation, measured by USI, has been shown to be a valid representation of EMG activity for low-level contraction (Kiesel et al. 2007a), with excellent reliability for intra- and interexaminer in individuals with and without LBP (ICC = 0.92 and 0.97) (Wallwork et al. 2007); for a review see (Hebert et al. 2009).
Surface electromyography (EMG)
MF were bilaterally recorded using parallel-bar EMG sensors with adhesive skin interfaces (16-channel Bagnoli EMG System, Delsys Inc., Boston, MA). Electrodes were placed at the level of the L5 spinous process along the line joining the posterosuperior iliac spine (PSIS) and L1–L2 vertebral interspace (www.seniam.com). A common ground was positioned on the iliac crest. EMG signals were band-pass-filtered (Butterworth, 10–450 Hz), amplified before digitization (2 kHz) and computer-stored for online display and offline analysis (PowerLab acquisition system, LabChart-ADInstruments, Colorado Springs, CO).
TMS testing
Participants were seated on a chair, the feet flat on the floor and no arm support (O’Connell et al. 2007; Tsao et al. 2011a, b). They were instructed to lean slightly forward with the lumbar spine in lordosis for activating MF (Dankaerts et al. 2006; O’Sullivan et al. 2006; Claus et al. 2009a, b). This preactivation is known to ease the generation of MEP in the paravertebral muscles (O’Connell et al. 2007; Tsao et al. 2011a). The posture was maintained with minimal pain or discomfort and moderate exertion. Prior to TMS testing, the mean rectified MF EMG activity associated with this posture was calculated and used as a target EMG level during testing. This method was used to avoid increase in pain that could have influenced TMS data.
During TMS testing, participants sat in the same posture, with real-time EMG activity of MF (low-pass filtered at 2 Hz) displayed on a screen, along with the target EMG level. Participants were asked to match their MF EMG output to the target line. This served to stabilize motoneuronal excitability and spinal cord output (Schneider et al. 2004). EMG background activity of MF contralateral to M1 stimulated was displayed as a target line. Trials in which EMG fell outside the acceptance window were rejected (EMG associated with sitting posture ±35 %). M1 of both hemispheres was stimulated alternatively, and only data from the contralateral MF were analyzed. Rest was imposed between each TMS trial to avoid fatigue or pain.
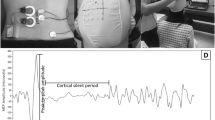
Magnetic stimuli were applied using a double-cone coil (7 cm outer diameter each wing, Magstim Company Limited, Whitland, UK) optimal for the activation of M1 cells of trunk muscles (Nowicky et al. 2001; Davey et al. 2002; Tsao et al. 2011a). The TMS coil was positioned over the M1 area of MF that was first located by means of the 10–20 EEG coordinates at 2 cm lateral to the midline and 2 cm anterior to the vertex (Tsao et al. 2011a). The position was slightly adjusted to determine the “hot spot,” namely the location eliciting the largest amplitudes of MEP. Scalp location was marked in each participant using a surgical marker to ensure reliable positioning and orientation of the coil. The active motor threshold (AMT) was determined as the TMS intensity required to elicit at least 5 measurable MEP out of 10 trials. Test TMS was set at 120 % AMT to elicit MEP in the preactivated MF and probe the corticospinal excitability.
Double TMS paradigms (coil connected to two Magstim 2002 monophasic stimulators) were used to test SICI and SICF (Fig. 2). In SICI, a subthreshold conditioning TMS (70 % AMT) was delivered 2 ms before a test TMS which was set at an intensity (e.g., 120 % AMT Ortu et al. 2008) eliciting an unconditioned (test) MEP of sufficient amplitude, i.e., about 100 µV. Of note, the only study having used different ISI for testing SICI of trunk muscles reported that ISI of 2 ms was better than 5 ms (Chen et al. 1998). In SICF, a subthreshold conditioning TMS (90 % AMT) was delivered 1 ms after a test TMS at 100 % AMT (Ziemann et al. 1998; Ortu et al. 2008). For both SICI and SICF, the amplitude of the conditioned MEP was expressed relative to the amplitude of the corresponding test MEP. This provided measures of pure M1 GABAA inhibition (SICI: conditioned MEP lower than MEP120%AMT) and pure glutamate facilitation (SICF: conditioned MEP higher than MEP100%AMT) in preactivated muscle conditions (Ortu et al. 2008). Of note, SICF (test elicited 1 ms before conditioning) was preferred to the long-interval ICF (LICF, test 10–15 ms after conditioning) because LICF is difficult to test in preactivated conditions (Ridding et al. 1995) as compared to SICF (Tokimura et al. 1996) and its neuronal population remains unknown (Reis et al. 2008). Eight to ten test MEP and 8–10 conditioned MEP were elicited for testing SICI and the same for testing SICF. The test TMS intensity was adjusted between sides in each participant to match test MEP amplitudes and for valid comparisons of conditioned MEP amplitudes between sides.
Raw traces of unconditioned (upper graphs) and conditioned (lower graphs) motor-evoked potentials (MEP) testing the short-interval intracortical inhibition (SICI, left graphs) and facilitation (SICF, right graphs) of multifidus muscle in a participant. Dotted lines and arrows figure the times of test and conditioning pulses, respectively: In SICI, the conditioning pulse was elicited 2 ms before the test TMS and in SICF 1 ms after the test. In SICI, the conditioning pulse was set at 70 % of active motor threshold (AMT) and the test at 120 % AMT, whereas in SICF the conditioning pulse was set at 90 % AMT and the test at 100 % AMT. Note the decrease in the conditioned MEP amplitude in SICI and the increase in SICF relative to their respective unconditioned MEP
Data reduction and statistical analysis
Five TMS outcomes associated with MF were acquired per side for each participant: (1) AMT—the basic transsynaptic M1 excitability during voluntary contraction; (2) test MEP—the peak-to-peak amplitude of the MEP (120 % AMT), reflecting the volume of M1 cells synchronized by TMS and the synchronicity of descending volleys at the motoneuronal level; (3) SP—the duration of the EMG silent period following the test MEP, likely indicating M1 GABAB inhibition (Werhahn et al. 1999); (4) SICI—the peak-to-peak amplitude of the conditioned MEP testing SICI, involving post-synaptic GABAA receptors (expressed in % MEP120%AMT); and (5) SICF—the peak-to-peak amplitude of the conditioned MEP testing SICF, involving post-synaptic glutamate receptors (expressed in % MEP100%AMT).
MF activation was calculated from USI recordings. Figure 1 presents the linear measurements of muscle thickness between the L4–L5 zygapophyseal joint and subcutaneous tissues, done at rest (a) and during volitional contraction (b). The % of MF activation ([b-a]/a) was calculated as follows (Kiesel et al. 2007a):
A two-way analysis of variance (ANOVA) with mixed design, and factors Group (LBP vs. CTL) × Side (Right vs. Left), with repeated measures on Side, was applied on all outcomes. A contrast analysis of ANOVA (planned comparisons) tested where differences took place. A one-way ANOVA with factor Pain Side (More vs. less painful) was applied on all outcomes in CLBP group. Since three left-handed participants were included, a second ANOVA with Handedness (Right vs. Left) as a covariate was also applied. Unpaired t tests were used on the individual TMS raw data to verify the existence of SICI and SICF in each participant, i.e., whether the conditioned MEP amplitudes were significantly decreased (in SICI paradigm) and increased (SICF paradigm) compared to the respective test MEP. Paired t test was then applied on raw group data to test the existence of SICI and SICF in each group. Pearson’s matrices tested the correlations between TMS outcomes and USI recordings, for both groups, and between pain intensity (last week and spontaneous) and AMT, for the CLBP group. Significance level, for all tests, was set at p < 0.05.
Results
SICI data were not analyzed in eight subjects (N = 3 in CLBP group; N = 5 in CTL group) whose raw EMG traces were contaminated by double TMS artifacts. This brought SICI analysis to a number of 8 participants per group. Testing did not aggravate pain, and rest periods were strictly respected between trials.
Cortical motor excitability of MF muscle
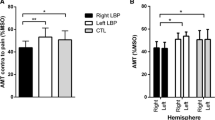
In SICI, the two-way ANOVA applied on the conditioned MEP revealed no main effects but detected a Group × Side interaction (F (1,14) = 5.53; p = 0.03) with higher amplitudes for the left hemisphere in the CLBP group (mean = 117.1 ± 41.4 %) compared with their right hemisphere (mean = 84.1 ± 35 %; F (1,14) = 6.71; p = 0.02) and with the left hemisphere of the CTL group (mean = 77.3 ± 25.5 %; F (1,14) = 4.84; p = 0.045, Fig. 3a). Individual data showed that, in the left hemisphere (Fig. 3b), SICI conditioned MEP amplitudes were greater than 100 % test MEP in 6/8 participants with CLBP (instead of being reduced), whereas this occurred in only 1/8 CTL participant. No such contrast was observed for the right hemisphere (Fig. 3c). T tests on the group means per hemisphere did not detect significant differences between conditioned and test MEP (p > 0.05).
Short-interval intracortical inhibition (SICI) of multifidus muscle. a Group mean amplitudes in CLBP and pain-free participants (controls, CTL) of conditioned MEP expressed in percentage of mean unconditioned (test) MEP for the right (black histogram) and the left hemispheres (white histogram), i.e., respectively, for the left and right multifidus muscles. b, c Individuals’ conditioned MEP amplitudes (% test MEP) for the left and right hemispheres. Note the lower level of SICI in the left hemisphere for most CLBP participants. Bars represent SD. *p < 0.05; **p < 0.01
In SICF, ANOVA did not show any main effect or Group × Side interaction (F (1,20) = 0.98; p = 0.33, Fig. 4a). T tests on the group means detected that the conditioned MEP amplitudes were higher than test MEP in each hemisphere (p < 0.01). Individual data showed that most participants presented with a significant SICF in each hemisphere (Fig. 4b, c).
Short-interval intracortical facilitation (SICF) of multifidus muscle. a Group mean amplitudes in CLBP and pain-free participants (controls, CTL) of conditioned MEP expressed in percentage of mean unconditioned (test) MEP for the right (black histogram) and the left hemispheres (white histogram), i.e., respectively, for the left and right multifidus muscles. b, c Individuals’ conditioned MEP amplitudes (% test MEP) for the left and right hemispheres. Note that SICF was detected in each hemisphere for each group
No main effect or interaction was detected for AMT, test MEP and SP (Table 2). Handedness, as a covariate, did not influence the results.
MF isometric activation in relation to TMS outcomes and pain
The two-way ANOVA applied on MF isometric activation percentage did not detect any difference between groups or between sides. A significant, inverse relationship, however, was found between MF isometric activation, for the left and right MF, and AMT in the contralateral hemisphere for the CTL group, thus indicating increased M1 excitability (lower AMT) in pain-free individuals with greater MF isometric activation (Fig. 5a, b). These correlations were absent in participants with CLBP (Fig. 5c, d).
Correlations between the active motor threshold (AMT, % maximal TMS stimulator output) and the volitional activation of multifidus (MF) muscle [% (active-rest)/rest in ultrasound imaging] in pain-free (CTL, upper graphs) and CLBP participants (lower graphs). Note that, in CTL group, higher activation of contralateral and ipsilateral MF corresponded to lower AMT in motor cortical area (M1) of the right (a) and left hemispheres (b), whereas this link was absent in the CLBP group for both hemispheres (c, d)
A strong, positive correlation was also found between pain intensity (both last week and spontaneous) and the absolute between-side difference of MF isometric activation, regardless of pain side (Fig. 6). This indicates greater asymmetry of MF isometric activation for individuals in the CLBP group reporting higher pain intensities.
Correlation in CLBP participants between the intensity of low back pain (as assessed by the visual analog scale, VAS) and the difference of multifidus volitional activation between the left and right sides of the spine [activation = % (active-rest)/rest from ultrasound imaging]. Note that larger between-side differences of MF activation corresponded to higher intensity of pain for the week before (last-week pain, white squares) and at time of experiment (spontaneous pain, black squares)
ANOVA did not detect any other side difference when the more and less painful sides were contrasted for TMS or USI outcomes (p > 0.05).
Discussion
This study was the first to test the SICI and SICF mechanisms of the MF muscle in concurrence with ultrasound imaging, in an effort to assess whether corticomotor and motor control differences exist between individuals with CLBP and pain-free counterparts. Our findings indicate that the level of SICI was lower in the left MF M1 area of CLBP participants as compared to pain-free people, whereas SICF was present in each hemisphere of each group. A relationship was also found, in pain-free subjects, between M1 excitability in both hemispheres and the volitional activation of the contralateral and ipsilateral MF. This correlation was missing in the CLBP group. Also, pain intensity was correlated with the difference of MF activation between sides. These results are discussed in terms of corticomotor and functional changes associated with MF control in CLBP.
Impaired corticomotor control of MF in CLBP
Motor control depends on the integrity of primary sensory (S1) and motor (M1) cortical circuits and on sensorimotor integration that contributes to shape M1 transsynaptic connections (Kaneko et al. 1994). In CLBP, the lower SICI level of MF found in the left hemisphere (in the absence of any other disorder) could be related to local S1 or M1 maps rearrangement (Flor et al. 1997; Tsao et al. 2011b) or to changes in other areas (Apkarian et al. 2011) and to modification of sensory coding and corticomotor excitability (Strutton et al. 2003, 2005; Tsao et al. 2008, 2011b; Masse-Alarie et al. 2012). This is in line with our previous studies on lower levels of SICI for abdominal muscle in CLBP (Masse-Alarie et al. 2012, 2013) and with reports of missing SICI in different pain conditions (Schwenkreis et al. 2003, 2010; Lefaucheur et al. 2006; Mhalla et al. 2010). However, although both hemispheres usually process pain, the frontal areas of the right hemisphere are more active in unilateral pain (Hsieh et al. 1996; Coghill et al. 2001; Symonds et al. 2006). More precisely, the right anterior cingulate cortex (ACC) is up-regulated (Symonds et al. 2006) and, via the recruitment of corticocortical connections to motor areas, this can up-regulate the right M1 excitability (Dum et al. 2002). In our participants with lateralized CLBP, a persistent up-regulation of the right M1 could have increased the recruitment of the interhemispheric pathways to the left M1. This may have resulted in sustained interhemispheric inhibition of SICI and facilitation of SICF in the left M1, in regard to what is known from double TMS testing on these inhibitory (SICI) and excitatory circuits (SICF) surrounding the corticospinal cells somas in M1’s layer 5 (Reis et al. 2008). Thus, interhemispheric inhibition of SICI could explain the lower levels of left M1 SICI in our CLBP group. This is in line with neuroimaging studies in people with CLBP that showed atrophy and altered connectivity in the left S1/M1 networks (Baliki et al. 2012; Seminowicz et al. 2011; Pijnenburg et al. 2015). These anatomical changes were of functional significance (slower performances of a mobility task) and were related to the fact that the left hemisphere was more engaged in motor action than the right thus being more altered by the pain-related preclusion of movement (Pijnenburg et al. 2015). That said, future longitudinal studies on the chronicization of LBP should question whether a persistent decrease in SICI levels in the left M1 could be related to anatomical brain changes, i.e., could be an indicator of upcoming cerebral atrophy.
SICF was the other mechanism probed by double TMS to assess the pure glutamatergic facilitation within M1 circuits under preactivated conditions (Ortu et al. 2008; Peurala et al. 2008). Unlike SICI, SICF of MF was equivalent between groups and between hemispheres. However, owing to SICI and SICF interactions to control for M1 homeostasis (Peurala et al. 2008; Wagle-Shukla et al. 2009; Shirota et al. 2010), SICI decrease should have been accompanied by SICF changes. One putative explanation could be that the regulation of SICF levels for homeostatic balance was masked by the increase in interhemispheric facilitation onto SICF circuits. However, SICF mechanisms of MF muscles are probed for the first time in CLBP and in pain-free participants; thus, their functional role remains unclear. Among our participants with CLBP, many other mechanisms could have regulated the excitability of SICF circuits such as other interactions in M1, subcortical influences or frontal loops (Apkarian et al. 2011). Further studies are thus warranted to more thoroughly address the intertwined M1 mechanisms of trunk muscles control in CLBP and in pain-free participants.
Functional changes associated with MF control in CLBP
The volitional and postural control of axial muscles activity is bilateral in nature (Ferbert et al. 1992; Carr et al. 1994) and ensured by different descending pathways originating from M1, brainstem, propriospinal networks, etc. (Nathan et al. 1996). Responses of axial muscles to TMS of M1 representation and in association with timing of anticipatory postural activation during ballistic movements reflect that M1 is involved in the control of axial muscles (Tsao et al. 2010). In that vein, and supported by previous studies on the topic (Chen et al. 1998; Masse-Alarie et al. 2012), our findings of a different modulation of MF MEP by SICI and SICF paradigms tend to show that the functional organization of M1 area controlling the trunk muscles resembles the M1 hand area organization. Yet, and unlike for distal muscles, the impact of a decrease in SICI on axial motor control is not known. For distal muscles, it is acknowledged that the GABAA-related inhibition of M1 has a determining role in motor planning, e.g., by tuning movement-related neural activity in cortical networks to determine the level of dexterity of complex or individuated finger movements (Keller 1993), synchronized movements (Stinear and Byblow 2003) and proximodistal synergy (Gagné and Schneider 2008a). The modulation of SICI is thus deemed to influence the cortical maps by the regulation of M1 plasticity (Keller 1993; Bachtiar and Stagg 2014), i.e., its increase disrupts M1 use-dependent plasticity, as tested by GABAA agonist administration in pain-free subjects (Butefisch et al. 2000), whereas its decrease favors brain plasticity, as tested by GABAA antagonist administration (Ziemann et al. 2001). Thus, the decrease in left M1 SICI of MF muscles in our study could reflect a sustained plastic disinhibition still acting on the modification of sensorimotor maps (Flor et al. 1997; Tsao et al. 2011b). This disinhibition could reflect a functional plasticity to seek and learn alternate motor strategies to recover the control of trunk muscles precluded by pain (Hodges and Tucker 2011; Schabrun et al. 2015). The persistence of these plastic phenomena could be related to the persistence of pain and of axial disorders in CLBP, such as impairment of MF motor control (Kiesel et al. 2007b; Wallwork et al. 2009), the contraction of task-inefficient muscles and the selection of inefficient strategies or trunk muscle synergies for postural adjustments, gait or protection of spine integrity (Hodges and Tucker 2011; Tsao et al. 2011b; Schabrun et al. 2015). Studies using chronic pain treatment showed that the decrease in pain was accompanied by brain changes, e.g., for hip replacement (Gwilym et al. 2010), facet infiltration (Seminowicz et al. 2011), exercises (Tsao et al. 2010) and neurostimulation (Lefaucheur et al. 2006; Masse-Alarie et al. 2013; Schabrun et al. 2015). More precisely, restoration of a normative level of SICI by neurostimulation in neuropathic pain (Lefaucheur et al. 2006, 2012) and in combination with exercises in CBLP (Masse-Alarie et al. 2013) significantly reduced pain and also improved the impaired function of trunk muscles (Masse-Alarie et al. 2013). A correlation was even found between the decrease in neuropathic pain intensity at upper extremities and the increase in SICI level (Lefaucheur et al. 2006, 2012). By extension, these studies support that a proper M1 function should be restored (including SICI level and M1 maps) to recover the motor control of MF muscles in CLBP and alleviate pain. Future studies should further probe the extent to which MF SICI is related to functional plasticity in CLBP.
Another interesting finding is the presence in pain-free subjects and the absence in CLBP of a negative correlation between AMT and the ability to voluntarily activate MF. Given that AMT provides information related to the transsynaptic excitability of M1 tissues under preactivated conditions (Ziemann et al. 2014), the lack of correlation in CLBP suggests a desynchronization between functional settings of M1 excitability and the capacity for volitional activation. A potential cause could be smudging of MF and ES M1 sites (Tsao et al. 2011b). Thus, the AMT obtained for MF M1 area in our participants with CLBP could represent M1 excitability related to different paravertebral muscles recruitment and surface EMG electrodes at the low back could not discriminate between these muscles (O’Connell et al. 2007; Tsao et al. 2011b). The missing correlation in CLBP thus further supports a reorganization of M1 circuits. Therefore, the lack of corticospinal coherence between the excitability of the M1 area controlling MF and the volitional activation of MF provides new evidence of motor planning disorders for trunk muscles control in CLBP. It is also possible that, following changes of brain functional organization, the control of postural muscles depends more on extrapyramidal systems than on corticospinal in CLBP, thus rendering obsolete any putative correlation between M1 excitability and MF activation. Of note, this correlation in pain-free participants between M1 excitability and the ability to voluntarily activate both the contralateral and ipsilateral MF provides further support for the bilateral organization of corticospinal pathways controlling MF muscles (Ferbert et al. 1992).
Surprisingly, we did not find any difference in MF activation or TMS outcomes between the more and less painful sides, and no relation of left SICI decrease with pain side. A potential explanation may come from the idea that the CNS uses a generic strategy to protect the spine, rather than a strategy specific to the side of pain (Tsao et al. 2011b). This has been shown for other joints, like the knee, for which both experimental (Kosek and Hansson 2002) and chronic patellofemoral pain syndrome (Jensen et al. 2007) are reported to alter somatosensory perception of the non-painful side. In our CLBP group, low levels of spontaneous and last-week pain were related to smaller differences of MF activation between sides and vice versa, regardless of pain side. We thus propose that the generic strategy to protect spine works with a bilateral integration of painful signals, a lateralized processing of pain in the right hemisphere (e.g., ACC up-regulation leading to left M1 disinhibition) and bilateral descending controls of muscles, regardless of pain side. Further studies, however, are warranted to better understand the cerebral mechanisms of spine motor control in CLBP.
Methodological considerations
Unlike previous studies, we did not find lower activation of MF in CLBP group (Wallwork et al. 2009; Hides et al. 2011), nor did we find higher AMT reflecting lower M1 excitability (Strutton et al. 2005). We assume that this might have been due to higher levels of physical activity in our CLBP sample presenting with only low level of pain and minor disability at the time of the experiment (see Table 1). For example, the mean spontaneous pain level of our participants was 2/10 and Wallwork et al. (2009) excluded participants with pain below 3/10 on the visual analog scale. Ultrasound imaging is a valid representation of MF activation for low to moderate muscle contractions (at 19–34 % MVC, see (Kiesel et al. 2007a) with excellent psychometric properties (Hebert et al. 2009). Thus, correlations between MF activation calculated by ultrasound imaging and TMS outcomes are valid to test the corticomotor control of MF. The generalizability of our results could be questioned by the small sample size; however, results were significant and data consistent across participants. MEP amplitudes are far smaller in axial muscles than in distal muscles (Ferbert et al. 1992; Strutton et al. 2004); thus, potential “floor effects” under preactivated conditions may explain, in part, why, in SICI testing, the group means of conditioned MEP were not significantly smaller than the unconditioned MEP. Also, the parameters used for the double TMS paradigms (interstimulus intervals, intensity) were related to the properties of M1 circuits controlling upper limb muscles (Ortu et al. 2008). Yet, it is not responded whether M1 circuits controlling trunk muscles share similar properties; thus, future studies should determine the optimal parameters to probe SICI and SICF of trunk muscles. Given possible contamination (cross-talk) of surface MF EMG recordings by the activity in adjacent muscles (Stokes et al. 2003), these studies should consider the use of fine-wire electrodes to investigate different paravertebral muscles or different portions (superficial, deep) of MF.
Conclusion
This original study on concurrent TMS and ultrasound imaging outcomes provides further support for impaired corticomotor control of MF muscles among individuals with CLBP. A sustained disinhibition of the left hemisphere and the lack of correlation between M1 excitability and MF activation may reflect cortical reorganization leading to motor planning disorders. Future studies should address whether impairments in spine motor control, including anticipatory postural adjustments, are also reflected in other motor systems (e.g., extrapyramidal) and the extent to which physical therapy can help redress corticomotor and functional changes in CLBP.
References
Apkarian AV, Hashmi JA, Baliki MN (2011) Pain and the brain: specificity and plasticity of the brain in clinical chronic pain. Pain 152:S49–S64. doi:10.1016/j.pain.2010.11.010
Bachtiar V, Stagg CJ (2014) The role of inhibition in human motor cortical plasticity. Neuroscience 278:93–104. doi:10.1016/j.neuroscience.2014.07.059
Baliki MN, Petre B, Torbey S et al. (2012) Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci 15:1117–1119. doi:10.1038/nn.3153
Butefisch C, Davis B, Wise S, Sawaki L, Kopylev L, Classen J, Cohen L (2000) Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci USA 97:3661–3665
Byblow WD, Stinear CM (2006) Modulation of short-latency intracortical inhibition in human primary motor cortex during synchronised versus syncopated finger movements. Exp Brain Res 168:287–293. doi:10.1007/s00221-005-0205-9
Carr LJ, Harrison LM, Stephens JA (1994) Evidence for bilateral innervation of certain homologous motoneurone pools in man. J Physiol 475:217–227
Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, Cohen LG (1998) Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol 80:2870–2881
Claus AP, Hides JA, Moseley GL, Hodges PW (2009a) Different ways to balance the spine: subtle changes in sagittal spinal curves affect regional muscle activity. Spine (Phila Pa 1976) 34:E208–E214. doi:10.1097/BRS.0b013e3181908ead00007632-200903150-00021
Claus AP, Hides JA, Moseley GL, Hodges PW (2009b) Is ‘ideal’ sitting posture real? Measurement of spinal curves in four sitting postures. Man Ther 14:404–408. doi:10.1016/j.math.2008.06.001
Coghill RC, Gilron I, Iadarola MJ (2001) Hemispheric lateralization of somatosensory processing. J Neurophysiol 85:2602–2612
Dankaerts W, O’Sullivan P, Burnett A, Straker L (2006) Altered patterns of superficial trunk muscle activation during sitting in nonspecific chronic low back pain patients: importance of subclassification. Spine (Phila Pa 1976) 31:2017–2023. doi:10.1097/01.brs.0000228728.11076.8200007632-200608010-00021
Davey NJ, Lisle RM, Loxton-Edwards B, Nowicky AV, McGregor AH (2002) Activation of back muscles during voluntary abduction of the contralateral arm in humans. Spine (Phila Pa 1976) 27:1355–1360
D’Hooge R, Cagnie B, Crombez G, Vanderstraeten G, Dolphens M, Danneels L (2012) Increased intramuscular fatty infiltration without differences in lumbar muscle cross-sectional area during remission of unilateral recurrent low back pain. Man Ther 17:584–588. doi:10.1016/j.math.2012.06.007S1356-689X(12)00148-8
Dum RP, Li C, Strick PL (2002) Motor and nonmotor domains in the monkey dentate. Ann N Y Acad Sci 978:289–301
Ferbert A, Caramia D, Priori A, Bertolasi L, Rothwell JC (1992) Cortical projection to erector spinae muscles in man as assessed by focal transcranial magnetic stimulation. Electroencephal Clin Neurophysiol Evoked Potent Sect 85:382–387
Ferreira PH, Ferreira ML, Hodges PW (2004) Changes in recruitment of the abdominal muscles in people with low back pain: ultrasound measurement of muscle activity. Spine (Phila Pa 1976) 29:2560–2566
Ferreira PH, Ferreira ML, Maher CG, Refshauge K, Herbert RD, Hodges PW (2010) Changes in recruitment of transversus abdominis correlate with disability in people with chronic low back pain. Br J Sports Med 44:1166–1172. doi:10.1136/bjsm.2009.061515
Flor H, Braun C, Elbert T, Birbaumer N (1997) Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett 224:5–8
Gagné M, Schneider C (2008a) Dynamic influence of wrist flexion and extension on the intracortical inhibition of the first dorsal interosseus muscle during precision grip. Brain Res 1195:77–88
Gagné M, Schneider C (2008b) Dynamic influence of wrist flexion and extension on the intracortical inhibition of the first dorsal interosseus muscle during precision grip. Brain Res 1195:77–88. doi:10.1016/j.brainres.2007.12.021
Hebert JJ, Koppenhaver SL, Parent EC, Fritz JM (2009) A systematic review of the reliability of rehabilitative ultrasound imaging for the quantitative assessment of the abdominal and lumbar trunk muscles. Spine (Phila Pa 1976) 34:E848–E856. doi:10.1097/BRS.0b013e3181ae625c00007632-200911010-00029
Hides J, Stanton W, Mendis MD, Sexton M (2011) The relationship of transversus abdominis and lumbar multifidus clinical muscle tests in patients with chronic low back pain. Man Ther 16:573–577. doi:10.1016/j.math.2011.05.007
Hodges PW (2012) Functional implications of musculoskeletal pain: Mechanisms and rehabilitation. In: 14th world congress on pain, Milano, Italia
Hodges PW, Richardson CA (1996) Inefficient muscular stabilization of the lumbar spine associated with low back pain. A motor control evaluation of transversus abdominis. Spine (Phila Pa 1976) 21:2640–2650
Hodges PW, Tucker K (2011) Moving differently in pain: a new theory to explain the adaptation to pain. Pain 152:S90–S98. doi:10.1016/j.pain.2010.10.020
Hsieh JC, Hannerz J, Ingvar M (1996) Right-lateralised central processing for pain of nitroglycerin-induced cluster headache. Pain 67:59–68
Jensen R, Hystad T, Kvale A, Baerheim A (2007) Quantitative sensory testing of patients with long lasting Patellofemoral pain syndrome. Eur J Pain 11:665–676. doi:10.1016/j.ejpain.2006.10.007
Kaneko T, Caria MA, Asanuma H (1994) Information processing within the motor cortex. II. Intracortical connections between neurons receiving somatosensory cortical input and motor output neurons of the cortex. J Comp Neurol 345:172–184. doi:10.1002/cne.903450203
Keller A (1993) Intrinsic synaptic organization of the motor cortex. Cereb Cortex 3:430–441
Kiesel KB, Uhl TL, Underwood FB, Rodd DW, Nitz AJ (2007a) Measurement of lumbar multifidus muscle contraction with rehabilitative ultrasound imaging. Man Ther 12:161–166. doi:10.1016/j.math.2006.06.011
Kiesel KB, Underwood FB, Mattacola CG, Nitz AJ, Malone TR (2007b) A comparison of select trunk muscle thickness change between subjects with low back pain classified in the treatment-based classification system and asymptomatic controls. J Orthop Sports Phys Ther 37:596–607
Kjaer P, Bendix T, Sorensen JS, Korsholm L, Leboeuf-Yde C (2007) Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med 5:2. doi:10.1186/1741-7015-5-2
Kosek E, Hansson P (2002) The influence of experimental pain intensity in the local and referred pain area on somatosensory perception in the area of referred pain. Eur J Pain 6:413–425
Kujirai T, Caramia MD, Rothwell JC et al (1993) Corticocortical inhibition in human motor cortex. J Physiol 471:501–519
Lefaucheur JP, Drouot X, Menard-Lefaucheur I, Keravel Y, Nguyen JP (2006) Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology 67:1568–1574. doi:10.1212/01.wnl.0000242731.10074.3c
Lefaucheur JP, Ayache SS, Sorel M et al (2012) Analgesic effects of repetitive transcranial magnetic stimulation of the motor cortex in neuropathic pain: influence of theta burst stimulation priming. Eur J Pain 16:1403–1413. doi:10.1002/j.1532-2149.2012.00150.x
Masse-Alarie H, Flamand VH, Moffet H, Schneider C (2012) Corticomotor control of deep abdominal muscles in chronic low back pain and anticipatory postural adjustments. Exp Brain Res 218:99–109. doi:10.1007/s00221-012-3008-9
Masse-Alarie H, Flamand VH, Moffet H, Schneider C (2013) Peripheral neurostimulation and specific motor training of deep abdominal muscles improve posturomotor control in chronic low back pain. Clin J Pain 29:814–823. doi:10.1097/AJP.0b013e318276a058
Masse-Alarie H, Beaulieu LD, Preuss R, Schneider C (2014) Impairment of corticomotor control of lumbar multifidus in chronic low back pain. In: 35th annual scientific meeting of the Canadian pain society, Quebec city
Mhalla A, de Andrade DC, Baudic S, Perrot S, Bouhassira D (2010) Alteration of cortical excitability in patients with fibromyalgia. Pain 149:495–500. doi:10.1016/j.pain.2010.03.009
Moseley GL, Hodges PW, Gandevia SC (2002) Deep and superficial fibers of the lumbar multifidus muscle are differentially active during voluntary arm movements. Spine (Phila Pa 1976) 27:E29–E36
Nathan PW, Smith M, Deacon P (1996) Vestibulospinal, reticulospinal and descending propriospinal nerve fibres in man. Brain 119(Pt 6):1809–1833
Nowicky AV, McGregor AH, Davey NJ (2001) Corticospinal control of human erector spinae muscles. Mot Control 5:270–280
O’Connell NE, Maskill DW, Cossar J, Nowicky AV (2007) Mapping the cortical representation of the lumbar paravertebral muscles. Clin Neurophysiol 118:2451–2455. doi:10.1016/j.clinph.2007.08.006
Ortu E, Deriu F, Suppa A, Tolu E, Rothwell JC (2008) Effects of volitional contraction on intracortical inhibition and facilitation in the human motor cortex. J Physiol 586:5147–5159
O’Sullivan PB, Dankaerts W, Burnett AF, Farrell GT, Jefford E, Naylor CS, O’Sullivan KJ (2006) Effect of different upright sitting postures on spinal-pelvic curvature and trunk muscle activation in a pain-free population. Spine (Phila Pa 1976) 31:E707–E712. doi:10.1097/01.brs.0000234735.98075.5000007632-200609010-00024
Peurala SH, Muller-Dahlhaus JF, Arai N (2008) Ziemann U (2008) Interference of short-interval intracortical inhibition (SICI) and short-interval intracortical facilitation (SICF). Clin Neurophysiol 119:2291–2297. doi:10.1016/j.clinph.05.031S1388-2457(08)00584-1
Pijnenburg M, Brumagne S, Caeyenberghs K et al. (2015) Resting-state functional connectivity of the sensorimotor network in individuals with nonspecific low back pain and the association with the sit-to- stand-to-sit task. Brain Connect 5:303–311. doi:10.1089/brain.2014.0309
Reis J, Swayne OB, Vandermeeren Y et al (2008) Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol 586:325–351. doi:10.1113/jphysiol.2007.144824
Reynolds C, Ashby P (1999) Inhibition in the human motor cortex is reduced just before a voluntary contraction. Neurology 53:730–735
Richardson CA, Hides JA, Hodges PW (2004) Therapeutic exercise for lumbopelvic stabilization: a motor control approach for the treatment and prevention of low back pain. Elsevier, Edinburgh
Ridding MC, Inzelberg R, Rothwell JC (1995) Changes in excitability of motor cortical circuitry in patients with Parkinson’s disease. Ann Neurol 37:181–188. doi:10.1002/ana.410370208
Rossi S, Hallett M, Rossini PM, Pascual-Leone A (2009) Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120:2008–2039. doi:10.1016/j.clinph.2009.08.016
Schabrun SM, Christensen SW, Mrachacz-Kersting N, Graven-Nielsen T (2015) Motor cortex reorganization and impaired function in the transition to sustained muscle pain. Cereb Cortex. doi:10.1093/cercor/bhu319
Schneider C, Lavoie BA, Barbeau H, Capaday C (2004) Timing of cortical excitability changes during the reaction time of movements superimposed on tonic motor activity. J Appl Physiol 97:2220–2227
Schwenkreis P, Janssen F, Rommel O et al (2003) Bilateral motor cortex disinhibition in complex regional pain syndrome (CRPS) type I of the hand. Neurology 61:515–519
Schwenkreis P, Scherens A, Ronnau A-K, Hoffken O, Tegenthoff M, Maier C (2010) Cortical disinhibition occurs in chronic neuropathic, but not in chronic nociceptive pain. BMC Neurosci 11:73
Seminowicz DA, Wideman TH, Naso L et al. (2011) Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci 31:7540–7550 doi:10.1523/JNEUROSCI.5280-10.2011
Shirota Y, Hamada M, Terao Y et al (2010) Influence of short-interval intracortical inhibition on short-interval intracortical facilitation in human primary motor cortex. J Neurophysiol 104:1382–1391. doi:10.1152/jn.00164.2010
Stinear CM, Byblow WD (2003) Role of intracortical inhibition in selective hand muscle activation. J Neurophysiol 89:2014–2020. doi:10.1152/jn.00925.200200925.2002
Stokes IA, Henry SM, Single RM (2003) Surface EMG electrodes do not accurately record from lumbar multifidus muscles. Clin Biomech 18:9–13
Stokes M, Hides J, Elliott J, Kiesel K, Hodges P (2007) Rehabilitative ultrasound imaging of the posterior paraspinal muscles. J Orthop Sports Phys Ther 37:581–595
Strutton PH, Catley M, McGregor AH, Davey NJ (2003) Corticospinal excitability in patients with unilateral sciatica. Neurosci Lett 353:33–36
Strutton PH, Beith ID, Theodorou S, Catley M, McGregor AH, Davey NJ (2004) Corticospinal activation of internal oblique muscles has a strong ipsilateral component and can be lateralised in man. In: Experimental brain research, vol 158. Springer, Berlin, pp 474–479
Strutton PH, Theodorou S, Catley M, McGregor AH, Davey NJ (2005) Corticospinal excitability in patients with chronic low back pain. J Spinal Disord Tech 18:420–424
Symonds LL, Gordon NS, Bixby JC, Mande MM (2006) Right-lateralized pain processing in the human cortex: an FMRI study. J Neurophysiol 95:3823–3830. doi:10.1152/jn.01162.2005
Teyhen DS, Williamson JN, Carlson NH et al (2009) Ultrasound characteristics of the deep abdominal muscles during the active straight leg raise test. Arch Phys Med Rehabil 90:761–767. doi:10.1016/j.apmr.2008.11.011S0003-9993(09)00120-8
Tokimura H, Ridding MC, Tokimura Y, Amassian VE, Rothwell JC (1996) Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalogr Clin Neurophysiol 101:263–272
Tsao H, Galea MP, Hodges PW (2008) Reorganization of the motor cortex is associated with postural control deficits in recurrent low back pain. Brain 131:2161–2171. doi:10.1093/brain/awn154
Tsao H, Galea MP, Hodges PW (2010) Driving plasticity in the motor cortex in recurrent low back pain. Eur J Pain 14:832–839. doi:10.1016/j.ejpain.2010.01.001
Tsao H, Danneels L, Hodges PW (2011a) Individual fascicles of the paraspinal muscles are activated by discrete cortical networks in humans. Clin Neurophysiol 122:1580–1587. doi:10.1016/j.clinph.2011.01.048
Tsao H, Danneels LA, Hodges PW (2011b) ISSLS prize winner: smudging the motor brain in young adults with recurrent low back pain. Spine (Phila Pa 1976) 36:1721–1727. doi:10.1097/BRS.0b013e31821c4267
van Dieen JH, Selen LP, Cholewicki J (2003) Trunk muscle activation in low-back pain patients, an analysis of the literature. J Electromyogr Kinesiol 13:333–351
Van K, Hides JA, Richardson CA (2006) The use of real-time ultrasound imaging for biofeedback of lumbar multifidus muscle contraction in healthy subjects. J Orthop Sports Phys Ther 36:920–925
Wagle-Shukla A, Ni Z, Gunraj CA, Bahl N, Chen R (2009) Effects of short interval intracortical inhibition and intracortical facilitation on short interval intracortical facilitation in human primary motor cortex. J Physiol 587:5665–5678. doi:10.1113/jphysiol.2009.181446
Wallwork TL, Hides JA, Stanton WR (2007) Intrarater and interrater reliability of assessment of lumbar multifidus muscle thickness using rehabilitative ultrasound imaging. J Orthop Sports Phys Ther 37:608–612
Wallwork TL, Stanton WR, Freke M, Hides JA (2009) The effect of chronic low back pain on size and contraction of the lumbar multifidus muscle. Man Ther 14:496–500. doi:10.1016/j.math.2008.09.006
Werhahn KJ, Kunesch E, Noachtar S, Benecke R, Classen J (1999) Differential effects on motorcortical inhibition induced by blockade of GABA uptake in humans. J Physiol 517(Pt 2):591–597
Whittaker JL (2007) Ultrasound imaging for rehabilitation of the lumbopelvic region: a clinical approach. Churchill Livingstone, Philadelphia
Ziemann U, Tergau F, Wassermann EM, Wischer S, Hildebrandt J, Paulus W (1998) Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J Physiol 511(Pt 1):181–190
Ziemann U, Muellbacher W, Hallett M, Cohen LG (2001) Modulation of practice-dependent plasticity in human motor cortex. Brain 124:1171–1181
Ziemann U, Reis J, Schwenkreis P, Rosanova M, Strafella A, Badawy R, Muller-Dahlhaus F (2014) TMS and drugs revisited 2014. Clin Neurophysiol. doi:10.1016/j.clinph.2014.08.028
Acknowledgments
The authors acknowledge the support of the Canadian Foundation for Innovation (CS equipment), the Fonds de Recherche en Santé du Québec (HMA and LDB PhD studentship), the Canadian Institute for Health Research (HMA PhD studentship), Université Laval (HMA PhD studentship) and Ms. Mélaine Henry for recruitment and study coordination.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they do not have any financial relationship relevant to this article to disclose and no conflict of interest.
Ethical approval
All procedures were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Massé-Alarie, H., Beaulieu, LD., Preuss, R. et al. Corticomotor control of lumbar multifidus muscles is impaired in chronic low back pain: concurrent evidence from ultrasound imaging and double-pulse transcranial magnetic stimulation. Exp Brain Res 234, 1033–1045 (2016). https://doi.org/10.1007/s00221-015-4528-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-015-4528-x