Abstract
Many situations in our everyday life call for a mechanism deputed to outright stop an ongoing course of action. This behavioral inhibition ability, known as response stopping, is often impaired in psychiatric conditions characterized by impulsivity and poor inhibitory control. Transcranial direct current stimulation (tDCS) has recently been proposed as a tool for modulating response stopping in such clinical populations, and previous studies in healthy humans have already shown that this noninvasive brain stimulation technique is effectively able to improve response stopping, as measured in a stop-signal task (SST) administered immediately after the stimulation. So far, the right inferior frontal gyrus (rIFG) has been the main focus of these attempts to modulate response stopping by the means of noninvasive brain stimulation. However, other cortical areas such as the right dorsolateral prefrontal cortex (rDLPFC) have been implicated in inhibitory control with other paradigms. In order to provide new insight about the involvement of these areas in response stopping, in the present study, tDCS was delivered to 115 healthy subjects, using five stimulation setups that differed in terms of target area (rIFG or rDLPFC) and polarity of stimulation (anodal, cathodal, or sham). The SST was performed 15 min after the offset of the stimulation. Consistently with previous studies, only anodal stimulation over rIFG induced a reliable, although weak, improvement in the SST, which was specific for response stopping, as it was not mirrored in more general reaction time measures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
On many occasions in our everyday life, we face situations that require suddenly stopping an ongoing course of action. Often, this ability is essential to ensure ours or others’ safety. For example, if while cooking we accidentally drop a boiling pot, we could instinctively try to catch it, as we often do when an object we are currently using falls toward the floor. However, this would probably result in getting burnt; hence, a process for outright stopping of an overlearned response to a situation which is similar, but not identical, to the one where such behavior would have been appropriate is needed.
There is now a growing amount of evidence from neuroimaging studies (e.g., Aron et al. 2007; Chevrier et al. 2007; Li et al. 2006) that response stopping is associated with activation in prefrontal areas, such as the inferior frontal gyrus (IFG), the dorsolateral prefrontal cortex (DLPFC), and the medial frontal gyrus, as well as in the basal ganglia. Among these areas, the right portion of the IFG (rIFG) has been proposed as the core component of a prefrontal-basal ganglia network selectively deputed to response stopping (e.g., Aron et al. 2014; but see Swick and Chatham 2014, for a different viewpoint).
The involvement of rIFG in response stopping processes is also supported by lesion (e.g., Aron et al. 2003), and brain stimulation studies including both transcranial direct current stimulation (tDCS; Ditye et al. 2012; Jacobson et al. 2011) and Transcranial Magnetic Stimulation, (TMS, Chambers et al. 2006). Recently, noninvasive brain stimulation techniques (NIBS) such as tDCS and TMS have gained credit as promising tools for investigating and modulating the neural substrates of high-level cognitive functions (e.g., Vannorsdall et al. 2012; Metuki et al. 2012; Penolazzi et al. 2010, 2013; see also Jacobson et al. 2012b) and inhibitory control processes (Juan and Muggleton 2012). Indeed, the same techniques are being tested for use as therapeutic tools to improve symptoms in many psychiatric disorders, with a particular attention to tDCS, given its relative inexpensiveness and ease of use (e.g., Brunoni et al. 2014; Feil and Zangen 2010; Krause and Cohen Kadosh 2013). Since inhibitory deficits have been implicated in many psychiatric conditions, inhibitory processes are among the favored cognitive processes targeted in brain stimulation studies (see Juan and Muggleton 2012, for a review on both tDCS and TMS studies).
As regards response stopping, for instance, a recent study by Jacobson et al. (2011) has shown that anodal tDCS could be effectively used to modulate performance in a commonly used behavioral inhibitory task called stop-signal task (SST) (e.g., Logan and Cowan 1984). This modulation was obtained by targeting the rIFG. A subsequent study (Jacobson et al. 2012a) with EEG recordings provided supporting evidence for the efficacy of a rIFG direct current stimulation, showing a selective theta band reduction over the rIFG after anodal tDCS administration. On a later study, Ditye et al. (2012) found that combining anodal tDCS over the rIFG with training in a SST yielded a better improvement in response stopping than training alone, but only after the third session of four combined training and stimulation sessions.
Remarkably, different stimulation loci have been shown to successfully modulate performance in other inhibitory tasks. For example, Beeli et al. (2008) found an increase in false alarms in a go/no-go task that followed cathodal stimulation of the right prefrontal region. In the same vein, Penolazzi et al. (2014) showed that cathodal tDCS over the right dorsolateral prefrontal cortex (rDLPFC) during a retrieval-practice task induced a reduction in retrieval-induced forgetting, a measure of forgetting which is thought to reflect the intervention of an inhibitory process deputed to selective retrieval from competing memories (Anderson 2003).
In the present study, we aimed to address two main questions related to the literature discussed above. Firstly, we aimed to address the persistence of the modulatory effects of tDCS in response stopping reported in previous studies that delivered electrical stimulation over the prefrontal cortex (Ditye et al. 2012; Jacobson et al. 2011). To this end, we adopted a tDCS protocol where participants were asked to perform a standard SST 15 min after the offset of the stimulation (delayed task).
The SST probes inhibitory motor control by requiring participants to withhold a response that has already been triggered. In a typical SST, participants take part in a choice RT task (e.g., a shape judgment task) and are instructed to withdraw their response whenever they hear a stop signal (e.g., a sound), which can be presented shortly after the target stimulus has appeared. Trials that include the stop signal are usually quite infrequent (e.g., 25 %) compared to trials where participants must respond (go trials). This is assumed to elicit a bias in the participants, who are somehow “pushed” into responding. According to the horse-race model of response inhibition in the SST (e.g., Logan and Cowan 1984; Osman et al. 1986), during a stop trial, the inhibitory process triggered by the stop signal races against the ongoing response process triggered by the target. Response inhibition is therefore successful whenever the former process acts faster, leading to inhibition of the initiated response. Critically, the individual probability of successful inhibition in a given stop trial is a function of the stop-signal delay (SSD), i.e., the time elapsed between the target stimulus and the stop-signal in that particular trial. Indeed, longer SSD mean that the response process will be closer to execution when the competing inhibitory process is triggered. Inhibitory performance in the SST is typically measured with the stop-signal reaction time (SSRT) index, which is computed as the difference between mean RT in the go trials (no-signal RT, NSRT) and the mean SSD in the trials where they must interrupt response. SSRT is interpreted as the covert latency of the response stopping process, so that shorter SSRTs indicate a more efficient response inhibition. The task is often kept challenging by using an adaptive staircase procedure which adjusts the SSD in a trial-wise fashion. This procedure is intended to keep the probability of effectively inhibiting response at ~0.5. Previous work has shown that SSRT could also yield clinical relevance, since high SSRTs had been associated with several psychiatric conditions such as attention-deficit hyperactivity disorder (Depue et al. 2010), eating disorders (Wu et al. 2013), obsessive–compulsive disorder (Boisseau et al. 2012), schizophrenia (Enticott et al. 2008), and substance abuse disorder (Fillmore and Rush 2002).
In the context of our study, we decided not to administer the SST both immediately after tDCS and after this short delay, because we did not want to make the experimental session too demanding for our participants (which, in turn, also allowed us to test a reasonably larger sample compared to standard tDCS studies). As for the effects observed immediately after tDCS, we relied on the pattern observed in previously published reports attesting that stimulation over both the right IFG and right DLPFC is effective in modulating inhibitory processing (Beeli et al. 2008; Jacobson et al. 2011, 2012a; see Juan and Muggleton 2012, for a review). In addition, we decided to test participants after 15 min because this time delay seemed a good compromise between our aim of estimating the short-term effects of single session tDCS and the need to keep the duration of the experimental session not too long for our participants. In this regard, assessing the persistence of tDCS-induced effects on behavior is particularly relevant. Indeed, on the one hand, many studies have shown that, depending on stimulation parameters and montage, tDCS is able affect cortical excitability up to several hours after the current has been delivered (Batsikadze et al. 2013). However, on the other hand, much less effort has been devoted to assess whether measures of behavioral performance mirror this long-lasting effects. Hence, although some recent studies have already suggested tDCS effects on delayed cognitive tasks related to high-level cognitive processes (Falcone et al. 2012; Penolazzi et al. 2010, 2013), the durability of stimulation effects is in need of further investigation. The second aim of the present study was to clarify the role of areas other than the rIFG in response stopping. To this purpose, anodal, cathodal, or sham stimulations were delivered to either the rIFG or the rDLPFC in five groups of human participants. We targeted the rDLPFC to probe the involvement of this area in response stopping, thus contributing to the debate about the specificity of the neural underpinnings of inhibitory processes. Assuming that our tDCS protocol was capable of inducing long-lasting neuromodulatory effects, and in light of preexisting evidence of the association between anodal tDCS and faster SSRT (i.e., more effective response inhibition; Ditye et al. 2012; Jacobson et al. 2011), our main prediction was to observe beneficial effects in inhibitory performance—if any—in the experimental group that received anodal stimulation over the rIFG. In light of the findings reported by Beeli et al. (2008) and Penolazzi et al. (2014), we expected to observe also a possible modulation of SSRT when administering tDCS over rDLPFC.
Methods
Participants
One hundred and fifteen undergraduate students participated in the study (29 males, M = 23.37, SD = 2). All participants met the inclusion criteria for taking part in brain stimulation protocols (Bikson et al. 2009; Nitsche et al. 2003), had normal or corrected-to-normal vision, and did not suffer from hearing impairment. All participants gave a written informed consent before taking part in the study, which was performed in accordance with the principles of the Declaration of Helsinki and approved by the local ethical committee. Participants were randomly assigned to one of four experimental groups or to a control group and were naïve to the purpose of the experiment.
Procedure
The experiment began with a 20-min tDCS session. About 15 min after the end of the stimulation, participants performed the SST. During the stimulation and the 15-min interval prior to the SST, participants performed filler tasks (i.e., they were required to learn word-pairs and to fill paper-and-pencil questionnaires) aimed at delaying SST administration but unrelated to motor inhibition processes.
SST
We administered the SST provided within the STOP-IT software (Verbruggen et al. 2008). The task consisted of two experimental blocks of 64 trials each (128 total), and a shorter practice block (32 trials) at the beginning to ensure that participants understood the instructions. The primary task engaged participants in a choice reaction time test, where they had to respond as fast and accurately as possible. Each trial began with a 250-ms central fixation (+), followed by a visual stimulus (either a square or a circle) which stayed centrally on screen until participants responded or 1.250 ms had elapsed. Both fixation and stimuli were presented in a white font on a black background. The ISI was 2000 ms and was independent of RTs. Participants used the keyboard to respond, and they had to press “A” for squares or “L” for circles. On 25 % of the trials, shortly after stimulus onset, a sound (750 Hz, 75 ms) was presented through loudspeakers as a stop-signal. When the stop-signal was presented, participants had to hold back their response. The task began with a stop-signal delay of 250 ms, which then increased or decreased by 50 ms after each successful or unsuccessful stopping trial, respectively. Under this tracking procedure, participants correctly stopped half the responses, which is required by the method used to calculate SSRT. According to the horse-race model (Logan and Cowan 1984; Osman et al. 1986), SSRT is calculated as the difference between mean RT in the trials where participants must respond and mean SSD in the trials where they must withhold response.
The SST used here is schematically represented in Fig. 1.
tDCS
The study adopted the procedures for safe administration of NIBS (Bikson et al. 2009; Nitsche et al. 2003). In the active stimulation conditions, we delivered a 1.5 mA direct current for 20 min (fade-in/fade-out time: 60 s) with a battery-driven current stimulator (BrainStim, EMS, Italy), wired to a pair of surface saline-soaked sponge electrodes (16 cm2, resulting in a current density of 0.094 mA/cm2). In the sham (i.e., control) condition, instead, we delivered a 1.5 mA direct current for 15 s at the beginning and 15 s at the end of the stimulation time. We choose to stimulate with parameters that lead to a higher current density (i.e., intensity/electrode size) than previous studies (Ditye et al. 2012; Jacobson et al. 2011), in order to increase the spatial focality of tDCS effects (Nitsche et al. 2007).
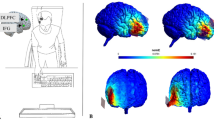
We used a single blind, between-group design: Depending on the random assignment to conditions, participants could receive either anodal stimulation over the right IFG (N = 20; 6 males, M = 23.95, SD = 2.26), cathodal stimulation over the right IFG (N = 20; 8 males, 23.35, SD = 1.53), anodal stimulation over the right DLPFC (N = 20; 3 males, M = 23.65, SD = 2.08), cathodal stimulation over the right DLPFC (N = 20; 3 males, 23.10, SD = 2.57), or sham stimulation on either right DLPFC or right IFG (N = 35; 9 males, M = 23.06, SD = 1.61). In all conditions, electrode placement followed the 10–20 EEG system (Jasper 1958). The rIFG was identified as the area underlying the crossing point between T4-Fz and F8-Cz (Jacobson et al. 2011), the rDLPFC was identified as the area underlying F4, and the reference electrode was positioned above the left supraorbital area in all groups. An overview of the different tDCS montages used here is shown in Fig. 2 (see both Panel a and b). As anticipated earlier, we choose the right IFG as a stimulation site because we sought to extend previous findings on SST targeting this area with tDCS. Furthermore, we stimulated the right DLPFC, since previous studies suggest its involvement in several tasks probing response stopping (Hughes et al. 2014) as well as other inhibition-related phenomena (Beeli et al. 2008; Bermpohl et al. 2006; Penolazzi et al. 2014). Both at the beginning and at the end of the procedure, participants completed a self-report questionnaire about arousal and mood as further control to rule out alternative accounts of tDCS effects on response stopping. At the very end of the experiment, participants completed a self-report questionnaire (Fertonani et al. 2010) dealing with unpleasant sensations (if any) due to tDCS stimulation.
a Schematic illustration of the tDCS montages used in the study. Anodal electrodes are gray, cathodal electrodes have an oblique texture, inactive electrodes are transparent. Dotted lines in the Sham group indicate that for half participants the montage involved the rIFG, whereas for the remaining participants it included the rDLPFC. Electrodes are not drawn to scale. b Modeled image of the human head schematically showing the position of the electrodes in the two montages aimed at targeting the rIFG and the rDLPFC (Jung et al. 2013)
Results
SSRT and NSRT
Data from one participant of the Cathodal rIFG Group were lost due to a technical failure of the software. In order to investigate whether tDCS effectively and selectively modulated inhibitory performance in the SST, we first calculated SSRT and NSRT separately for each participant using the ANALYZE-IT software (Verbruggen et al. 2008), which comes as companion software to STOP-IT. To calculate individual SSRT, ANALYZE-IT first computes the mean RTs for all trials without a stop signal and then subtracts the mean stop-signal delay from this value (Verbruggen et al. 2008). First, we performed a between-participant ANOVA with Group as factor. Subsequently, we performed a series of independent samples t tests to compare SSRT of each experimental group with SSRT of the control, sham stimulation group. Independent samples t tests on NSRT were also carried out to assess any effect of the stimulation on RTs in go trials, which, if found, could be attributed to mechanisms different from those responsible of SSRT, thus undermining the selective effect of tDCS on response stopping. To minimize the occurrence of type II error while controlling for type I error, we adjusted the α level for the number of comparisons according to the False Discovery Rate procedure for multiple testing (Benjamini and Hochberg 1995). This latter approach is well established (e.g., Betta et al. 2007; Galfano et al. 2004; Stefan et al. 2005) and is particularly suited and powerful for analyzing RT data, as shown by Montecarlo studies (Pastore et al. 2008).
The tracking procedure was effective in keeping the overall probability (respond/signal) at about 0.5 for all participants. The main effect of Group in the ANOVA approached significance, F (4,114) = 2,221, p = 0.07. The FDR-corrected t tests revealed that only the comparison between SSRT of anodal rIFG group and control group showed a significant difference, t(53) = 2.281, p < 0.02, with lower SSRT (indicating better inhibitory performance) for the anodal right IFG group compared to the control group (see Fig. 3; Table 1). No significant differences between groups emerged on NSRT.
Analyses of questionnaires revealed no effect of stimulation on any of the items (i.e., mood/arousal and sensations perceived during stimulation) for participants assigned to sham and real stimulation groups. No differences in the percentage of correctly recalled word pairs (filler task) emerged as a function of group.
Discussion
Previous research has shown that delivering tDCS to the right prefrontal cortex can improve the ability to outright stop an initiated course of action (Jacobson et al. 2011), an inhibitory process often termed response stopping, which is recruited whenever a change in the context occurs and an overlearned, prepotent, behavioral response needs to be suppressed because inappropriate to the updated environment.
For the purpose of extending previous findings on the modulation of the response stopping ability, the present study tested the hypothesis that tDCS to the rIFG could improve SSRT even on a delayed SST, whereas in previous studies (Ditye et al. 2012; Jacobson et al. 2011) participants engaged in the SST immediately following tDCS application. Overall, the observed results confirmed this hypothesis, as SSRTs were lower for participants assigned to the rIFG anodal tDCS condition as compared to those assigned to the control group. Interestingly, the magnitude of such improvement was similar to that reported in previous studies (Ditye et al. 2012; Jacobson et al. 2011). This finding indicates that, in the domain explored by the present study, at least for brief post-stimulation periods (i.e., about 15 min), the magnitude of behavioral effects induced by tDCS does not seem to diminish. It is worth noting that this response stopping improvement is unlikely to result from a general cognitive enhancement. Indeed, it is more likely to reflect a specific effect on a process selectively deployed during stop trials, because NSRT analysis failed to show any significant between-group difference.
In sharp contrast, delivering tDCS to the rDLPFC did not affect response stopping. This is remarkable if one considers that the stimulation sites were closely contiguous (but see Penolazzi et al. 2013, for similar results with partially overlapping tDCS montages), and that tDCS is generally described as characterized by a low spatial resolution (especially when compared to other, more invasive, neurostimulation techniques such as TMS). This finding might be prone to several interpretations. One possibility is that rDLPFC may be not involved in the process of response stopping as measured in the SST. Notably, however, this would not necessarily imply that rDLPFC plays no role in inhibitory processing, given that this area is known to be involved in other tasks that probe this cognitive function (e.g., Beeli et al. 2008; Penolazzi et al. 2014). Another possibility is that the stimulation-induced engagement of rDLPFC is short-lasting and hence not evident in the present (delayed) protocol. Alternatively, tDCS parameters implemented in the protocol adopted in the present study, which were higher in both intensity and duration compared to previous studies (Ditye et al. 2012; Jacobson et al. 2011), could have been sub-optimal to produce an effective modulation of the rDLPFC. Likely, all the different factors illustrated above played some role in accounting for the absence of tDCS-induced modulations when targeting the rDLPFC. Further studies focusing on the manipulation of both tDCS parameters (e.g., density and duration) and stimulation-task delay will possibly shed light on the relative weight of the different alternatives illustrated above.
One may wonder whether the present findings may reflect a different engagement of the rIFG and rDLPFC networks by the filler tasks used during stimulation to delay the SST administration. We discard this alternative account based on neuroimaging evidence (e.g., Kuhl et al. 2007) revealing that both the rIFG and the rDLPFC are critically involved in the cognitive processes called into play by our filler memory tasks as parts of a broad prefrontal network considered to support cognitive control.
Recently, Hughes et al. (2014) have proposed that performance in the SST would be supported by two dissociable networks, one including the rIFG responsible for phasic, transiently activated, response stopping and the other comprising the rDLPFC involved in tonically maintaining the stopping rule (see also Chikazoe et al. 2009). Within this perspective, it could be well possible that perturbing the neural underpinnings of either process would produce different effects on response stopping, as the two processes could be not only differently sensitive to disruption or enhancement by means of tDCS, but even differently related to behavioral performance in the SST. The version of the SST implemented in the present experiment was more apt to probe the phasic, reactive, component of response inhibition. In this regard, a recent study by Cunillera et al. (2014) used a hybrid response stopping task which allowed investigating both the tonic and the phasic components of response inhibition. Both components were modulated by stimulating the rIFG. Using a similar task by targeting both the rIFG and the rDLPFC might represent a promising avenue to disentangle the specific contribution of these areas in the two types of response stopping.
Research so far suggests a role of the rIFG in response stopping. However, there is far from unanimous agreement on whether this inhibitory process is critically orchestrated by the IFG and mainly dependent on the right hemisphere (Aron et al. 2014; Banich and Depue 2015), or else results from the combined action of a more widespread network of areas (Schall and Godlove 2012; Swick et al. 2011). Moreover, studies addressing the role of areas other than the rIFG in response stopping obtained mixed results. For example, Hsu et al. (2011) modulated inhibition as measured by noncanceled rates in a SST by delivering tDCS over the pre-supplementary motor area, but failed to observe a significant effect on SSRT. Finally, Berryhill et al. (2014) failed to find any effect of a stimulation protocol similar to the one used by Hsu et al. (2011) on response inhibition in a go/no-go task (Swick et al. 2011).
As a final remark, given the importance of the reference electrode in determining the current flow distribution, it is worth noting that, in tDCS studies, findings should generally be ascribed to the combined effect of the active and the reference electrodes than to the effect of stimulated target areas in isolation. Therefore, our results are more likely to reflect the joint effect of stimulation of the rDLPFC and left frontal pole on the one hand, and stimulation of the rIFG and left frontal pole on the other hand. Nevertheless, it is important to note that, although the same reference was used, stimulation of two close but distinct areas resulted in different behavioural effects related to the phenomenon under investigation which, in turn, highlights that these two areas contributed to the investigated process to a different extent.
In summary, the results obtained in the present study support the notion that tDCS-induced effects can be relatively long-lasting by exploring a different cognitive domain with respect to those already investigated in the literature (Falcone et al. 2012; Penolazzi et al. 2010, 2013). Interestingly, the present findings add to the growing amount of evidence that the rIFG is critically involved in response stopping. In our opinion, the current state of the literature suggests that the rIFG is the most reliable target for brain stimulation studies aimed to modulate response stopping in the SST, and perhaps favored target for clinical investigations interested in developing therapeutic protocols based on NIBS (especially tDCS) with regard to clinical populations that suffer from lack of inhibitory control.
References
Anderson MC (2003) Rethinking interference theory: executive control and the mechanisms of forgetting. J Mem Lang 49:415–445
Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW (2003) Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci 6:115–116
Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA (2007) Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 27:3743–3752
Aron AR, Robbins T, Poldrack RA (2014) Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 18:177–185
Banich MT, Depue B (2015) Recent advances in understanding neural systems that support inhibitory control. Curr Opin Behav Sci 1:17–22
Batsikadze G, Moliadze V, Paulus W, Kuo M-F, Nitsche MA (2013) Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol 591:1987–2000
Beeli G, Casutt G, Baumgartner T, Jäncke L (2008) Modulating presence and impulsiveness by external stimulation of the brain. Behav Brain Funct 4:33
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57:289–300
Bermpohl F, Fregni F, Boggio PS, Thut G, Northoff G, Otachi PTM, Rigonatti SP, Marcolin MA, Pascual-Leone A (2006) Effect of low-frequency transcranial magnetic stimulation on an affective go/no-go task in patients with major depression: role of stimulation site and depression severity. Psychiatry Res 141:1–13
Berryhill ME, Peterson DJ, Jones KT, Stephens JA (2014) Hits and misses: leveraging tDCS to advance cognitive research. Front Psychol 5:800. doi:10.3389/fpsyg.2014.00800
Betta E, Galfano G, Turatto M (2007) Microsaccadic response during inhibition of return in a target-target paradigm. Vision Res 47:428–436
Bikson M, Datta A, Elwassif M (2009) Establishing safety limits for transcranial direct current stimulation. Clin Neurophysiol 120:1033–1034
Boisseau CL, Thompson-Brenner H, Caldwell-Harris C, Pratt E, Farchione T, Harrison Barlow D (2012) Behavioral and cognitive impulsivity in obsessive–compulsive disorder and eating disorders. Psychiatry Res 200:1062–1066
Brunoni AR, Shiozawa P, Truong D, Javitt DC, Elkis H, Fregni F, Bikson M (2014) Understanding tDCS effects in schizophrenia: a systematic review of clinical data and an integrated computation modeling analysis. Expert Rev Med Devices 11:383–394
Chambers CD, Bellgrove MA, Stokes MG, Henderson TR, Garavan H, Robertson IH, Morris AP, Mattingley JB (2006) Executive “brake failure” following deactivation of human frontal lobe. J Cogn Neurosci 18:444–455
Chevrier AD, Noseworthy MD, Schachar R (2007) Dissociation of response inhibition and performance monitoring in the stop signal task using event-related fMRI. Hum Brain Mapp 28:1347–1358
Chikazoe J, Jimura K, Hirose S, Yamashita K, Miyashita Y, Konishi S (2009) Preparation to inhibit a response complements response inhibition during performance of a stop-signal task. J Neurosci 29:15870–15877
Cunillera T, Fuentemilla L, Brignani D, Cucurell D, Miniussi C (2014) A simultaneous modulation of reactive and proactive inhibition processes by anodal tDCS on the right inferior frontal cortex. PLoS ONE 9:e113537. doi:10.1371/journal.pone.0113537
Depue BE, Burgess EG, Willcutt L, Ruzic MT, Banich MT (2010) Inhibitory control of memory retrieval and motor processing associated with the right lateral prefrontal cortex: evidence from deficits in individuals with ADHD. Neuropsychologia 48:3909–3917
Ditye T, Jacobson L, Walsh V, Lavidor M (2012) Modulating behavioral inhibition by tDCS combined with cognitive training. Exp Brain Res 219:363–368
Enticott PG, Ogloff JRP, Bradshaw JL (2008) Response inhibition and impulsivity in schizophrenia. Psychiatry Res 157:251–254
Falcone B, Coffman B, Clark VP, Parasuraman R (2012) Transcranial direct current stimulation augments sensitivity and 24-hour retention in a complex threat detection task. PLoS ONE 7:e34993. doi:10.1371/journal.pone.0034993
Feil J, Zangen A (2010) Brain stimulation in the study and treatment of addiction. Neurosci Biobehav Rev 34:559–574
Fertonani A, Rosini S, Cotelli M, Rossini PM, Miniussi C (2010) Naming facilitation induced by transcranial direct current stimulation. Behav Brain Res 208:311–318
Fillmore MT, Rush CR (2002) Impaired inhibitory control of behavior in chronic cocaine users. Drug Alcohol Depend 66:265–273
Galfano G, Betta E, Turatto M (2004) Inhibition of return in microsaccades. Exp Brain Res 159:400–404
Hsu T-Y, Tseng L-Y, Yu J-X, Kuo W-J, Hung DL, Tzeng OJL, Walsh V, Muggleton NG, Juan Juan C-H (2011) Modulating inhibitory control with direct current stimulation of the superior medial frontal cortex. Neuroimage 56:2249–2257
Hughes ME, Budd TW, Fulham WR, Lancaster S, Woods W, Rossell SL, Michie PT (2014) Sustained brain activation supporting stop-signal task performance. Eur J Neurosci 39:1363–1369
Jacobson L, Javitt DC, Lavidor M (2011) Activation of inhibition: diminishing impulsive behavior by direct current stimulation over the inferior frontal gyrus. J Cogn Neurosci 23:3380–3387
Jacobson L, Ezra A, Berger U, Lavidor M (2012a) Modulating oscillatory brain activity correlates of behavioral inhibition using transcranial direct current stimulation. Clin Neurophysiol 123:979–984
Jacobson L, Koslowsky M, Lavidor M (2012b) tDCS polarity effects in motor and cognitive domains: a meta-analytical review. Exp Brain Res 216:1–10
Jasper H (1958) The ten-twenty system of the International Federation. Electroencephalogr Clin Neurophysiol 10:371–375
Juan C-H, Muggleton NG (2012) Brain stimulation and inhibitory control. Brain Stimul 5:63–69
Jung Y-J, Kim J-H, Im C-H (2013) COMETS: A MATLAB toolbox for simulating local electric fields generated by transcranial Direct Current Stimulation (tDCS). Biomed Eng Lett 3:39–46
Krause B, Cohen Kadosh R (2013) Can transcranial electrical stimulation improve learning difficulties in atypical brain development? A future possibility for cognitive training. Dev Cogn Neurosci 6:176–194
Kuhl BA, Dudukovic NM, Kahn I, Wagner A (2007) Decreased demands on cognitive control reveal the neural processing benefits of forgetting. Nat Neurosci 10:908–914
Li CSR, Huang C, Constable RT, Sinha R (2006) Imaging response inhibition in a stop-signal task: neural correlates of signal monitoring and post-response processing. J Neurosci 26:186–192
Logan GD, Cowan WB (1984) On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev 91:295–327
Metuki N, Sela T, Lavidor M (2012) Enhancing cognitive control components of insight problem solving by anodal tDCS of the left dorsolateral prefrontal cortex. Brain Stimul 5:110–115
Nitsche MA, Liebetanz D, Lang N, Antal A, Tergau F, Paulus W (2003) Safety criteria for transcranial direct current stimulation (tDCS) in humans. Clin Neurophysiol 114:220–222
Nitsche MA, Doemkes S, Karakose T, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W (2007) Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol 97:3109–3117
Osman A, Kornblum S, Meyer DE (1986) The point of no return in choice reaction time: controlled and ballistic stages of response preparation. J Exp Psychol Hum Percept Perform 12:243–258
Pastore M, Nucci M, Galfano G (2008) Comparing different methods for multiple testing in reaction time data. J Mod App Stat Meth 7:120–139
Penolazzi B, Di Domenico A, Marzoli D, Mammarella N, Fairfield B, Franciotti R, Brancucci A, Tommasi L (2010) Effects of transcranial direct current stimulation on episodic memory related to emotional visual stimuli. PLoS ONE 5:e10623. doi:10.1371/journal.pone.0010623
Penolazzi B, Pastore M, Mondini S (2013) Electrode montage dependent effects of transcranial direct current stimulation on semantic fluency. Behav Brain Res 248:129–135
Penolazzi B, Stramaccia DF, Braga M, Mondini S, Galfano G (2014) Human memory retrieval and inhibitory control in the brain: beyond correlational evidence. J Neurosci 34:6606–6610
Schall JD, Godlove DC (2012) Current advances and pressing problems in studies of stopping. Curr Opin Neurobiol 22:1012–1021
Stefan K, Cohen LG, Duque J, Mazzocchio R, Celnik P, Sawaki L, Ungerleider L, Classen J (2005) Formation of a motor memory by action observation. J Neurosci 25:9339–9346
Swick D, Chatham CH (2014) Ten years of inhibition revisited. Front Hum Neurosci 8:329. doi:10.3389/fnhum.2014.00329
Swick D, Ashley V, Turken U (2011) Are the neural correlates of stopping and not going identical? Quantitative meta-analysis of two response inhibition tasks. Neuroimage 56:1655–1665
Vannorsdall TD, Schretlen DJ, Andrejczuk M, Ledoux K, Bosley LV, Weaver JR, Skolasky RL, Gordon B (2012) Altering automatic verbal processes with transcranial direct current stimulation. Front Psychiatry 3:73
Verbruggen F, Logan GD, Stevens MA (2008) STOP-IT: windows executable software for the stop-signal paradigm. Behav Res Methods 40:479–483
Wu M, Giel KE, Skunde M, Schag K, Rudofsky G, de Zwaan M, Zipfel S, Herzog W, Friederich HC (2013) Inhibitory control and decision making under risk in bulimia nervosa and binge-eating disorder. Int J Eat Disord 46:721–728
Acknowledgments
Part of this research was funded by grants awarded from the University of Padua to G.G., B.P., and S.M.
Conflict of interest
None of the authors have potential conflicts of interest to be disclosed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Stramaccia, D.F., Penolazzi, B., Sartori, G. et al. Assessing the effects of tDCS over a delayed response inhibition task by targeting the right inferior frontal gyrus and right dorsolateral prefrontal cortex. Exp Brain Res 233, 2283–2290 (2015). https://doi.org/10.1007/s00221-015-4297-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-015-4297-6