Abstract
The physical stumble caused by stepping onto a stationary (broken) escalator represents a locomotor aftereffect (LAE) that attests to a process of adaptive motor learning. Whether such learning is primarily explicit (requiring attention resources) or implicit (independent of attention) is unknown. To address this question, we diverted attention in the adaptation (MOVING) and aftereffect (AFTER) phases of the LAE by loading these phases with a secondary cognitive task (sequential naming of a vegetable, fruit and a colour). Thirty-six healthy adults were randomly assigned to 3 equally sized groups. They performed 5 trials stepping onto a stationary sled (BEFORE), 5 with the sled moving (MOVING) and 5 with the sled stationary again (AFTER). A ‘Dual-Task-MOVING (DTM)’ group performed the dual-task in the MOVING phase and the ‘Dual-Task-AFTEREFFECT (DTAE)’ group in the AFTER phase. The ‘control’ group performed no dual task. We recorded trunk displacement, gait velocity and gastrocnemius muscle EMG of the left (leading) leg. The DTM, but not the DTAE group, had larger trunk displacement during the MOVING phase, and a smaller trunk displacement aftereffect compared with controls. Gait velocity was unaffected by the secondary cognitive task in either group. Thus, adaptive locomotor learning involves explicit learning, whereas the expression of the aftereffect is automatic (implicit). During rehabilitation, patients should be actively encouraged to maintain maximal attention when learning new or challenging locomotor tasks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stepping onto a broken (stationary) escalator may cause a stumble and an odd sensation (Fukui et al. 2009), termed the ‘locomotor aftereffect’ (LAE) (Reynolds and Bronstein 2003, 2004; Bronstein et al. 2009), that results from prior adaptation to a moving escalator. The LAE occurs despite prior knowledge that the escalator is broken and will not move (Reynolds and Bronstein 2003, 2004; Bronstein et al. 2009). Indeed, transcranial direct current stimulation (tDCS) applied over the motor cortex before the adaptation task has been shown to enhance the LAE (Kaski et al. 2012), suggesting that the aftereffect relies upon cortical processing. The terms ‘adaptation’ and ‘motor (skill) learning’ often fall under the general term ‘motor learning’ (Krakauer and Mazzoni 2011). However, in this manuscript, we refer to motor adaptation as an error-based motor learning process occurring over minutes to hours that allows modification of motor strategies to maintain motor control in the face of an external perturbation (Bastian 2008) and differs from motor learning, which is a higher level cognitive process that involves the acquisition of a new motor skill that takes longer to achieve. The expression of the LAE is best described as adaptive locomotor learning, with repetition resulting in better performance (motor adaptation) as well as the formation/alteration of motor strategies (learning) (Bastian 2008; Taylor and Ivry 2012). The acquisition and expression of motor skills necessarily involve different neural processes; acquisition relies more upon attention resources than the expression of a learnt motor skill (Brashers-Krug et al. 1996; Shadmehr and Holcomb 1997).
Regarding the experimental ‘broken escalator’ paradigm, one unanswered question is whether attention modulates the LAE. In other words, is the LAE principally explicit (skill learning, requiring attention resources) or implicit (adaptive, independent of attention) or does it have components of both? Implicitly learnt motor strategies are less susceptible to dual-task interference than explicit tasks since they require less attentional resources for their execution (Liao and Masters 2001). Studying the LAE whilst imposing a secondary cognitive task (i.e. dual-tasking) in the adaptation (MOVING) and aftereffect (AFTER) phases allows us to address this question (Mazzoni and Krakauer 2006). If implicit, the LAE would be mainly unaffected by dual-tasking because adaptive locomotor learning occurs even when attentional resources are diverted by the simultaneous cognitive task. If explicit and attentional resources are needed for the cognitive task and for adaptive locomotor learning, adaptive learning in the MOVING phase would be significantly reduced, resulting in a reduced after effect. We thus investigated whether a secondary cognitive task (dual-tasking) would affect the adaptive learning and expression of the LAE. We hypothesised that dual-tasking during the adaptation phase would reduce the LAE, but not when dual-tasking during the expression of the LAE.
Methods
Experimental procedures
Subjects
Forty-eight healthy, naïve, consenting, adult participants were recruited from the student and staff at the local University Hospital; age ranges were 18–39 (further details below, under ‘Dual-Tasking’). The study was approved by the local ethics committee.
Equipment
Moving sled
The computer-controlled linear sled, running on a level track, was powered by two linear induction motors (Reynolds and Bronstein 2003; Bronstein et al. 2009). Sled velocity was recorded with a tachometer.
Movement analysis
Anterior–posterior upper trunk position was measured using a Fastrak™ electromagnetic tracking system (Polhemus, VT, USA) sampled at 250 Hz. The movement sensor was secured at the level of the C7 vertebra to measure linear trunk displacement, and the transmitter was attached to the sled. A second wall-mounted sensor recorded sled movement in the MOVING trials. Step timing was measured by contact plates on each foot and corroborated with a sled-mounted linear accelerometer.
EMG activity was quantitatively analysed from the medial gastrocnemius (MG) muscle of the left leg. This is the first leg to contact the sled and EMG activity responsible for braking (gait termination) is best visualised here (Bunday and Bronstein 2008, 2009). Signals were band-pass filtered (10–600 Hz) and sampled at 500 Hz.
Procedure
‘Broken escalator’ paradigm
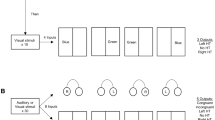
The experimental sequence (Fig. 1) comprised BEFORE (5 trials, stationary sled), MOVING (5 trials, moving sled, adaptation phase) and AFTER trials (5 trials, stationary sled, locomotor aftereffect phase). Performing 5 MOVING trials produces a robust LAE (Bunday et al. 2006; Kaski et al. 2012).
Illustration of the study design and examples of verbal and trunk responses in a single subject in one of the MOVING trials (a) and in the first AFTER trial (b). An error is exposed in Figure b (‘Onion’ is repeated twice). The auditory cue for stepping onset was activated when subjects spoke their first word on the cognitive task. The cognitive task was thereafter performed continually over the entire 16 s trial. DTM/DTAE: dual verbal task carried out during the moving and after trials, respectively
In all BEFORE, MOVING and AFTER trials, subjects stepped from a stationary platform onto the sled. All subjects began by standing 55 cm from the front of the sled, facing the direction of movement. The motor task was always to walk forwards from a stationary stance prompted by a single, brief auditory cue (beep), step with their right foot onto the fixed platform and then onto the sled with their left foot and thereafter stop and remain still with both feet in line.
In the MOVING trials, the onset of platform motion was triggered by breaking an infra-red light beam when the subject stepped forwards from the ‘start’ platform onto the sled. After breaking the beam, the sledmoved, with a 600 ms delay, and travelled a distance of approximately 3.7 m in 4.2 s; maximum velocity of 1.4 m/s was achieved at 1.3 s. Participants were asked to avoid using the handrails unless absolutely necessary. On completing the MOVING trials, participants were given the following information ‘I want you to step onto the sled as before. Only this time it is not going to move, and the motor is now going to be turned off. The sled will be stationary just like in the first test’—and the motor was ostensibly turned off, indicated by a key turning and the sound of the running motor ceasing. Each trial lasted 16 s after which the participants were returned to the original starting position.
Dual-tasking
The secondary cognitive task was to spontaneously verbalise names of vegetables, fruits and colours, in this order, prior to hearing the starting ‘beep’ and to repeat the task sequentially with different names until the end of that trial (e.g. ‘carrot, apple, green, potato, banana, blue’ etc., Fig. 1). Participants were asked not to repeat the same names used in a previous trial. Fruits were defined as ‘sweet and fleshy product of a tree or other plant that contains seed and can be eaten as food’, whereas a vegetable is ‘any edible part of a plant with a savoury flavour’. Where common ambiguities existed in fruit and vegetable categories (e.g. tomato), such responses were accepted as being correct. Participants were randomly assigned to three equally sized groups: the ‘control’ group (7 females/5 males; mean age 25 years) performed no dual task, the ‘Dual-Task MOVING (DTM)’ group (5 females/7 males; mean age 25 years) performed the dual task in the MOVING trials only and the ‘Dual-Task AFTEREFFECT (DTAE)’ group (6 females/6 males; mean age 22 years) performed the dual task in the AFTER trials only. To establish baseline values for performance of this dual task, 12 naïve subjects (5 females/7 males; mean age 28 years), age and intelligence-matched to subjects performing the motor task were asked to perform the cognitive task only. They performed five trials, each lasting 16 s. These subjects did not perform a motor task and will be referred to as the ‘Baseline’ group.
The responses were recorded in order to quantify verbal task performance. All participants were either native or bi-lingual English speakers.
Analysis
All locomotor measurements were as in our previous studies, where further details can be obtained (Reynolds and Bronstein 2003; Bronstein et al. 2009). Foot-sled contact was detected both from contact plates strapped under the feet and a sled-mounted accelerometer. Trunk displacement in the BEFORE and AFTER trials was the maximum forwards deviation of the trunk relative to the mean final trunk position in the last 3 s of the trial, providing a measure of the magnitude of the locomotor aftereffect. In MOVING trials, trunk displacement was measured as the maximum backwards–forwards (peak-to-peak) displacement after stepping onto the sled (Bunday and Bronstein 2008; Kaski et al. 2012). Gait velocity was calculated as the mean linear trunk velocity over a 0.5 s period prior to foot-sled contact. EMG signals from the left MG were rectified and integrated over a 500 ms time frame after foot-sled contact, and analysed as the area under curve. BEFORE trials 3–5 were averaged and used in the analyses (Kaski et al. 2012).
Cognitive task
We calculated the total number of words spoken during the entire 16 s recording and the total number of word errors (incorrect order, e.g. fruit, vegetable, colour; word repetition; or a word unrelated to the task). For the latter, an error percentage (Brown 1967; de Fockert et al. 2001) was calculated thus: number of errors/number of words spoken × 100; where a higher value would correspond to a higher error percentage. We did not observe any responses where there existed ambiguity about whether an item belonged to a fruit or a vegetable category.
Statistical analysis
Due to the different time course of the motion data in the three experimental phases, e.g. changing markedly as a function of trial number during MOVING trials but not during the BEFORE trials (see Fig. 2), the statistical approach consisted of performing three separate ANOVAs, one for each phase. Separate one-way ANOVAs were performed for BEFORE and AFTER trials to evaluate ‘Group’ effects (3 levels: Control, DTM and DTAE groups). For the MOVING trials, a two-way full factorial ANOVA (General linear model) was used with factors ‘Group’ (3 levels, Control, DTM and DTAE) and ‘Trial number’ (5 levels, trials 1–5). Additional information on the statistical approach for each condition is presented below.
Mean trunk displacement (±SD) for Control, Dual-Task MOVING (DTM) and Dual-Task AFTEREFFECT (DTAE) groups. A trunk displacement aftereffect was seen in all three subject groups shown by increased trunk displacement in AFTER trial 1 compared with the BEFORE phase (mean BEFORE trials 3–5). The DTM group had larger trunk sway in the MOVING trials but a smaller trunk displacement LAE, but there was no significant difference between the DTAE and Control groups
As in previous publications (Kaski et al. 2012) for the BEFORE condition, trials 1–2 are discarded as these are de facto practise trials. EMG data were not analysed in the MOVING trials as it becomes very noisy. To demonstrate the presence of an aftereffect, we compared AFTER versus BEFORE trials. As the aftereffect is mostly expressed in the first AFTER trial, we compare the data of AFTER trial 1 with baseline data (i.e. the average of BEFORE trials 3–5) using a one-way ANOVA, as in previous publications (Kaski et al. 2012). This statistical approach was applied to all motion variables (trunk displacement or ‘overshoot’, approach gait velocity and leg EMG) after log transformation.
The performance of the Cognitive task was assessed in terms of an error percentage (number of errors/number of words spoken × 100) per attempt. A two-way ANOVA was used to evaluate error percentages in the MOVING (DTM group), AFTER (DTAE group) and baseline conditions, with factors ‘Group’ (3 levels, baseline, DTM and DTAE) and ‘Attempt number’ (5 levels, 1–5).
When main effects were present: (a) ‘Group’ × ‘Trial/Attempt number’ interactions were examined and (b) post-hoc tests (Mann–Whitney) between groups were applied. For all analyses, P values <0.05 were considered significant.
Where additional tests (Spearman’s rank correlation coefficient) between variables were applied, these are explained in the “Results” section.
Results
As previously (Reynolds and Bronstein 2003; Bunday et al. 2006; Kaski et al. 2012), an aftereffect was observed for all variables (trunk sway, approach gait velocity and EMG) and all three subject groups. This was confirmed statistically with the one-way ANOVA comparing BEFORE trials 3–5 versus AFTER trial 1 for all variables and groups (F values range 5.5–58.7; P values range 0.029 − <0.001). Apart from this expected effect, our main finding was an increase in trunk sway during the MOVING trials and a reduction in the magnitude of the trunk displacement aftereffect in the DTM group. There now follows a detailed description of the results, displayed in Figs. 2, 3, 4 and 5.
Error percentages for the Baseline (dark grey), DTM (black) and DTAE (light grey) as a function of trial number. Error percentages were higher in the DTM (i.e. subjects who performed the dual task (cognitive/locomotor) in the MOVING trials) and DTAE (i.e. subjects who performed the dual task in the AFTER trials) groups compared to Controls with ANOVA. In addition, error percentages were higher in the DTM compared with the DTAE group attesting to dual-task interference
BEFORE trials
Gait velocity in all groups was within the range previously recorded for healthy subjects (Kaski et al. 2012) and accordingly one-way ANOVAs showed no main ‘Group’ effect for gait velocity, trunk overshoot or left MG EMG.
MOVING trials
As expected, during the MOVING trials, all subjects approached the sled at a faster velocity and showed larger trunk sway than during BEFORE trials (Fig. 2). Trunk sway was largest in the first MOVING trial in all three subject groups. Trunk sway diminished during successive trials in all groups (Fig. 2).
For trunk sway, we investigated ‘Group’ and ‘Trial number’ main effects by two-way ANOVA. As Fig. 2 illustrates, we found a significant main ‘Group’ effect [F(2,146) = 161, P < 0.001]. Post-hoc statistics showed larger trunk sway in the DTM group compared with controls (trial 4, P = 0.014) and in the DTM group compared with the DTAE group (trial 4, P = 0.049; trial 5, P = 0.006). As seen in Fig. 2, the DTM group had consistently greater levels of trunk sway in all trials than the other groups. As expected, we saw diminished trunk sway during successive trials as subjects adapted to the moving sled (i.e. main ‘Trial number’ effect, [F(4, 146) = 8.50, P < 0.001]). The rate of reduction in trunk sway was similar across the groups (i.e. no significant interaction between ‘Group’ and ‘Trial number’).
For gait velocity (Fig. 3), we investigated ‘Group’ and ‘Trial number’ main effects by two-way ANOVA. We found a significant main ‘Group’ effect [F(2, 164) = 7.25, P = 0.001]. Post-hoc statistics showed faster gait approach velocity in the DTAE group compared with controls in trial 1 (P = 0.030); this was owing to two faster walkers in this group [statistical significance was lost on removal of these two subjects]. There were no significant changes in gait velocity with successive trials i.e. no main ‘Trial number’ effect or ‘Group’ × ‘Trial number’ interaction, across all groups.
AFTER Trial 1
As in all previous studies with this paradigm (Reynolds and Bronstein 2003; Green et al. 2010; Kaski et al. 2012; Tang et al. 2013), the LAE was present in AFTER trial 1 in all groups We investigated ‘Group’ differences by one-way ANOVA. We found a main ‘Group’ effect for the size of trunk overshoot [F(2,35) = 4.05, P = 0.027] (Fig. 2). Post-hoc statistics showed smaller trunk overshoot in the DTM group compared with controls (P = 0.021). There was no significant difference between the DTAE group compared with controls. No significant main ‘Group’ effect was found for gait velocity (Fig. 3). A marginal main ‘Group’ effect was found for Left MG EMG [F(2,35) = 3.22, P = 0.054]. EMG activity was discernibly smaller in the DTM group (Fig. 4).
Additional statistical tests showed that the reduced aftereffect magnitude in the DTM group was not associated with slower gait velocity in the MOVING trials (Spearman’s rank correlation coefficient = −0.466; P = 0.128).
Cognitive responses
The cognitive task was to spontaneously verbalise a series of categories; ‘vegetable, fruit, colour’ in this order. The task was scored in terms of an error percentage per attempt (5 attempts; error % = total number of errors/total number of words spoken × 100). As expected with this cognitive task, mean error percentages were smallest for the first attempt and increased during successive attempts (Fig. 5).
All three groups verbalised similar numbers of words, 4.9–6.0 words per attempt and, as Fig. 5 shows, all groups found the task progressively difficult (mean error percentages rose between attempts 1–5 i.e. a main ‘Attempt number’ effect [F(4, 165) = 13.27, P < 0.001]).
A significant main ‘Group’ effect was found [F(2, 165) = 30.24, P < 0.001]. These group differences were related to trial number as shown by a significant interaction between ‘Group’ × ‘Attempt number’ [F(8, 165) = 2.17, P = 0.032]. Post-hoc statistics showed higher mean error percentages in the DTM group compared with controls (attempts 3, 4 and 5, P values range 0.008 − <0.001) and in the DTM group compared with the DTAE group (attempts 3 and 4, P values range 0.002 − <0.001). A marginally higher mean error percentage was also seen in the DTAE group compared to controls (attempts 1 and 3, P values range 0.038–0.047).
There was no correlation between the trunk displacement aftereffect in AFTER trial 1 and mean error percentage in attempt 1 of the DTM group (Spearman correlation coefficient = 0.376, P = 0.229) or DTAE group (Spearman correlation coefficient = −0.044, P = 0.892).
Discussion
We show a smaller trunk displacement aftereffect when dual-tasking during the adaptation (MOVING) phase of the ‘broken escalator’ paradigm, but not during the AFTER phase. Given that the magnitude of the trunk displacement aftereffect is a reflection of the learning process during the MOVING trials, the decreased aftereffect size likely reflects impaired motor adaptation.
Contemporaneous cognitive tasks can affect the performance of a primary motor task if general resources are shared and insufficient to complete both simultaneously (Gresty and Golding 2009). Such dual-task interference was apparent in subjects performing a secondary cognitive task in the MOVING phase, manifesting as both greater trunk sway and by a reduced LAE size. Hence, adaptive locomotor learning depends on appropriate attention resources and involves an explicit mode of learning. This is consistent with functional imaging studies that have shown activation of similar neuronal systems [dorsolateral prefrontal cortex (Holtzer et al. 2011) and anterior cingulate gyrus (Shadmehr and Holcomb 1997; Grossman et al. 2002; Rosenthal et al. 2009)] during explicit motor learning and whilst performing cognitive characterisation tasks. In agreement with our current results, which suggests a cortical basis for the ‘broken escalator’ LAE is our previous finding that the LAE is enhanced with neurostimulation to midline primary motor and premotor cortex (Kaski et al. 2012).
Conversely, the expression of the LAE in the DTAE group (AFTER trial 1) was unaffected by dual-tasking, implying that resources are not shared between the cognitive task and the motor task in the AFTER phase i.e. an automatic (or implicit) expression of this adaptive learning response. As indicated by Schmidt (2005), ‘Automaticity is any process which can be performed without interference from a mental-task involving (conscious) information-processing activities’. Once learnt, certain motor strategies are executed automatically (implicitly) (Voss et al. 2008), hastening the response (Mazzoni and Krakauer 2006) and freeing attention resources for other activities (Malone and Bastian 2010). Contextual cues presumably dictate whether the previously learnt motor programme has to be released (the sled or escalator may move) or not (a solid platform or stairs will not move) (Reynolds and Bronstein 2004; Fukui et al. 2009), probably through an internal probabilistic risk assessment (Green et al. 2010). Breaching this contextual threshold releases the LAE even whilst performing a secondary cognitive task. Indeed, introspection suggests that the LAE occurs in everyday settings on a broken escalator even whilst talking or on a mobile phone.
Although the rates of trunk sway reduction in the MOVING trials were similar across all groups (see trunk displacement in Fig. 2), trunk displacement was greater in the DTM group compared with the DTAE and control groups across all trials, similar to dual-tasking on a split-belt treadmill (Malone and Bastian 2010). The smaller trunk displacement aftereffect observed in the DTM group may thus relate to a constant level of deficit induced by the cognitive interference (analogous to a DC offset, in engineering terms) and reflect reduced adaptive learning. Thus, dual-tasking did not alter the rate at which the motor task was learnt, but rather introduced an offset in the adaptation performance. Such a dissociation may reflect a temporal difference—the rate of adaptation may be less susceptible to cognitive interference, whereas the retention of novel motor strategies takes longer to achieve— and may be more readily affected by a secondary cognitive task.
Unlike the trunk displacement aftereffect in the DTM group, the gait velocity aftereffect was unaffected by dual-tasking, supporting the view that different neural mechanisms underpin these two aftereffect components (Tang et al. 2013). This is also evident in the low-level correlation present between the magnitude of the trunk displacement LAE and gait velocity LAE (Bronstein et al. 2009). In the current experiment, DTM subjects had greater trunk displacement amplitudes once upon the moving sled—at which point gait velocity is nil. It could be argued that our cognitive task was sufficient to interfere with a difficult motor task (swaying on a moving sled) but not to modify a relatively easy task such as unperturbed walking (i.e. gait velocity) in the DTM group. It is usually in advanced cerebral dysfunction when dual-tasking (Beauchet et al. 2009) interferes with simple walking. Two subjects in the DTAE group walked much faster than others during the MOVING trials, which increased the group average gait velocity in MOVING trials (Fig. 3); the variability between gait velocity in the MOVING trials and magnitude of the trunk LAE in all groups indicate that this finding can be disregarded.
Our cognitive task interfered with adaptive locomotor learning and vice versa. Poorer performance of the cognitive task was observed in the DTM compared with the DTAE group (Fig. 5), which may relate to task prioritisation, the ‘posture first principle’ (Lajoie et al. 1993; Yardley et al. 2001; Gresty and Golding 2009). In dual-task experiments of upper limb motor adaptation, performance of a cognitive task governed the level of motor learning; subjects who performed the cognitive task well had reduced motor learning (Taylor and Thoroughman 2008). Conversely, dual-tasking can improve motor performance in some tasks (Goh et al. 2012), perhaps because a secondary task that does not compete for shared resources may inadvertently increase arousal.
Study limitations
The interference between a cognitive and motor task may depend upon various factors including the nature and difficulty of the motor and secondary cognitive task (Hemond et al. 2010). One potential confound to our data is that the adaptation and LAE expression processes have different levels of motor difficulty, perhaps leading to differential susceptibility to dual-task interference. That the baseline group (cognitive task only) performed better than subjects in both DTM and DTAE groups (i.e. lower error percentage) suggests that the motor tasks were of sufficient difficulty to interfere with the cognitive task (and vice versa). Although we cannot comment on whether differences in motor task difficulty could account for differences in the LAE during dual-tasking between DTM and DTAE groups, we found no correlation between performance of the cognitive and locomotor tasks.
Secondly, we did not test whether other cognitive tasks, such as verbal or spatial Stroop tests (Barra et al. 2006), Brooks Matrix tests (Gresty et al. 2003), have similar effects. The advantages of our chosen dual-task are that it allowed error percentage scoring (Schmidt 2005) and was increasingly difficult to perform without errors. Indeed, our cognitive task was of sufficient difficulty to produce errors in a baseline group that did not perform a dual-task. When dual-tasking, cognitive tasks require a sufficient degree of complexity so as to influence the motor task and vice-versa (Chen et al. 2013), features apparent in our experiments, i.e. a bi-directional dual-task interference, was observed in the DTM group and cognitive-task interference in the DTAE group but without interference of LAE expression.
Conclusions
We have shown that the ‘broken-escalator’ paradigm involves an explicit mode of learning. An explicit mode of learning presumably offers flexibility to accommodate challenging environments (Torres-Oviedo et al. 2011) and multitask in day-to-day activities.
Diverting attention during the MOVING phase of the ‘broken escalator’ paradigm results in a reduced LAE size. This supports previous evidence suggesting cortical involvement in this task (Kaski et al. 2012). However, the first aftereffect is not subject to cognitive interference, suggesting that the degree of automaticity is greater when expressing the aftereffect. These findings may be clinically relevant for locomotor rehabilitation. When learning new or challenging locomotor tasks during rehabilitation, patients should be encouraged to maintain their full attention to enhance adaptive locomotor learning.
References
Barra J, Bray A, Sahni V, Golding JF, Gresty MA (2006) Increasing cognitive load with increasing balance challenge: recipe for catastrophe. Exp Brain Res 174:734–745. doi:10.1007/s00221-006-0519-2
Bastian AJ (2008) Understanding sensorimotor adaptation and learning for rehabilitation. Curr Opin Neurol 21:628–633. doi:10.1097/WCO.0b013e328315a293
Beauchet O, Annweiler C, Dubost V et al (2009) Stops walking when talking: a predictor of falls in older adults? Eur J Neurol 16:786–795. doi:10.1111/j.1468-1331.2009.02612.x
Brashers-Krug T, Shadmehr R, Bizzi E (1996) Consolidation in human motor memory. Nature 382:252–255
Bronstein AM, Bunday KL, Reynolds R (2009) What the “broken escalator” phenomenon teaches us about balance. Ann N Y Acad Sci 1164:82–88. doi:10.1111/j.1749-6632.2009.03870.x
Brown ID (1967) Measurement of control skills, vigilance, and performance on a subsidiary task during 12 hours of car driving. Ergonomics 10:665–673. doi:10.1080/00140136708930920
Bunday KL, Bronstein AM (2008) Visuo-vestibular influences on the moving platform locomotor aftereffect. J Neurophysiol 99:1354–1365. doi:10.1152/jn.01214.2007
Bunday KL, Bronstein AM (2009) Locomotor adaptation and aftereffects in patients with reduced somatosensory input due to peripheral neuropathy. J Neurophysiol 102:3119–3128. doi:10.1152/jn.00304.2009
Bunday KL, Reynolds RF, Kaski D, Rao M, Salman S, Bronstein AM (2006) The effect of trial number on the emergence of the ‘broken escalator’ locomotor aftereffect. Exp Brain Res 174:270–278. doi:10.1007/s00221-006-0446-2
Chen C, Leys D, Esquenazi A (2013) The interaction between neuropsychological and motor deficits in patients after stroke. Neurology 80:S27–S34. doi:10.1212/WNL.0b013e3182762569
de Fockert JW, Rees G, Frith CD, Lavie N (2001) The role of working memory in visual selective attention. Science 291:1803–1806. doi:10.1126/science.1056496
Fukui T, Kimura T, Kadota K, Shimojo S, Gomi H (2009) Odd sensation induced by moving-phantom which triggers subconscious motor program. PLoS ONE 4:e5782. doi:10.1371/journal.pone.0005782
Goh HT, Sullivan KJ, Gordon J, Wulf G, Winstein CJ (2012) Dual-task practice enhances motor learning: a preliminary investigation. Exp Brain Res 222:201–210. doi:10.1007/s00221-012-3206-5
Green DA, Bunday KL, Bowen J, Carter T, Bronstein AM (2010) What does autonomic arousal tell us about locomotor learning? Neuroscience 170:42–53. doi:10.1016/j.neuroscience.2010.06.079
Gresty MA, Golding JF (2009) Impact of vertigo and spatial disorientation on concurrent cognitive tasks. Ann N Y Acad Sci 1164:263–267. doi:10.1111/j.1749-6632.2008.03744.x
Gresty MA, Waters S, Bray A, Bunday K, Golding JF (2003) Impairment of spatial cognitive function with preservation of verbal performance during spatial disorientation. Curr Biol 13:R829–R830
Grossman M, Smith EE, Koenig P, Glosser G, DeVita C, Moore P, McMillan C (2002) The neural basis for categorization in semantic memory. Neuroimage 17:1549–1561
Hemond C, Brown RM, Robertson EM (2010) A distraction can impair or enhance motor performance. J Neurosci 30:650–654. doi:10.1523/JNEUROSCI.4592-09.2010
Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J (2011) fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci 66:879–887. doi:10.1093/gerona/glr068
Kaski D, Quadir S, Patel M, Yousif N, Bronstein AM (2012) Enhanced locomotor adaptation aftereffect in the “broken escalator” phenomenon using anodal tDCS. J Neurophysiol 107:2493–2505. doi:10.1152/jn.00223.2011
Krakauer JW, Mazzoni P (2011) Human sensorimotor learning: adaptation, skill, and beyond. Curr Opin Neurobiol 21:636–644. doi:10.1016/j.conb.2011.06.012
Lajoie Y, Teasdale N, Bard C, Fleury M (1993) Attentional demands for static and dynamic equilibrium. Exp Brain Res 97:139–144
Liao CM, Masters RS (2001) Analogy learning: a means to implicit motor learning. J Sports Sci 19:307–319. doi:10.1080/02640410152006081
Malone LA, Bastian AJ (2010) Thinking about walking: effects of conscious correction versus distraction on locomotor adaptation. J Neurophysiol 103:1954–1962. doi:10.1152/jn.00832.2009
Mazzoni P, Krakauer JW (2006) An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26:3642–3645. doi:10.1523/JNEUROSCI.5317-05.2006
Reynolds RF, Bronstein AM (2003) The broken escalator phenomenon. After-effect of walking onto a moving platform. Exp Brain Res 151:301–308. doi:10.1007/s00221-003-1444-2
Reynolds RF, Bronstein AM (2004) The moving platform after-effect: limited generalization of a locomotor adaptation. J Neurophysiol 91:92–100. doi:10.1152/jn.00495.2003
Rosenthal CR, Roche-Kelly EE, Husain M, Kennard C (2009) Response-dependent contributions of human primary motor cortex and angular gyrus to manual and perceptual sequence learning. J Neurosci 29:15115–15125. doi:10.1523/JNEUROSCI.2603-09.2009
Schmidt RA (2005) Motor control and learning: a behavioural emphasis, 4th edn
Shadmehr R, Holcomb HH (1997) Neural correlates of motor memory consolidation. Science 277:821–825
Tang KS, Kaski D, Allum JH, Bronstein AM (2013) The effect of gait approach velocity on the broken escalator phenomenon. Exp Brain Res 226:335–346. doi:10.1007/s00221-013-3438-z
Taylor JA, Ivry RB (2012) The role of strategies in motor learning. Ann N Y Acad Sci 1251:1–12. doi:10.1111/j.1749-6632.2011.06430.x
Taylor JA, Thoroughman KA (2008) Motor adaptation scaled by the difficulty of a secondary cognitive task. PLoS ONE 3:e2485. doi:10.1371/journal.pone.0002485
Torres-Oviedo G, Vasudevan E, Malone L, Bastian AJ (2011) Locomotor adaptation. Prog Brain Res 191:65–74. doi:10.1016/B978-0-444-53752-2.00013-8
Voss M, Ingram JN, Wolpert DM, Haggard P (2008) Mere expectation to move causes attenuation of sensory signals. PLoS ONE 3:e2866. doi:10.1371/journal.pone.0002866
Yardley L, Gardner M, Bronstein A, Davies R, Buckwell D, Luxon L (2001) Interference between postural control and mental task performance in patients with vestibular disorder and healthy controls. J Neurol Neurosurg Psychiatry 71:48–52
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, M., Kaski, D. & Bronstein, A.M. Attention modulates adaptive motor learning in the ‘broken escalator’ paradigm. Exp Brain Res 232, 2349–2357 (2014). https://doi.org/10.1007/s00221-014-3931-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-014-3931-z