Abstract
The interhemispheric interactions between homologous wrist extensor and flexor muscles representations in the right and left primary cortex (M1) were studied using a paired-pulse transcranial magnetic stimulation in healthy subjects. The magnitude of interhemispheric inhibition (IHI) was studied in 9 right-handed subjects at short (10 ms, SIHI) and long (40 ms, LIHI) interstimulus intervals between the magnetic conditioning (CS) and test stimulus in the motor dominant to non-dominant cortex and vice versa, while the right or left hand was at rest or performing a unimanual sustained tonic contraction (holding a pen with the hand contralateral to the CS). A bidirectional powerful interhemispheric inhibition could be elicited at the short and long IHI phases (SIHI and LIHI) in wrist extensor and flexor muscles in most of the subjects at rest. SIHI but not LIHI was significantly bidirectionally reduced during unimanual contraction of the hand contralateral to the CS stimulation in comparison with rest. The amount of IHI after the stimulation of the “non-dominant” right hemisphere was not reduced in comparison with IHI after stimulation of the “dominant” left hemisphere whatever the active or resting condition. IHI directed to the wrist muscles had a similar level than IHI directed to digit muscles (FDI) at rest. Our data indicate that contralateral wrist muscles activity evokes a global, bidirectional reduction in IHI which was more pronounced for SIHI. These results provide additional evidence that changes in interhemispheric interactions between the M1s are involved in the control of unimanual movements including suppression of unwanted motor activity in the opposite limb during unilateral movements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In humans, interhemispheric interactions between homologous muscle representations may be probed using a paired-pulse transcranial magnetic stimulation (TMS) technique whereby a conditioning stimulus (CS) is applied to the muscle representation in one hemisphere followed by a test stimulus (TS) to the contralateral hemisphere. This technique has revealed at least three facilitatory and two inhibitory distinct interactions, depending on the parameters used (interstimulus interval (ISI), coil orientation and intensities of CS and TS) (Ferbert et al. 1992; Hanajima et al. 2001; Daskalakis et al. 2002). Facilitation or inhibition can even be produced at overlapping ISIs, depending on the nature of the CS and TS, suggesting that such interactions are likely to occur in parallel.
A single CS inhibits the motor-evoked potential (MEP) generated by the second TS applied over the contralateral motor cortex M1 when the conditioning–test interval (ISI) is between 6 and 50 ms (Ferbert et al. 1992; Gerloff et al. 1998). Further studies have reported that IHI between homologous M1s at ISIs of ≈10 ms (short latency IHI, SIHI) and ≈40 ms (long latency IHI, LIHI) may have different physiological origins (Chen et al. 2003; Kukaswadia et al. 2005; Talelli et al. 2008). There is good evidence that inhibition of the test MEP occurs at the cortical level (Ferbert et al. 1992; Di Lazzaro et al. 1999; Hanajima et al. 2001) although subcortical sites may also be involved (Gerloff et al. 1998).
The technique described by Ferbert et al. (1992) was used to assess the modulation of IHI before and during the movement performed by the dominant and non-dominant hand in normal subjects (Chen et al. 2003; Duque et al. 2005b, 2007; Perez and Cohen 2008; Nelson et al. 2009; Hinder et al. 2010) in the chronic recovery phase of stroke patients (Murase et al. 2004; Duque et al. 2005a) or in focal hand dystonia (Beck et al. 2009; Nelson et al. 2010). Opposite changes of IHI during muscle contraction were reported depending on the methodology used to study this modulation in comparison with rest: increase in IHI during contraction if conditioning magnetic intensities were not adjusted between the rest and active condition (Ferbert et al. 1992; Perez and Cohen 2008; Talelli et al. 2008; Hinder et al. 2010), and decrease in IHI during contraction if the intensity of CS was decreased in the active condition in comparison with rest in order to match the amplitude of the conditioned MEPs across conditions (Chen et al. 2003; Chen 2004; Perez and Cohen 2008; Nelson et al. 2009, 2010).
There is also some evidence that task-related changes in SIHI may be distinct to the ones seen in LIHI (Chen et al. 2003; Talelli et al. 2008), but these differences in movement modulation between SIHI and LIHI were no longer observed in a more recent study (Nelson et al. 2009). SIHI and LIHI may be altered differently depending on the type of movement or level of muscle contraction, which was not always comparable in these previous studies.
Most of the previous IHI studies cited above were conducted on digit muscles (FDI, APB, ADM), except the pioneering studies of Ferbert et al. (1992) and Gerloff et al. (1998), which examined in control experiments whether IHI could be demonstrated in flexor carpi radialis (FCR) or biceps muscles. Differences in SIHI level at rest between proximal (triceps brachii) and distal (FDI) muscles were reported by Harris-Love et al. (2007). IHI studies on wrist muscles are sparse: SIHI in left FCR muscle was studied at rest and when the right wrist was flexed at different forces (Perez and Cohen 2008) or in left ECR during a right APB muscle contraction (Hinder et al. 2010). In these studies, IHI in wrist muscles was only studied at short interval (10 ms) and not bidirectionally.
The purpose of the present study was to investigate, with the paired-pulse TMS method, SIHI and LIHI in the wrist extensor and flexor muscles (FCR and ECR) of the dominant and non-dominant hand of normal right-handed subjects, at rest and during a unimanual sustained tonic contraction of wrist muscles performed by the contralateral hand (holding a pen with the dominant or non-dominant hand). Our aim was to see any evidence of a hand dominance effect for wrist muscles regarding the direction of IHI (interhemispheric asymmetry of transcallosal inhibition) and any difference in modulation between SIHI and LIHI during the motor task.
Methods
Subjects
Nine right-handed healthy subjects (4 women, 5 men, aged 22–54 years; mean age ± SD, 36 ± 14) were studied. Handedness (laterality quotient) was confirmed at 0.86 ± 8.6 using the Oldfield Handedness Inventory (Oldfield 1971). All subjects provide written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the Toulouse Hospital Ethic Board.
Recordings
Surface electromyograms (EMG) were simultaneously recorded from the left and right extensor carpi radialis (ECR) and flexor carpi radialis (FCR) muscles with Ag–AgCl surface electrodes. EMG signals were amplified (1,000×, DIGITIMER D360), filtered (20 Hz–2 kHz), digitized and fed via a CED (Cambridge Electronic Design, OXFORD Instruments, UK) laboratory interface (sampling frequency, 5 kHz) to a PC for display, storage and off-line analysis. The EMG signal of the four channels was also displayed on an oscilloscope (TEXTRONIX) in order to monitor the resting and active conditions (see below).
Transcranial magnetic stimulation
TMS was delivered to the motor cortex bilaterally using two Magstim 200 stimulators (The Magstim Co., Dyfed, UK) and through two figures of eight coils (outside diameter of each wing 9.5 cm) with the handle of the coils pointed backward at approximately 45° from the mid-sagittal line. The optimal site for evoking responses in the contralateral resting wrist muscles was determined for each hemisphere.
A conditioning stimulus (CS) to the optimal site on the scalp for wrist muscle of one hemisphere was followed by a test stimulus applied to the optimal homologous hot spot in the opposite hemisphere. Two CS–TS interstimulus intervals (ISI) were studied: a short one (10 ms) for the short IHI (SIHI) and a longer one (40 ms) for the long (IHI) (Nelson et al. 2009). Single data blocks of 30 trials randomly alternated every 5 s consisted of 10 trials of paired-pulse CS–TS with a 10-ms ISI, 10 trials of paired-pulse CS–TS with a 40-ms ISI and 10 trials of TS alone.
Experimental protocol
Two conditions were tested: 1/rest, whereby both hands were completely relaxed as determined by online EMG recordings; and 2/pen hold in the right or left hand with the contralateral hand at rest: around 10–20 % MVC isometric left or right wrist muscle contraction (mainly ECR) while holding a pen between the thumb and index finger with the pen tip in contact with the paper. During the acquisition period, subjects were also asked to imagine they were writing a sentence with the hand holding the pen, but without moving the hand. This was proposed to improve their attention and better focus on the hand doing the task.
For each subject, we recorded firstly IHI at rest in both directions (IHI from the right to left M1 and vice versa with the order of IHI directions randomized across participants). Visual feedback of the 4 EMG channels was given to ensure that subjects remained relaxed during the resting condition. Following the two blocks recorded in the resting condition, two successive further blocks during isometric left or right wrist contraction (the sides of isometric contraction being randomized across participants) were recorded, each of them with the CS contralateral to the hand holding the pen and the TS contralateral to the resting hand (active condition). The upper part of the Figs. 1 and 2 illustrates the experimental set-up and the different conditions tested. The constant level of wrist EMG isometric contraction in the active hand was monitored by the oscilloscope, and visual feedback of contralateral homologous EMG was given to ensure that the wrist muscles of the contralateral hand remained at rest.
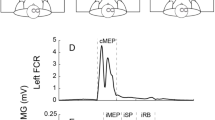
Upper part experimental set-up for studying with paired-pulse TMS IHI in left ECR in the rest and active conditions (right pen hold with the CS contralateral to the active hand). Bottom part superposition of 8 MEP tests and 8 MEPs conditioned at both ISIs obtained in one representative subject for the left ECR muscle, in the rest (left panel) and active conditions (right panel). The size of the MEPs decreased after the CS (interhemispheric inhibition) in comparison with the TS for both ISIs and in both conditions, but the decrease was more marked in the rest condition than in the active
Upper part experimental set-up for right FCR muscle: left rest, right active condition with left pen hold and CS contralateral to the left hand. Bottom part superposition of 8 MEP tests and 8 MEPs conditioned at both ISIs obtained in one representative subject for the right FCR muscle, in the rest (left panel) and active conditions (right panel). The size of the MEPs decreased after the CS (interhemispheric inhibition) in comparison with the TS for both ISIs and in both conditions
The intensities of both the conditioning and test stimuli were adjusted to elicit a MEP of ≈0.8–1.5 mV in their respective contralateral ECR and FCR muscles, depending on whether the contralateral wrist muscles were at rest or active. When the hand contralateral to the TS pulse was at rest, the TMS intensity was adjusted to evoke a 0.8–1.5 mV MEP in that relaxed hand. When the hand contralateral to the CS pulse was active, TMS intensity was adjusted to evoke a 0.8–1.5 mV MEP in the active muscle (slightly decreased in comparison with rest) in order to normalize IHI to the increase in corticospinal excitability caused by voluntary contraction (Perez and Cohen 2008). We used a ‘matching’ technique (Nelson et al. 2009) whereby the CS intensity was set at the output to evoke ~0.8–1.5 mV MEP amplitude when wrist muscles were active or relaxed since it has been previously shown that IHI modulation is dependent on the MEP size (Ferbert et al. 1992). We adjusted the CS TMS intensity to maintain MEP amplitudes across the two conditions (rest/active) and to match the degree of corticospinal output across conditions, assuming that both the corticospinal system and the IHI are modulated in a similar manner by the voluntary activity (Avanzino et al. 2007). Trials contaminated by EMG in the resting hand were rejected on- and offline.
Data analyses
Because of the frequent polyphasic shape of the MEPs in the wrist muscles, responses were rectified offline to measure the area of the ECR and FCR MEPs, using Signal V4 software. The paired-pulse MEP area was expressed as a ratio of the mean unconditioned MEP area (TS alone) for each subject and each condition. Ratios below one represent inhibition of the MEP, and the ratios above one represent facilitation. The mean area of the prestimulus EMG was calculated for a 30-ms window prior to the first TMS pulse for each trial in each condition, using Signal V4 software. This measure was used to analyse the background EMG in ECR and FCR wrist muscles during the postural task (holding a pen) and to compare it to the background EMG obtained in the resting condition.
Significant reduction in MEP amplitude with conditioning stimulation was assessed using one-sample two-tailed Student’s t tests against the null hypothesis of a population mean ratio of 1 (conditioned responses = unconditioned response). Significance for multiple t tests was set at 0.01 level.
Repeated measures ANOVA was used to test on MEP ratios (MEP CS/MEP TS), the effect of IHI direction (2 levels: non-dominant to dominant/dominant to non-dominant), CONDITION (3 levels: rest/right pen hold/left pen hold), CS–TS interval ISI (2 levels: 10 (SIHI)/40 ms (LIHI)) and MUSCLE (2 levels: ECR/FCR). The prestimulus EMG levels and MEP test sizes were tested each separately using ANOVA with 3 factors: SIDE (2 levels: left/right), CONDITION (3 levels: rest/right pen hold/left pen hold) and MUSCLE (2 levels: ECR/FCR) and 2 factors for TMS intensities (SIDE and CONDITION). Post hoc tests were performed using the Bonferroni t test. The level of significance was set at p < 0.05.
Results
TMS intensities
The mean ± SD TMS intensities used for the experiments in the 4 conditions are given in Table 1. There were no significant SIDE differences for the TS (right M1, 56.6 ± 8.3/left M1, 58 ± 7.9; p = 0.16; F(1.8) = 3) TMS stimulator intensities used to achieve a MEP size of ≈0.8–1.5 mV in ECR and FCR muscles, demonstrating similar level of excitability across the two hemispheres. For the CS, there was a trend for a higher intensity used to stimulate left M1 (58.2 ± 6.2) in comparison with right M1 (56.4 ± 6.6), but the differences were not statistically significant (p = 0.06; F(1.8) = 5.21). During contralateral muscles contraction, CS TMS intensities, required to evoke MEPs of similar amplitude than at rest, were significantly slightly decreased (CONDITION CS, p = 0.007, F(1.8) = 12.91; TS, p = 0.1, F(1.8) = 2.3, see Table 1).
MEP test area
There was no significant SIDE (p = 0.1; F(1.8) = 2.95), MUSCLE (p = 0.1; F(1.8) = 3.2) or CONDITION (p = 0.7; F(1.8) = 0.16) differences for the mean MEPs test areas ±SD (Table 1), confirming that the “matching technique” used to obtain MEP test areas of similar sizes in the resting and active conditions was effective.
Prestimulus EMG
Group-averaged prestimulus EMG area from the right and left ECR and FCR muscles in the different conditions (rest, left or right pen hold) is shown in Fig. 3. White bars indicate prestimulus EMG for the hand at rest, and grey bars indicate the EMG level during the task of holding a pen with the right or left hand. EMG was significantly greater for the hand performing the task compared to that at rest for ECR (left and right) but not for FCR muscle (MUSCLE, p = 0.01, F(1.8) = 10.75; CONDITION, p = 0.0001, F(1.8) = 73.55; SIDE, p = 0.3, F(1.8) = 1.08) with a significant interaction MUSCLE x CONDITION (p = 0.001 F(1.8) = 25). Post hoc test revealed that prestimulus EMG area of ECR muscle during the task of holding a pen was significantly greater than EMG area of ECR muscle at rest (p < 0.0001), FCR muscle at rest (p < 0.0001) and also FCR muscle under active conditions (p = 0.003). FCR EMG area was not statistically different between rest and active conditions.
Prestimulus EMG area recorded from left and right ECR (left panel) and left and right FCR (right panel) during rest (conditioning stimulus (CS) to right M1, or left M1) and active conditions (pen hold with the right or left hand with CS contralateral to the active hand). Background EMG increases during the task of holding the pen, but the difference with background EMG at rest was only significant for ECR (p < 0.0001)
Interhemispheric inhibition
A decrease in the MEP size after the paired-pulse TMS in comparison with TS alone was observed in most of the subjects studied except in one at long ISI (40 ms) for the right ECR and in two further subjects at short ISI (10 ms) for the right FCR and long ISI (40 ms) for the left FCR.
The bottom part of Figs. 1 and 2 shows the superposition of 8 MEP tests and 8 MEPs conditioned at both ISIs obtained in two representative subjects for left ECR and right FCR muscles, respectively, in the rest (left panel) and active condition (right panel). The size of the MEPs decreased after the CS (interhemispheric inhibition) in comparison with the TS in both muscles and in both conditions, but the decrease was more marked in the rest condition than in the active, especially for the ECR muscle. Thus, the level of IHI decreased in the active condition in comparison with rest.
The two-tailed t test pointed to a significant reduction in the MEP area for the CS in comparison with the TS for both muscles, whatever the SIDE, the ISI and the CONDITION (p < 0.0001 for all comparisons, Table 2).
The repeated measures ANOVA on the MEP ratios showed no main effect of IHI direction, CONDITION or MUSCLE or interaction between these factors but a significant effect of ISI (p = 0.01 F(1.8) = 10.23). Therefore, we performed further analysis of SIHI and LIHI separately. The MEP ratio decreased in the active condition (left or right pen hold) in comparison with the rest condition for both ISIs, but the decrease was only statistically significant for the SIHI (CONDITION, p = 0.01, F(1.8) = 9.5). There was no significant effect of IHI direction (SIHI, p = 0.6, F(1.8 = 0.17); LIHI, p = 0.86, F(1.8) = 0.03) and MUSCLE (SIHI, p = 0.07, F(1.8) = 4.35; LIHI, p = 0.9, F(1.8) = 0.005) and no interaction between these factors for both SIHI and LIHI.
When we tested ANOVA for each muscle separately, we found a significant effect for CONDITION in ECR (p = 0.01, F(1.8) = 10.09) but not in FCR muscle (p = 0.1, F(1.8) = 3.1), which means that the modulation of IHI in the active condition was more pronounced in ECR than in FCR muscle. Figure 4 illustrates these results. Although there was a trend for a greater reduction in IHI at rest for ECR and for IHI from non-dominant to dominant hemisphere (left ECR) than IHI from the dominant to non-dominant hemisphere (right ECR), the differences were not statistically significant (ECR IHI direction, p = 0.74, F (1.8) = 0.11).
Amount of SIHI and LIHI during rest (black bars) and isometric contraction of the hand contralateral to CS (grey bars). Histograms display mean data (with standard errors) of SIHI (left panel) and LIHI (right panel) for right and left ECR (top) and right and left FCR (bottom) muscles during rest and active conditions (left or right pen hold). A bidirectional powerful IHI could be elicited at the two phases in both muscles at rest and during the active task. Contralateral wrist muscles activity evokes a global, bidirectional reduction in IHI which was more pronounced for SIHI and ECR
Discussion
The magnitude of interhemispheric inhibition (IHI) in wrist muscles was investigated at short (10 ms, SIHI) and long (40 ms, LIHI) interstimulus intervals from the dominant towards the non-dominant motor cortex and vice versa, while the right or left hand was at rest or performing a unimanual sustained tonic contraction (holding a pen with the hand contralateral to the CS).
A bidirectional powerful interhemispheric inhibition could be elicited at the short and long IHI phases in wrist extensor and flexor muscles in all the right-handed healthy subjects at rest and during the active task. SIHI but not LIHI was significantly bidirectionally reduced during unimanual contraction of the hand contralateral to the CS stimulation in comparison with rest. The amount of IHI from the non-dominant right hemisphere towards the dominant left hemisphere was not reduced in comparison with the amount of IHI from dominant to non-dominant hemisphere.
IHI in wrist muscles at rest
To date, paired-pulse suprathreshold TMS-induced IHI has been mainly studied in digit muscles (Ferbert et al. 1992; Gerloff et al. 1998; Duque et al. 2005b; Beck et al. 2009; Nelson et al. 2009; Ni et al. 2009) in healthy subjects or patients. Paired-pulse TMS-induced IHI was observed in FCR muscle at rest (Gerloff et al. 1998). Using another TMS-EMG method to study transcallosal pathways named iSP (interruption of ipsilateral voluntary EMG activity after a single-pulse magnetic stimulus), Ferbert et al. (1992) observed a suppression of EMG activity in the flexor carpi radialis (FCR) muscle but more variable results in the biceps muscle. However, it has been suggested that iSP and IHI do not represent the same phenomenon and should be considered complementary measures of ipsilateral inhibition (Chen et al. 2003). SIHI in wrist muscles was further studied in the left ECR during a contralateral ballistic or tonic contraction of APB muscle (Hinder et al. 2010).
Our study extends the previous ones studying the modulation of IHI on human upper limb and provides additional evidence that a suprathreshold TMS pulse applied to one motor cortex can reduce the size of MEPs evoked in wrist extensor and flexor muscles by a TMS pulse applied on the contralateral motor cortex. Although we did not study the precise time course of the inhibition, we found a significant inhibition of both ECR and FCR MEPs at an interstimulus interval of 10 and 40 ms, which are in the range of intervals more often chosen to study the two phases of interhemispheric inhibition, the short and long IHI (Nelson et al. 2009; Ni et al. 2009). The inhibition of FCR and ECR MEPs induced by the contralateral CS TMS at ISIs 8 and 40 ms was similar to that reported in FDI muscles using the same conditioning–test TMS paradigm with comparable suprathresholds CS and TS intensities, adequate to simultaneously elicit a MEP of ≈0.8–1.5 mV in amplitude in ECR and FCR muscles (Kukaswadia et al. 2005; Ni et al. 2009).
The similarity of IHI observed in wrist muscles compared to IHI in digit muscles, regarding the interval between CS–TS, and the amount of inhibition obtained with same CS–TS intensities paradigm, strongly suggests that the inhibitory effect induced by the contralateral conditioning TMS on FCR and ECR MEP was mainly mediated through transcallosal pathways. Transcallosal projections between the two M1 hand areas are known to exist in cat (digit zones) and monkeys (Pappas and Strick 1981; Gould et al. 1986; Rouiller et al. 1994). The proposed view that inhibitory interhemispheric effects are mediated through transcallosal pathways is strongly supported by studies in patients with agenesis of the corpus callosum (Meyer et al. 1995). Direct evidence of the cortical origin of the inhibition induced by the paired-pulse technique in later I-waves (I3) was provided by Di Lazzaro et al. (1999) recording descending volleys with epidural electrodes. With regard to the cell populations involved, it is likely that even in the case of IHI, the transcallosal projections are excitatory, synapsing onto local inhibitory circuits within the target hemisphere (Reis et al. 2008).
Previous results of FCR H reflex control experiments (Gerloff et al. 1998; Ni et al. 2009) confirmed that spinal inhibitory mechanisms do not substantially contribute to SIHI/LIHI since FCR H reflex amplitude was not changed by the conditioning–test TMS IHI paradigm.
The part of subcortical routes to the descending modulatory influence on the human M1 on muscles of the ipsilateral limb has been studied using transcranial electrical stimulation at the level of pyramidal decussation (pdTES), and these experiments have shown that this ipsilateral inhibition is to a significant extent mediated below the cortical level (Gerloff et al. 1998). Given the greater strength of the bilaterally organized descending projections of the C3–C4 cervical propriospinal system onto wrist and proximal upper limb muscles in comparison with digit muscles, and the absence of propriospinal projections to motoneurons of intrinsic hand muscles (Pierrot-Deseilligny and Burke 2005), it is possible that part of the ipsilateral ECR and FCR MEPs inhibition observed here is mediated by the propriospinal pathway or the reticulo-spinal tract.
Modulation of IHI during a postural active task
IHI was modulated during muscle contraction. Our data indicate that contralateral wrist muscles activity evokes a global, bidirectional reduction in IHI which was more pronounced for SIHI. As for FDI muscle, we did not observe a facilitation of the CS MEP during the active task, and despite reduced, they remain a net inhibition between the two hemispheres. We did not observe any interaction between the effect of the CONDITION and IHI direction; therefore, IHI decreases similarly in the active task for IHI from dominant to non-dominant hemisphere and IHI from non-dominant to dominant hemisphere, providing no evidence of hand dominance effect for wrist muscles. Reduced cortical inhibition during voluntary contraction of either hand may be required to allow ongoing muscle activity.
In a first study, Chen et al. (2003) found that IHI at short ISI (8 ms) was reduced during 50 % maximum voluntary contraction (MVC) of the FDI muscle, similarly to the IHI results reported by Ridding et al. (2000). By contrast, they reported that IHI at 40 ms showed little change with muscle activation reinforcing the view that SIHI and LIHI were not mediated by the same mechanism. However, Nelson et al. (2009) found in a more recent study performed on FDI muscle, but with a different motor task (20 % isometric contraction while holding a pen), that both LIHI and SIHI were similarly reduced during unilateral contractions of either hand in comparison with rest. SIHI and LIHI may be altered differently depending on the type of movement or level of muscle contraction. Here, using the same postural context of holding a pen with the hand contralateral to the CS, we found a decrease in IHI in the active condition (left or right hand) vs rest for both wrist muscles and both SIHI and LIHI, but the difference was only statistically significant for SIHI as in the first study of Chen et al. (2003) and more pronounced for ECR in comparison with FCR muscle. Differences in the modulation of IHI between FCR and ECR muscles in the postural context of holding a pen compared to rest were not very surprising. Even if we have observed with the EMG monitoring a physiological cocontraction of ECR and FCR muscles in order to maintain the wrist in the good posture to hold the pen, postural extensor wrist muscles were more active than wrist flexor muscles in such task and this was confirmed by the prestimulus EMG analysis (Fig. 3). The large range of age of the subjects included (22–54) could also have explained the absence of significant modulation of LIHI in the active condition in comparison with rest since it has been previously reported a reduced ability to modulate IHI at ISI 40 ms during a force production task with advancing age (Talelli et al. 2008).
Finally, we cannot conclude that the decrease in IHI observed during the task of holding the pen was exclusively related to changes in wrist muscles IHI excitability since many other forearm and digit muscles (pinch grip APB, FDI) were also involved in our task and could influence this modulation to various extents (Hinder et al. 2010).
The differences observed in the modulation of IHI during an active task involving digit and wrist muscles between SIHI and LIHI reinforce the evidence that the two phases of IHI are not mediated by the same mechanism although a similar modulation by the movement for both ISIs would not have excluded a mediation by different mechanisms. Evidence for differing mechanisms of IHI at these two ISIs comes primarily from studies of their interactions with other inhibitory phenomena (Kukaswadia et al. 2005). As for LIHI, it was found that long intracortical inhibition (LICI) (Valls-Sole et al. 1992; Wassermann et al. 1996; Daskalakis et al. 2002) shows little change with voluntary muscle contraction. Daskalakis et al. (2002) have hypothesized that LICI and IHI may be mediated by a similar population of inhibitory neurons based on a few pieces of indirect evidence. This includes the tendency for both LICI and IHI to predominately affect motor cortical neurons activated at low intensities; both require suprathreshold conditioning stimulus to produce inhibitory effects, and they both inhibit SICI. Both are also believed to be dependent on GABA-B mediated neurotransmission in the target hemisphere. This was confirmed for LIHI by a recent study of pharmacological modulation by GABA agonists: IHI at ISIs of up to 200 ms was strengthened after application of the GABA-B agonist baclofen, suggesting that LIHI is most likely mediated by postsynaptic GABA-B receptors (Irlbacher et al. 2007). More recently, it has been suggested that circuits mediating SIHI are susceptible to sodium channel blockade (Sommer et al. 2012).
Bidirectional IHI
Although there was a trend for greater IHI (at both ISIs) during rest for ECR and for IHI from the motor dominant to non-dominant hemisphere than IHI from the non-dominant to dominant hemisphere, the differences were not significant (Fig. 4). An interhemispheric asymmetry of transcallosal inhibition (SIHI) was firstly reported in right-handed subjects by Netz et al. (1995) in FDI muscle, showing that inhibition from “dominant” left hemisphere to non-dominant right hemisphere was more marked than IHI from the right to the left hemisphere but no longer confirmed in later studies (Salerno and Georgesco 1996; De Gennaro et al. 2004; Nelson et al. 2009). We have now extended the absence of hemispheric dominance to IHI directed to wrist muscles for LIHI and SIHI and during active and resting states.
Conclusion
Although the precise respective role of SIHI and LIHI is still not entirely elucidated, data obtained from healthy subjects or patients suggest that changes in interhemispheric interactions between the M1s are involved in the control of unimanual and coordinated skilled bimanual movements including suppression of unwanted motor activity in the opposite limbs during unilateral movements and assistance in bilateral movements and also the acquisition and transfer of motor skills (Duque et al. 2005b; Reis et al. 2008). It has been recently suggested that the modulation of IHI during the movement can affect the motor performance depending on the motor task: individuals with greater IHI capacity demonstrated reduced mirror EMG activity in the left hand during unimanual right hand contraction but a poorest performance during a bimanual independent force production task (Fling and Seidler 2012).
An impaired interhemispheric inhibition of digit muscles has been recently reported in patients with movement disorders such as focal hand dystonia (FHD) (Beck et al. 2009; Nelson et al. 2010), but the role of IHI in the general pathophysiology of FHD is still discussed.
References
Avanzino L, Teo JT, Rothwell JC (2007) Intracortical circuits modulate transcallosal inhibition in humans. J Physiol 583:99–114
Beck S, Shamim EA, Richardson SP, Schubert M, Hallett M (2009) Inter-hemispheric inhibition is impaired in mirror dystonia. Eur J Neurosci 29:1634–1640
Chen R (2004) Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res 154:1–10
Chen R, Yung D, Li JY (2003) Organization of ipsilateral excitatory and inhibitory pathways in the human motor cortex. J Neurophysiol 89:1256–1264
Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R (2002) The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol 543:317–326
De Gennaro L, Bertini M, Pauri F, Cristiani R, Curcio G, Ferrara M, Rossini PM (2004) Callosal effects of transcranial magnetic stimulation (TMS): the influence of gender and stimulus parameters. Neurosci Res 48:129–137
Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC (1999) Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp Brain Res 124:520–524
Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG (2005a) Transcallosal inhibition in chronic subcortical stroke. Neuroimage 28:940–946
Duque J, Mazzocchio R, Dambrosia J, Murase N, Olivier E, Cohen LG (2005b) Kinematically specific interhemispheric inhibition operating in the process of generation of a voluntary movement. Cereb Cortex 15:588–593
Duque J, Murase N, Celnik P, Hummel F, Harris-Love M, Mazzocchio R, Olivier E, Cohen LG (2007) Intermanual differences in movement-related interhemispheric inhibition. J Cogn Neurosci 19:204–213
Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JG, Marsden CD (1992) Interhemispheric inhibition of the human motor cortex. J Physiol 453:525–546
Fling BW, Seidler RD (2012) Task-dependent effects of interhemispheric inhibition on motor control. Behav Brain Res 226:211–217
Gerloff C, Cohen LG, Floeter MK, Chen R, Corwell B, Hallett M (1998) Inhibitory influence of the ipsilateral motor cortex on responses to stimulation of the human cortex and pyramidal tract. J Physiol 510(Pt 1):249–259
Gould HJ 3rd, Cusick CG, Pons TP, Kaas JH (1986) The relationship of corpus callosum connections to electrical stimulation maps of motor, supplementary motor, and the frontal eye fields in owl monkeys. J Comp Neurol 247:297–325
Hanajima R, Ugawa Y, Machii K, Mochizuki H, Terao Y, Enomoto H, Furubayashi T, Shiio Y, Uesugi H, Kanazawa I (2001) Interhemispheric facilitation of the hand motor area in humans. J Physiol 531:849–859
Harris-Love ML, Perez MA, Chen R, Cohen LG (2007) Interhemispheric inhibition in distal and proximal arm representations in the primary cortex. J Neurophysiol 97:2511–2515
Hinder MR, Schmidt MW, Garry MI, Summers JJ (2010) Unilateral contractions modulate interhemispheric inhibition most strongly and most adaptively in the homologous muscle of the contralateral limb. Exp Brain Res 205:423–433
Irlbacher K, Brocke J, Mechow JV, Brandt SA (2007) Effects of GABA(A) and GABA(B) agonists on interhemispheric inhibition in man. Clin Neurophysiol 118:308–316
Kukaswadia S, Wagle-Shukla A, Morgante F, Gunraj C, Chen R (2005) Interactions between long latency afferent inhibition and interhemispheric inhibitions in the human motor cortex. J Physiol 563:915–924
Meyer BU, Roricht S, Grafin von Einsiedel H, Kruggel F, Weindl A (1995) Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain 118(Pt 2):429–440
Murase N, Duque J, Mazzocchio R, Cohen LG (2004) Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 55:400–409
Nelson AJ, Hoque T, Gunraj C, Ni Z, Chen R (2009) Bi-directional interhemispheric inhibition during unimanual sustained contractions. BMC Neurosci 10:31
Nelson AJ, Hoque T, Gunraj C, Ni Z, Chen R (2010) Impaired interhemispheric inhibition in writer’s cramp. Neurology 75:441–447
Netz J, Ziemann U, Homberg V (1995) Hemispheric asymmetry of transcallosal inhibition in man. Exp Brain Res 104:527–533
Ni Z, Gunraj C, Nelson AJ, Yeh IJ, Castillo G, Hoque T, Chen R (2009) Two phases of interhemispheric inhibition between motor related cortical areas and the primary motor cortex in human. Cereb Cortex 19:1654–1665
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Pappas CL, Strick PL (1981) Anatomical demonstration of multiple representation in the forelimb region of the cat motor cortex. J Comp Neurol 200:491–500
Perez MA, Cohen LG (2008) Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci 28:5631–5640
Pierrot-Deseilligny E, Burke D (2005) The circuitry of the human spinal cord. Cambridge University press, New York
Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG (2008) Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol 586:325–351
Ridding MC, Brouwer B, Nordstrom MA (2000) Reduced interhemispheric inhibition in musicians. Exp Brain Res 133:249–253
Rouiller EM, Babalian A, Kazennikov O, Moret V, Yu XH, Wiesendanger M (1994) Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Exp Brain Res 102:227–243
Salerno A, Georgesco M (1996) Interhemispheric facilitation and inhibition studied in man with double magnetic stimulation. Electroencephalogr Clin Neurophysiol 101:395–403
Sommer M, Gileles E, Knappmeyer K, Rothkegel H, Polania R, Paulus W (2012) Carbamazepine reduces short-interval interhemispheric inhibition in healthy humans. Clin Neurophysiol 123:351–357
Talelli P, Waddingham W, Ewas A, Rothwell JC, Ward NS (2008) The effect of age on task-related modulation of interhemispheric balance. Exp Brain Res 186:59–66
Valls-Sole J, Pascual-Leone A, Wassermann EM, Hallett M (1992) Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol 85:355–364
Wassermann EM, Samii A, Mercuri B, Ikoma K, Oddo D, Grill SE, Hallett M (1996) Responses to paired transcranial magnetic stimuli in resting, active, and recently activated muscles. Exp Brain Res 109:158–163
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sattler, V., Dickler, M., Michaud, M. et al. Interhemispheric inhibition in human wrist muscles. Exp Brain Res 221, 449–458 (2012). https://doi.org/10.1007/s00221-012-3187-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-012-3187-4