Abstract
The sleep–wake cycle is a major determinant of locomotor activity in humans, and the neural and physiological processes necessary for optimum postural control may be impaired by an extension of the wake period into habitual sleep time. There is growing evidence for such a contribution from sleep-related factors, but great inconsistency in the methods used to assess this contribution, particularly in control for circadian phase position. Postural control was assessed at hourly intervals across 14 h of extended wake in nine young adult participants. Force plate parameters of medio-lateral and anterior–posterior sway, centre of pressure (CoP) trace length, area, and velocity were assessed with eyes open and eyes closed over 3-min periods. A standard measure of psychomotor vigilance was assessed concurrently under constant routine conditions. After controlling for individual differences in circadian phase position, a significant effect of extended wake was found for anterior–posterior sway and for psychomotor vigilance. These data suggest that extended wake may increase the risk of a fall or other consequences of impaired postural control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The sleep–wake cycle is the most conspicuous behavioural rhythm in humans. The major biological determinants of sleep–wake behaviour have been described in models, such as Borbély’s 2-process model of sleep propensity (Borbely 1982), as an additive interaction between the core circadian rhythm and a homoeostatic drive to sleep. These components variably impact on a wide range of physiological processes (Duguay and Cermakian 2009), and on a wide range of performance tasks (Van Dongen and Dinges 2003). The two components can be manipulated through simple strategies such as increasing sleep deprivation (Lim and Dinges 2008), or through very sophisticated strategies such as forced desynchrony protocols (Dijk and Czeisler 1994) designed to differentiate the relative contribution of each component. The impact of the sleep–wake cycle, and particularly of extended wake periods, has been described for simple and complex reaction time tasks (Lisper and Kjellberg 1972; Cajochen et al. 1999), cognitive throughput tasks (Jewett and Kronauer 1999), and tasks of ‘executive function’ (Manly et al. 2002; Nilsson et al. 2005; Tucker et al. 2010). The general pattern of performance during extended wake, for example, on the psychomotor vigilance task (Dorrian et al. 2005), is curvilinear (Graw et al. 2004) with poorest performance typically around the circadian temperature nadir (Wyatt et al. 1999). The potential consequences of impairment in these skills may be seen, for example, in the high rate of severe road crashes associated with sleepiness (Connor et al. 2002); however, the peak in fatigue-associated accidents appears to occur some hours before peak sleep propensity, suggesting differential impact on specific skills (Williamson et al. 2011).
There are strong indications of a link between disturbed sleep and impaired balance at the population level. For example, sleep disturbance and subsequent daytime sleepiness has been identified as a potential risk factor for falls in older adults (Brassington et al. 2000; Kawamoto and Doi 2002; Avidan et al. 2005; Stone et al. 2006; Hill et al. 2007), but the potential mechanisms underlying this relationship are uncertain. The impact of the sleep–wake cycle on fundamental mechanisms associated with postural control may be critical. A number of laboratory-based studies have examined the effects of sleepiness, extended wake, and circadian rhythm variation on specific components of postural control derived from computerised posturographic tests, typically utilising force plates (Schlesinger et al. 1998; Nakano et al. 2001; Gribble and Hertel 2004; Fabbri et al. 2006; Morad et al. 2007; Gomez et al. 2008; Patel et al. 2008; Ma et al. 2009; Bougard et al. 2010; Robillard et al. 2011), including a thorough series of studies by Forsman and colleagues (Haeggstrom et al. 2006; Forsman et al. 2007a, b, c, 2008, 2010a; b; Tietavainen et al. 2007). These studies support an association between time awake and specific indices of postural control, including centre of pressure (CoP) velocity (Gribble et al. 2007), CoP area (Bougard et al. 2010), CoP anterior–posterior range (Robillard et al. 2011), and others. While some studies have attempted to distinguish specific time-of-day effects (Bougard et al. 2010), in most cases they provide no experimental or statistical control for individual differences in circadian phase. This aspect may be important, as individual participants included in these studies may vary in their circadian phase position by several hours. Circadian phase position is associated with significant variation in a wide range of physiological processes, including core body temperature, hormone excretion, and some aspects of gross motor performance (Jasper et al. 2009). As such time-of-day, or clock time, may be less biologically meaningful than individual circadian phase position. Further, sampling resolution in previous studies has varied from 2-hourly across 36 h to repeat measurement before and after 24-h of wake (Ma et al. 2009) or more irregular sampling (Gribble et al. 2007; Morad et al. 2007). Despite these limitations, differential impact of sleep homoeostasis (Robillard et al. 2011) and circadian rhythm components (Haeggstrom et al. 2006) are suggested. A single forced desynchrony study has assessed postural control at different points of the circadian cycle after different durations of prior wake with CoP area during 1 min of quiet standing (Sargent et al. 2010). This study suggested that neither circadian phase nor duration of prior wake (up to 17 h) had a significant impact on postural control. Our aim was to assess the impact of extended wake on postural control, with sampling at hourly intervals to assess nonlinear variation in balance parameters, with regard to individual variation in circadian phase position, and with convergent measurement of objective vigilance with a psychomotor task.
Materials and methods
Participants
The sample consisted of 3 male and 6 female university students aged 18–25 years (M = 21.56, SD = 2.51). Height and weight ranged from 164 to 175 cm and 51–70 kg (M = 169.33 and 59.78, SD = 3.97 and 7.58), respectively. The participants were recruited through word of mouth, with fourth-year psychology students asked to participate.
Exclusion criteria
Participants were excluded if they were currently sick, consumed drugs that could impede balance abilities (e.g. drugs with a sedative effect), engaged in transmedian travel and/or shiftwork 1 week prior to the study, excessive daily caffeine consumption (>4 cups), sleep difficulties (Pittsburgh Sleep Quality Index Score >5 (Buysse et al. 1989), sleep disorder diagnoses, and self-reported balance problems, physical conditions, and/or injuries to feet, ankles, legs, and arms (which could impede balance).
The study was approved by the Queensland University of Technology ethics committee (EC00171; approval 0900000337), and informed consent was provided by all participants.
Measures
Standing balance
Participants stood quietly, their barefeet positioned with heels 15 cm apart at 30° angle, arms at their sides on a force plate (HUR laboratory force platform, Model BT4, Tampere Finland) with eyes open and closed. Centre of pressure changes in the anterior–posterior (AP) and medial–lateral (ML) directions were recorded over a duration of three minutes at a sampling rate of 100 Hz. The CoP changes in motion were used to derive additional balance parameters: trace length (total distance of CoP change during a trial), C90 area (mm2; fitted ellipse in which 90 % of the trace length points fit into), and velocity. Higher values reflect greater balance instability. Data were acquired for analysis with Finsole Orthotic Analysing suite, version 2.0.
Perceptual psychomotor vigilance task (PPVT; Mueller 2010)
The computer-based PPVT (version 0.07) from the PEBL psychological test battery (version 0.1) was a visual attention task used to measure arousal and sleepiness. In line with conventional sleep deprivation research (Dinges and Powell 1985), the intervals between stimuli presentation ranged from 1 to 10 s. A block approach was used for this study. Each inter-interval stimulus interval (ISI) was presented eight times, was sampled without replacement and randomly presented, and resulted in 80 RTs for each trial block. This task required participants to press the space bar as quickly as possible when the stimulus (red circle) appeared on the computer screen. RTs in milliseconds were shown on screen following each response. A false start was recorded if the space bar was pressed prior to the presentation of the stimulus, and this was counted as a ‘too fast’ trial. Reaction times exceeding 5 s were considered to be a lapse. Lapses in particular are sensitive to the effects of sleep deprivation and circadian effects on performance (Rajaraman et al. 2008).
Actigraphy
Habitual sleep–wake behaviour was assessed using Actigraphy (wristwatch style recording accelerometers; Minimitter-Respironices Actiwatch II) for 1 week prior to attending the laboratory. Sleep indices, including daily sleep onset and wake times, were identified from the objective activity count data (Actiware 5.2 software). Actigraphy typically has good concordance with polysomnographically determined sleep (Chesson et al. 2007), but has an advantage for longer-term behavioural assessment.
Procedure
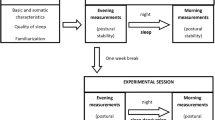
Participants attended the laboratory to complete the screening measures and study induction. They were fitted with an actigraph on their nondominant wrist and took the Pittsburgh Sleep Diary (PghSD; Monk et al. 1994) home with them to record sleep–wake times for a 6-day period prior to an extended wake testing day in the laboratory. Participants awoke at 5:30 am on that day, arrived at the laboratory at 5 p.m. in the evening, and commenced hourly testing of balance and PVT from 6 pm (12½ h since wake time) until 7 am the next morning (22 ½ h since wake time).
During the extended 14-h wake period, hourly measures were made of postural sway and the 10-min computer-based PVT task. Food consumption was restricted to hourly 500 kj aliquots, and lighting was standardised in both the testing and waiting areas to <40 lux at eye level (Gossen Mavolux 5032B digital luxmeter), similar to other constant routine protocols (Smith and Eastman 2009). Caffeine and alcohol consumption was not permitted, and participants watched DVDs when not being tested. Screen brightness was reduced to minimum settings on the monitors, and illumination from this source was measured at <15 lux at eye level from the normal viewing distance of approximately 1 metre.
Statistical analyses
Circadian phase position for each participant was estimated from their individual sleep–wake times recorded by Actigraphy and cross-checked with the Pittsburgh Sleep Diary reports (Monk et al. 1994). The mean of habitual wake time (but not sleep onset time) provides robust prediction of dim-light melatonin onset (DLMO) and hence the circadian core body temperature nadir (Martin and Eastman 2002; Burgess et al. 2003; Crowley et al. 2006; Bjorvatn and Pallesen 2009). Mean wake time for each sleep period was therefore calculated for the prior 7-day period for each participant. Participant data were then re-binned into hours since mean habitual wake time (from 10 to 23 h posthabitual wake) for further analysis.
To test whether postural sway parameters would vary across the 14 h of extended wake, a 2 × 14 repeated-measures ANOVA was conducted for each of the five dependent variables (ML sway, AP sway, trace length, C90 area, and velocity). To control for family-wise error, a Bonferroni adjustment was applied. There were two levels of the eye condition factor (open or closed) and 14 levels of the time factor. The main effects of eye condition and time are reported in Table 1, with conservative Greenhouse–Geiser-corrected estimates of degrees of freedom.
Results
A significant main effect of eye condition (open or closed) was found for the majority of the balance metrics (apart from AP sway; Table 1), in the direction of increased deviation with eyes closed. A main effect for the time condition was found only for AP sway. The pattern of this variation in AP sway was assessed with planned contrasts (simple), with each hourly score compared to the baseline start score. Planned contrasts revealed significant increase in AP sway with increasing wake duration for both the eyes open and eyes closed conditions, with the pattern of change described in Fig. 1a.
a Variation in mean AP sway (mm from centre) across 10–24 h extended wake for eyes open (solid line) and eyes closed (dashed line) conditions. Note reversed scale. b Variation in the mean number of false starts (solid line) and lapses (dashed line) across the same period. Error bars represent standard error of the mean. Significant differences from baseline are indicated with asterisks
Participants varied in their habitual sleep duration, and the fixed wake time of 5:30 am also meant that sleep duration on the night prior to the study was restricted to a varying degree between individuals (from 4.2- to 7.1-h sleep), depending on their habitual sleep onset and wake times. The relationship between duration of prior sleep and balance variables was assessed with Pearson correlation to determine eligibility as covariates. Correlation coefficients (r 2) varied between 0 and 0.28, all nonsignificant, and prior sleep was not included in further analyses as a covariate.
Variation in the PVT metrics of lapses and false starts across the extended wake period was assessed with one-way repeated-measures ANOVA with a Bonferroni adjustment. There was a significant main effect of time for PVT lapses (Table 1). To examine the pattern of this variation with time, post hoc simple contrasts were conducted for both (Fig. 1b). A significant increase in both lapses and false starts was observed in the hours 20–24 h after habitual wake (approximately 4–7 am for most participants).
In summary, an effect of extended wake was found only for AP sway, with increased AP sway observed relative to the baseline measure after 15 h of extended wake. A main effect of eye condition was observed for trace length, c90 area, ML sway, and velocity. Except for AP sway, higher values of all the other balance measures, indicative of decreased stability, were found when participants had their eyes closed. PVT lapses, but not PVT false starts, demonstrated a main effect for an increase across 14 h of extended wake.
Discussion
Postural control is a complex physiological process that requires preserved function across a range of specific brainstem and cortical structures (Winter et al. 1990). This process certainly changes abruptly with sleep onset and may be impaired with increased sleepiness associated with extended wake. The impact of extended wake demonstrated in this study was restricted to a single control parameter (AP sway), but with a potentially large effect on this parameter observed in the small sample. This finding is consistent with the previous findings of time-of-day effects, in particular those of Robillard et al. (2011), and suggests that these effects may be robust. The relationship between time and both PVT measures and AP sway suggests a general effect on psychomotor control. The variation in the PVT measures was also consistent with that observed in other extended wake studies (Smith et al. 2007). The findings partly contradict the findings by Sargent et al. (2010), at least for the single index of AP sway. The duration of posturographic assessment and duration of prior wake used in that study may not have been sufficient to identify degraded postural control, despite very careful manipulation of sleep–wake and circadian function. Conversely, it is recognised that control for circadian phase outside of a forced desynchrony methodology is partly confounded by time awake (as in the current study). Nevertheless, these two factors appear to interact to produce variation in postural control.
Several large prospective studies have identified that postural sway is associated with falls in older people (Lord et al. 2003) and in people with neurological disease (Kerr et al. 2010). While lateral sway has the strongest association with falls risk in these populations, oscillations in the AP plane are consistently reported (Lajoie and Gallagher 2004), including higher AP speed with eyes open (Topper et al. 1993). These measures form the basis of clinical assessment tools for predicting falls in these populations. However, it has been noted that methods used to measure postural sway and analyse sway parameters in clinical populations differ widely (Piirtola and Era 2006), making comparison of the magnitude of effects very difficult. The clinical significance of increased AP sway in young sleep-deprived subjects remains to be determined.
It remains possible that more subtle variation in other postural control indices could be observed in a larger sample, after more extreme sleep restriction (e.g. chronic partial sleep deprivation), or in special populations such as older adults. It is also possible that some participants in the current study could ‘rally’ to the relatively brief performance tasks, especially as standing itself has a transient alerting effect (Bonnet and Arand 1999). The light levels experienced during the study may have been sufficient to suppress or delay expression of melatonin in the evening (Gooley et al. 2011; Zele et al. 2011) with concomitant increase in alertness relative to normal dim-light expression (Cajochen et al. 2005). Assessment of postural control with melatonin expression either controlled (e.g. in a constant dim-light protocol) or manipulated (e.g. with exogenous melatonin or bright short-wavelength light) may be necessary to determine these effects. Continuous assessment of postural control (e.g. via measurement of gait) could provide a more sensitive measure with specific workplace validity, as could a ‘dual-task’ paradigm (Woollacott and Shumway-Cook 2002) requiring effort to be oriented to an executive function task. Higher resolution of homoeostatic and circadian contributions could be achieved with a forced desynchrony protocol or other unmasking strategies, or with more refined measurement with direct melatonin or core body temperature measurement.
A recently recognised issue in sleep and circadian rhythm research is that of individual differences in sensitivity to sleep loss (Galliaud et al. 2008). This variation may be due to a range of factors, including differences in clock genetics, body mass, and age. Intra-individual differences in muscle tone and ‘fitness’ may also contribute to individual difference in postural control, even in a healthy young sample. Multi-level modelling approaches have been proposed to assess such differences (Kristjansson et al. 2007), but studies using this approach would require quite large samples. Despite these considerations, this study provides further support for the impairment of postural control after extended wake and is consistent with circadian variation in this control.
References
Avidan AY, Fries BE, James ML, Szafara KL, Wright GT, Chervin RD (2005) Insomnia and hypnotic use, recorded in the minimum data set, as predictors of falls and hip fractures in Michigan nursing homes. J Am Geriatr Soc 53:955–962
Bjorvatn B, Pallesen S (2009) A practical approach to circadian rhythm sleep disorders. Sleep Med Rev 13:47–60
Bonnet MH, Arand DL (1999) Level of arousal and the ability to maintain wakefulness. J Sleep Res 8:247–254
Borbely AA (1982) A two process model of sleep regulation. Hum Neurobiol 1:195–204
Bougard C, Lepelley MC, Davenne D (2010) The influences of time-of-day and sleep deprivation on postural control. Exp Brain Res 209:1–7
Brassington GS, King AC, Bliwise DL (2000) Sleep problems as a risk factor for falls in a sample of community-dwelling adults aged 64–99 years. J Am Geriatr Soc 48:1234–1240
Burgess HJ, Savic N, Sletten T, Roach G, Gilbert SS, Dawson D (2003) The relationship between the dim light melatonin onset and sleep on a regular schedule in young healthy adults. Behav Sleep Med 1:102–114
Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28:193–213
Cajochen C, Khalsa SBS, Wyatt JK, Czeisler CA, Dijk DJ (1999) EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol Reg Integ Compar Physiol 277:R640
Cajochen C, Munch M, Kobialka S et al (2005) High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab 90:1311–1316
Chesson M Jr, Coleman M, Lee-Chiong M, Pancer D (2007) Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep 30:519
Connor J, Norton R, Ameratunga S et al (2002) Driver sleepiness and risk of serious injury to car occupants: population based case control study. Br Med J 324:1125
Crowley SJ, Acebo C, Fallone G, Carskadon MA (2006) Estimating dim light melatonin onset (DLMO) phase in adolescents using summer or school-year sleep/wake schedules. Sleep 29:1632
Dijk DJ, Czeisler CA (1994) Paradoxical timing of the circadian rhythm of sleep propensity serves to consolidate sleep and wakefulness in humans. Neurosci Lett 166:63–68
Dinges DF, Powell JW (1985) Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Inst Comput 17:652–655
Dorrian J, Rogers NL, Dinges DF (2005) Psychomotor vigilance performance: neurocognitive assay sensitive to sleep loss. Sleep deprivation: clinical issues, pharmacology and sleep loss effects. Marcel Dekker, Inc, New York, pp 39–70
Duguay D, Cermakian N (2009) The crosstalk between physiology and circadian clock proteins. Chronobiol Int 26:1479–1513
Fabbri M, Martoni M, Esposito MJ, Brighetti G, Natale V (2006) Postural control after a night without sleep. Neurpsychologia 44:2520–2525
Forsman P, Hæggström E, Wallin A (2007a) Reducing trial length in force platform posturographic sleep deprivation measurements. Meas Sci Technol 18:2893
Forsman P, Haeggström E, Wallin A, Toppila E, Pyykkö I (2007b) Daytime changes in postural stability and repeatability of posturographic measurements. J Occup Environ Med 49:591
Forsman P, Wallin A, Tietäväinen A, Hæggström E (2007c) Posturographic sleepiness monitoring. J Sleep Res 16:259–261
Forsman P, Tietäväinen A, Wallin A, Hæggström E (2008) Modeling balance control during sustained waking allows posturographic sleepiness testing. J Biomech 41:2892–2894
Forsman P, Hæggström E, Wallin AE, Toppila E, Pyykkö I (2010a) Principal component analysis detects sleepiness-related changes in balance control. Gait Posture 32:419–421
Forsman P, Wallin A, Hæggström E (2010b) Validation of a posturographic approach to monitor sleepiness. J Biomech 43:3214–3216
Galliaud E, Taillard J, Sagaspe P, Valtat CED, Bioulac B, Philip P (2008) Sharp and sleepy: evidence for dissociation between sleep pressure and nocturnal performance. J Sleep Res 17:11–15
Gomez S, Patel M, Berg S, Magnusson M, Johansson R, Fransson P (2008) Effects of proprioceptive vibratory stimulation on body movement at 24 and 36 h of sleep deprivation. Clin Neurophysiol 119:617–625
Gooley JJ, Chamberlain K, Smith KA et al (2011) Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab 96:E463–E472
Graw P, Krauchi K, Knoblauch V, Wirz-Justice A, Cajochen C (2004) Circadian and wake-dependent modulation of fastest and slowest reaction times during the psychomotor vigilance task. Physiol Behav 80:695–701
Gribble PA, Hertel J (2004) Changes in postural control during a 4-hour sleep deprivation period. Percept Motor Skills 99:1035–1045
Gribble PA, Tucker WS, White PA (2007) Time-of-day influences on static and dynamic postural control. J Athletic Train 42:35
Haeggstrom E, Forsman PM, Wallin AE, Toppila EM, Pyykko IV (2006) Evaluating sleepiness using force platform posturography. Biomed Eng IEEE Trans 53:1578–1585
Hill EL, Cumming RG, Lewis R, Carrington S, Couteur DGL (2007) Sleep disturbances and falls in older people. J Gerontol A Biol Sci Med Sci 62:62
Jasper I, Häußler A, Baur B, Marquardt C, Hermsdörfer J (2009) Circadian variations in the kinematics of handwriting and grip strength. Chronobiol Int 26:576–594
Jewett ME, Kronauer RE (1999) Interactive mathematical models of subjective alertness and cognitive throughput in humans. J Biol Rhythms 14:588
Kawamoto R, Doi T (2002) Sleep problems as a risk factor for fall in community dwelling older persons. Geriatrics Gerontol Int 2:16–22
Kerr GK, Worringham CJ, Cole MH, Lacharez PF, Wood JM, Silburn PA (2010) Predictors of future falls in Parkinson’s disease. Neurology 75:116–124
Kristjansson SD, Kircher JC, Webb AK (2007) Multilevel models for repeated measures research designs in psychophysiology: an introduction to growth curve modeling. Psychophysiology 44:728–736
Lajoie Y, Gallagher S (2004) Predicting falls within the elderly community: comparison of postural sway, reaction time, the Berg balance scale and the Activities-specific Balance Confidence (ABC) scale for comparing fallers and non-fallers. Arch Gerontol Geriatr 38:11–26
Lim J, Dinges DF (2008) Sleep deprivation and vigilant attention. Ann N Y Acad Sci 1129:305–322
Lisper HO, Kjellberg A (1972) Effects of 24-hour sleep deprivation on rate of decrement in a 10-minute auditory reaction time task. J Exp Psychol 96:287
Lord SR, Menz HB, Tiedemann A (2003) A physiological profile approach to falls risk assessment and prevention. Phys Ther 83:237–252
Ma J, Yao Y, Ma R et al (2009) Effects of sleep deprivation on human postural control, subjective fatigue assessment and psychomotor performance. J Int Med Res 37:1311–1320
Manly T, Lewis GH, Robertson IH, Watson PC, Datta AK (2002) Coffee in the cornflakes: time-of-day as a modulator of executive response control. Neuropsychologia 40:1–6
Martin SK, Eastman CI (2002) Sleep logs of young adults with self-selected sleep times predict the dim light melatonin onset. Chronobiol Int 19:695–707
Monk TH, Reynolds CF 3rd, Kupfer DJ et al (1994) The Pittsburgh sleep diary. J Sleep Res 3:111–120
Morad Y, Azaria B, Avni I, Barkana Y, Zadok D, Kohen-Raz R, Barenboim E (2007) Posturography as an indicator of fatigue due to sleep deprivation. Aviat Space Environ Med 78:859–863
Mueller ST (2010) The PEBL manual: programming and usage guide for the Psychology Experiment Building Language PEBL, Version 0.11. Lulu Press, Raleigh. ISBN:978-0-557-65817-6
Nakano T, Araki K, Michimori A, Inbe H, Hagiwara H, Koyama E (2001) Nineteen hour variation of postural sway, alertness and rectal temperature during sleep deprivation. Psychiatry Clin Neurosci 55:277–278
Nilsson JP, Söderström M, Karlsson AU, Lekander M, Åkerstedt T, Lindroth NE, Axelsson J (2005) Less effective executive functioning after one night’s sleep deprivation. J Sleep Res 14:1–6
Patel M, Gomez S, Berg S et al (2008) Effects of 24-h and 36-h sleep deprivation on human postural control and adaptation. Exp Brain Res 185:165–173
Piirtola M, Era P (2006) Force platform measurements as predictors of falls among older people–a review. Gerontology 52:1–16
Rajaraman S, Gribok AV, Wesensten NJ, Balkin TJ (2008) Individualized performance prediction of sleep-deprived individuals with the two-process model. J Appl Physiol 104:459–468
Robillard R, Prince F, Boissonneault M, Filipini D, Carrier J (2011) Effects of increased homeostatic sleep pressure on postural control and their modulation by attentional resources. Clin Neurophysiol 122:1771–1778
Sargent C, Ferguson SA, Darwent D, Kennaway DJ, Roach GD (2010) The influence of circadian phase and prior wake on neuromuscular function. Chronobiol Int 27:911–921
Schlesinger A, Redfern MS, Dahl RE, Jennings JR (1998) Postural control, attention and sleep deprivation. NeuroReport 9:49
Smith MR, Eastman CI (2009) Phase delaying the human circadian clock with blue-enriched polychromatic light. Chronobiol Int 26:709–725
Smith S, Kilby S, Jorgensen G, Douglas JA (2007) Napping and nightshift work: effects of a short nap on psychomotor vigilance and subjective sleepiness in health workers. Sleep Biol Rhyth 5:117–125
Stone KL, Ewing SK, Lui LY et al (2006) Self reported sleep and nap habits and risk of falls and fractures in older women: the study of osteoporotic fractures. J Am Geriatr Soc 54:1177–1183
Tietavainen A, Forsman P, Wallin A, Korsback A, Haeggstrom E (2007) Model-based posturographic sleepiness monitor tested on 20 subjects. In: IEEE, pp 3573–3576
Topper A, Maki B, Holliday P (1993) Are activity-based assessments of balance and gait in the elderly predictive of risk of falling and/or type of fall? J Am Geriat Soc 41:479–487
Tucker AM, Whitney P, Belenky G, Hinson JM, Van Dongen HPA (2010) Effects of sleep deprivation on dissociated components of executive functioning. Sleep 33:47
Van Dongen HP, Dinges DF (2003) Investigating the interaction between the homeostatic and circadian processes of sleep-wake regulation for the prediction of waking neurobehavioural performance. J Sleep Res 12:181–187
Williamson A, Lombardi DA, Folkard S, Stutts J, Courtney TK, Connor JL (2011) The link between fatigue and safety. Accid Anal Prev 43:498–515
Winter DA, Patla AE, Frank JS (1990) Assessment of balance control in humans. Med Prog Technol 16:31–51
Woollacott M, Shumway-Cook A (2002) Attention and the control of posture and gait: a review of an emerging area of research. Gait Posture 16:1–14
Wyatt JK, Cecco ARD, Czeisler CA, Dijk DJ (1999) Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol Reg Integ Compar Physiol 277:R1152
Zele AJ, Feigl B, Smith SS, Markwell EL (2011) The circadian response of intrinsically photosensitive retinal ganglion cells. PLoS One 6:e17860
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smith, S.S., Cheng, T. & Kerr, G.K. The effect of extended wake on postural control in young adults. Exp Brain Res 221, 329–335 (2012). https://doi.org/10.1007/s00221-012-3175-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-012-3175-8