Abstract
In a choice reaction time (RT) paradigm, providing partial advance information (a precue) about the upcoming response has been shown to decrease RT, presumably due to preprogramming of the precued parameters. When advance information about a particular aspect of a movement is provided (precued), several different strategies might be used to prepare the motor system during the foreperiod. For example, in studies where response preparation time was manipulated, precues were provided specifying the required arm and direction but movement amplitude was left uncertain. In this case it was shown that a default movement was preprogrammed whose amplitude was intermediate between the alternatives (Favilla et al. in Exp Brain Res 75(2):280–294, 1989, Exp Brain Res 79(3):530–538, 1990; Ghez et al. in Exp Brain Res 115(2):217–233, 1997). However, this strategy did not appear to be used in a RT task since there was an absence of online adjustments to movement. Therefore, it appeared movements were not initiated until all parameters had been correctly specified and programmed by the nervous system (Bock and Arnold in Exp Brain Res 90:(1):209–216, 1992). The present study reinvestigated the notion of a default movement preparation strategy in a choice RT paradigm, employing the triggering effect of a startling acoustic stimulus. On control trials (80 dB imperative stimulus), the movements were initiated toward the correct targets. Providing a startle stimulus (124 dB) resulted in the early initiation of a “default” movement whose amplitude fell in between the potential response alternatives. Thus, the current experiment found behavioral evidence of default intermediate-amplitude movement preparation as a strategy under conditions of response amplitude uncertainty.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
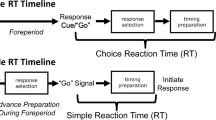
In a simple reaction time (RT) paradigm, it is presumed that response selection and response programming typically occur prior to the imperative stimulus, because all aspects of the upcoming response are known in advance. In a choice RT paradigm, however, it has been traditionally thought that preprogramming of movements is not possible because the required response is not identified until the RT interval begins (Klapp 1996). One variation of the choice RT task is the precue method (Rosenbaum 1980), which was developed to uncover how different aspects of an upcoming movement might be specified and programmed if only partial information about the required response is provided in advance.
In this precue method, participants are required to perform movements that might differ from trial to trial on various response parameters. For example, Rosenbaum (1980) used movements that might have involved two possible directions (e.g., forward or backward), hands (right or left), and/or amplitudes (near or far). Prior to onset of the imperative stimulus, a precue is provided specifying some, none, or all of the information about the upcoming response, presumably permitting some degree of response preprogramming. Thus, a precue might instruct that the upcoming movement will be made in the forward direction, but which hand to be used and the movement amplitude might be left unspecified. Thus, the programming of unspecified parameters must occur following presentation of the imperative stimulus (when the remaining parameters are provided). It has been consistently found that as more parameters of a response are precued, RT decreases (Goodman and Kelso 1980; Rosenbaum 1980). However, the manner in which partial information is used to preprogram various parameters may change with the nature of the precue. For example, Rosenbaum (1980) proposed a parameter specification model whereby preprogramming is a serial process and response parameters can be programmed independently of one another, with each parameter taking a differential amount of time. The increase in RT seen with a partial precue compared to a full precue (simple RT condition) presumably reflected the time needed to process and program remaining parameters following the imperative stimulus. An alternative argument to Rosenbaum’s (1980) parameter specification model is that the nervous system might have prepared multiple “holistic” movements during the foreperiod. It was suggested that because providing precues acts to decrease the number of response alternatives, selection time from among fewer response alternatives was shorter (Goodman and Kelso 1980). Thus, any increase in RT seen with partial precues compared to a full precue condition was indicative of the time required to choose the appropriate movement from among the multiple prepared responses.
In contrast to the notion of preparing multiple movements, it has been suggested that if the required amplitude of an upcoming response was left unknown, a single “default” response whose amplitude was intermediate between the precued alternatives could be initially prepared (Favilla et al. 1989, 1990; Ghez et al. 1997). This research was based upon use of a timed response paradigm in which movement preparation time was directly manipulated. Specifically, precues would specify both a short amplitude and a long amplitude target, and participants would have to initiate a response concurrent with a go signal at the end of a known constant foreperiod. However, the time at which the correct target was provided during the foreperiod was temporally manipulated such that on some trials, participants had very little time (e.g., 100 ms) to process and accurately program the correct parameters. In this case a default intermediate-amplitude movement falling between the target alternatives was indeed observed.
A novel precue paradigm was introduced by Bock and Arnold (1992) to further investigate the response preparation strategies employed depending on precued information. Precues in this study were not limited to a small number of potential targets, but rather either specified a variable range of amplitudes while holding response direction constant, or specified various directions while holding response amplitude constant. RT was found to be shorter when the range of possible amplitudes was smaller, as well as when the range of possible directions was smaller. Importantly, accurate knowledge of one parameter was not a prerequisite for preprogramming the other. As RT was found to depend on the size of the amplitude or direction range, support was afforded to the view that preparation of movements is a gradual process, in which the precued range gets progressively constrained around the final target. Bock and Arnold (1992) acknowledged that intermediate or “default” preparation might be used as a potential strategy in a RT paradigm, whereby the movement would be adjusted toward the correct target once it was underway; however, they ultimately rejected it due to a lack of observed online kinematic changes. It was concluded that movements were not initiated until after the correct target had been presented and accurately programmed by the nervous system. It could, however, be argued that participants in Bock and Arnold’s (1992) study did initially preprogram an intermediate/default movement, and upon specification of the correct target, the default parameters were adjusted to be compatible with the final target location, prior to movement initiation. In order to determine whether this strategy was utilized, one would need to examine the content of the programmed response prior to final target specification.
A startle method has been previously used to investigate the content of prepared responses as it has been shown to act as an early trigger for whatever is prepared (see Carlsen et al. 2011, in press; Valls-Solé et al. 2008 for reviews), and thus may be able to provide insight into whether a default programming strategy may be used in a precued RT paradigm. Specifically, when the imperative stimulus in a simple RT task was replaced with a loud (e.g., 124 dB) tone, the entire preprogrammed response appeared to be elicited at latencies suggested to be too short to have involved the usual voluntary cortical initiation mechanisms (Carlsen et al. 2004a, b; in press; Valls-Solé et al. 1999). This conclusion was based on two findings. Firstly, the observed premotor RT following the startle (<70 ms) was greatly reduced compared to control trials and sufficiently short to call into question the use of normal voluntary stimulus response processing pathways (see Carlsen et al. 2004b for details). Secondly, the observed triphasic electromyography (EMG) pattern following startle was similar to control conditions in which no startle was presented.
Since early movement triggering by startle appeared to be dependent on response preprogramming, it was argued that startle could thus be used as a probe to investigate the conditions that allow for full preprogramming (Carlsen et al. 2004a). For example, presenting a startling acoustic stimulus in a choice RT task does not shorten RT to nearly the same extent as that seen in simple RT (Carlsen et al. 2004a; Kumru et al. 2006; Maslovat et al. 2011; Nijhuis et al. 2007; Reynolds and Day 2007), suggesting that the effects of a startling stimulus are different depending on the level of advance preparation achieved by the performer. However, in contrast to a typical choice RT task, a precued RT task may allow for either partial programming or programming of multiple full responses (as discussed above). A startling stimulus has been previously shown to trigger prepared movements in a precued choice RT paradigm, but only if the precue provided the choice between two independent movements (Carlsen et al. 2009). The results indicated that when two response alternatives were precued involving opposite hands, a strategy was adopted to fully prepare both response alternatives. Thus, when unexpectedly startled, both of the preprogrammed response alternatives were subsequently triggered at short latencies (Carlsen et al. 2009). Thus, it was suggested that it was possible for full preprogramming of multiple responses to occur and thus be triggered by a startle stimulus.
In the current study, we presented a precue that provided knowledge of limb and direction but not movement amplitude, thus limiting advance preparation of both response amplitude alternatives. Of interest was whether a “default” amplitude movement would be involuntarily initiated by the startle since this is one of the few choice RT situations in which sufficient motor preparation might allow for a movement to be triggered early by a startle stimulus. If as previously suggested, participants adopted a strategy whereby they initially prepared a default movement (Favilla et al. 1989, 1990; Ghez et al. 1997), it was expected that an auditory startle would trigger this default preprogrammed movement. The first part of the present experiment was a simple RT paradigm, in which the amplitude of movement was known in advance and therefore the auditory startle was expected to decrease the RT of the preprogrammed response, without altering the kinematics or EMG activation patterns (Carlsen et al. 2004a, b; Valls-Solé et al. 1999). Part two was a modified choice RT paradigm in which arm and direction were held constant but amplitude of the movement was not provided until presentation of the imperative stimulus. Since early trigger by a startle stimulus is dependent upon response preprogramming, we expected any response preparation to be evidenced by a large reduction in RT. If movement initiation occurred at very short latencies (i.e., <100 ms) any parameter adjustments during the RT interval was unlikely. Analysis of the initial EMG patterns was expected to provide insight into the strategy of preparation used by participants. Specifically, the duration of the first EMG burst in the agonist muscle (agonist duration) has been suggested to be the modulated variable when moving fast and accurate to targets of different amplitude (Gottlieb et al. 1989) and this relationship is preserved following presentation of a startle stimulus (Maslovat et al. in press). Therefore in the present study, if startle triggered a default movement between the targets, the agonist duration was expected to be similar for both amplitudes of movement. On control trials, if default parameters were adjusted appropriately prior to movement initiation, the short and the long movements were expected to have clearly distinct EMG activation patterns in addition to a long RT.
Methods
Participants
Thirteen (8 females, 5 males; ages 25 ± 4 years) right-handed subjects free of any obvious upper body abnormalities or sensory/motor dysfunctions participated in the study after providing informed consent. Three of the participants did not show a consistent startle response in the sternocleidomastoid and were removed from subsequent analysis (see Carlsen et al. 2011 for inclusion criteria). All participants were naïve to the hypothesis under investigation and this study was carried out in accordance with the ethical guidelines set forth by the University of British Columbia as well as the 1964 Declaration of Helsinki.
Apparatus and task
Participants sat in a height-adjustable chair facing a 22-inch color monitor (Acer X233W, 1,152 × 864 pixels, 75 Hz refresh) placed on a table. Also attached to the table, to the right of the monitor, was a lightweight manipulandum that moved in the horizontal plane and was used to perform flexion–extension movements around the right elbow joint. The participant’s right arm was secured to the manipulandum with a Velcro strap such that the elbow joint was aligned with the rotational axis and was positioned at shoulder height to restrict motion to the elbow joint. Participants were instructed to grasp onto a vertical metal rod at the end of the manipulandum such that the right hand was in a semi-supinated position. The resting position for the right arm was with the shoulder abducted 90 degrees, and the elbow flexed 120 degrees. Metal brackets (i.e., targets) were placed at 30 and 60 degrees of angular displacement from the resting position such that movements resulted in the tip of the manipulandum being lined up with the end of a bracket.
Participants were instructed to look straight ahead at the computer monitor and following an auditory imperative signal, make a right arm extension movement as fast and accurately as possible to either the short (30°) or long (60°) target. All trials started with a warning tone consisting of a short beep (80 ± 2 dB, 100 ms, 100 Hz) presented simultaneously with the word “Ready” on the computer monitor. In the simple RT paradigm, a visual cue was presented on the monitor concurrent with the warning tone consisting of either a short or a long arrow (representing the 30° and 60° targets, respectively). In contrast, the visual cue was presented simultaneously with the auditory imperative stimulus during the choice RT paradigm.
Following the warning tone was a random foreperiod of 2,500–3,500 ms. The imperative stimulus occurred at the end of the foreperiod and was either a control stimulus (80 ± 2 dB, 100 ms, 1,000 Hz) or a startle stimulus (124 ± 2 dB, 40 ms, 1,000 Hz, <1 ms rise time). Auditory signals were generated by a customized computer program, amplified, and delivered 30 cm behind the participant’s head via a loudspeaker. Prior to each testing session, the auditory stimuli were measured and calibrated via a sound level meter (Cirrus Research model CR252B set to measure on impulse) at a 30-cm distance from loudspeaker.
After completion of the movement, feedback was provided on the monitor following each trial that included displacement reaction time (in ms) and accuracy (in degrees), which was expressed as constant error between the required target and maximal displacement achieved. A monetary bonus was also provided on each trial to encourage both fast reaction times and accurate movements.
Participants performed one testing session, which lasted approximately 50 min. The first part of the experiment involved a simple RT paradigm while the second part involved a choice RT paradigm. In both paradigms, participants performed one block of 10 practice trials followed by three testing blocks of 24 test trials. Each testing block included four startle trials (see below) pseudorandomly presented (with the stipulation that no 2 consecutive trials were startle trials and that no startle trials occurred in the first three trials of each block). During the simple RT paradigm, blocks consisted of random presentation of either a short (30°) movement or a long (60°) movement, precued with the warning signal (see above). For the choice RT paradigm, a similar random presentation of short and long movements was performed; however, the amplitude of the movement was not precued in advance and was instead provided concurrent with the imperative stimulus.
Recording equipment
Preamplified electrodes were used to collect surface EMG from muscle bellies of the following four muscles: right lateral head of the triceps brachii (agonist), right long head of the biceps brachii (antagonist), and the right and left sternocleidomastoid (startle indicator). These electrodes were connected with a shielded cable to an external amplifier (Delsys Model DS-80). The recording sites were cleansed and prepared in order to minimize electrical impedance. A ground electrode was attached to the right ulnar styloid process and the electrodes were attached using double-sided adhesive, oriented parallel to the muscle fibers. A potentiometer (Precision, MD157) (precision of 0.07°/bit) was attached to the rotational axis of the manipulandum and was used to measure angular displacement of the arm. A LabView customized computer program was used to control stimulus presentation and feedback. The same program was used to collect EMG and displacement data at a rate of 1,000 Hz via an analog to digital interface (National Instruments, PC-MIO-16E−1). Data collection was initiated 500 ms prior to presentation of the imperative stimulus and terminated 2,500 ms later.
Data reduction
Analysis was performed only on test trials (practice trials were excluded). A total of 86 of the 1,440 testing trials were omitted (6.0%). Reasons for exclusion included errors in performance consisting of trials in which abnormal movements were performed (e.g., very slow movement times, 16 trials). Fifty-eight trials were also excluded when participants responded too quickly (i.e., anticipation) or too slowly (i.e., inattention). Trials discarded due to long or short RTs were determined by calculating the means and standard deviations (SD) of agonist onset times for both startle and control trials within each participant (in both simple RT and choice RT). The criterion was set such that trials falling greater than 2 SDs outside of the respective mean for each trial type of every participant were discarded. Additionally, startle trials in which no observable bilateral sternocleidomastoid EMG activity was detected within 90 ms of the acoustic stimulus (Carlsen et al. 2011) were labeled as non-startle trials and subsequently discarded. This criterion resulted in the omission of 12 startle trials (5.0%).
Dependent measures and statistical analysis
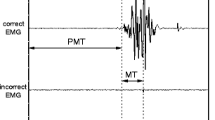
Surface EMG burst onset was defined as the point at which the rectified raw EMG first began a sustained rise above the baseline levels (the calculated mean of activity for 100 ms preceding the imperative stimulus on a trial-by-trial basis). The location of this point was determined by displaying the EMG pattern on a computer monitor with a superimposed line indicating the calculated point at which activity increased to more than 2 standard deviations above baseline. Onset was subsequently verified by visually locating and adjusting (if needed) the onset marker to the point at which activity first began a sustained increase above baseline. This method allowed for correction of errors due to the strictness of the algorithm. A similar method was used to mark EMG offset. Premotor RT was defined as the interval between presentation of the imperative stimulus and EMG activity onset in the right triceps brachii. We chose to examine premotor RT as this variable has been previously utilized in startle studies to support triggering of preprogrammed movements (e.g., Carlsen et al. 2004b; Valls-Solé et al. 1999).
To provide insight into the amplitudes of movements prepared and initiated under both stimulus conditions, we analyzed agonist duration. This variable was defined as the interval following the onset of EMG activity in right triceps brachii to the offset of activity. When movements are performed as fast and as accurately as possible, the agonist duration is thought to be modulated in order to reach targets of varying distance (Gottlieb et al. 1989).
In addition to EMG, we also examined kinematic measures to determine how the movements were performed. Displacement onset was defined as the first point of change of greater than 0.2° following onset of the imperative stimulus. Rather than using final displacement as a measure of movement amplitude, we used (initial) peak displacement as this more accurately reflects the preprogrammed ballistic portion of the movement. We were concerned that the entire movements were of sufficiently long duration to allow for online corrections and thus final displacement may not reflect the prepared movement amplitude. Peak displacement was defined as the point at which angular acceleration crossed the zero line a second time after displacement onset. To determine velocity, displacement data were first passed through a digital, fourth-order Butterworth lowpass filter (cutoff frequency of 10 Hz), and then differentiated. This method was repeated once to obtain acceleration data. To examine how consistent the prepared movement was, within-subject variability for each condition was determined by calculating the standard deviation of displacement at each millisecond for the first (Var1) and second (Var2) 100 ms and then calculated overall averages (see Maslovat et al. 2011 for a similar measure). We did not anticipate the first 100 ms of movement to be influenced by feedback and/or online corrections (Latash and Gottlieb 1991; Wadman et al. 1979) and thus Var1 should remain small if a similar movement was being initiated for each trial type. The second 100 ms of movement on the other hand would presumably be susceptible to feedback and/or online corrections and Var2 should increase if participants are making online modifications to the movement. Although this is a within-subject measure, it was expected to reveal online corrections as lack of advance preparation has been shown to result in increased movement variability (Maslovat et al. 2011). In addition to measuring the trial-to-trial variability, we also examined movement time and time after peak velocity (TAPV). Movement time was defined as the interval following displacement onset to the point where velocity decrease below 8 deg/s and remained below this value for a minimum of 50 ms. Similarly, TAPV was defined as the time interval from peak velocity to the point at which velocity decreased below 8 deg/s for at least 50 ms. Elliott et al. (1991) determined that added time following peak velocity was the result of feedback processing and the subsequent correction of movement errors. To provide insight into the efficiency and effectiveness of online corrections, we also examined constant error (CE) and variable error (VE) of the final limb position.
Analysis was performed separately for the simple RT and the choice RT sections of the experiment, as the interaction between the two conditions was not our primary interest. All dependent measures (Premotor RT, Agonist Duration, Peak Displacement, Var1, Var2, Movement Time, TAPV, CE, VE) were analyzed for significant differences between stimulus type (control versus startle) and movement type (short versus long), using a 2 × 2 repeated measures analysis of variance (ANOVA). When a significant stimulus type × movement type interaction was found, we employed simple main effects analysis to determine the location of the differences. Greenhouse-Geisser corrected degrees of freedom were applied to correct any violations of the assumption of sphericity. Uncorrected degrees of freedom are reported, with the corrected P values. Differences were considered to be statistically significant if a probability of less than 0.05 was determined. As a measure of effect size, partial eta (\( \eta_{\rho }^{2} \)) squared values were also reported.
Results
A summary of the results for all dependent measures, including means and standard deviations, are provided in Table 1.
Simple RT
In general, the presentation of an auditory startling stimulus in the simple RT condition replicated previous results (Carlsen et al. 2004b; Valls-Solé et al. 1999). That is, when the movement was known in advance and presumably preprogrammed, a startling stimulus resulted in early initiation of the movement, without altering the kinematics or triphasic EMG timing patterns. See Fig. 1a for typical movements to each target under both stimulus conditions (control and startle).
Exemplar trials of individual displacement profiles for each condition for subject 6. All profiles were normalized to displacement onset (time 0) and reveal displacement to the end of movement. a Simple RT task: note the early separation of short (gray) and long (black) movements under both control (dashed) and startle (solid) stimulus conditions. b Choice RT task: note on startle trials, the short movement overshoots significantly and the long and short movements are very similar for the first 150 ms of movement
EMG measures
For premotor RT, a main effect was found for stimulus type, F(1,9) = 26.009, P = 0.001, \( \eta_{\rho }^{2} \) = 0.743, indicating that the onset of the agonist burst was significantly shorter on startle trials (80.2 ms) than control trials (119.8 ms). As predicted, when the agonist duration (Fig. 2a) was analyzed, a main effect was found only for movement type, F(1,9) = 103.627, P < 0.001, \( \eta_{\rho }^{2} \) = 0.920, confirming a shorter agonist burst on short movements (99.2 ms) compared to long movements (119.7 ms), with no significant difference between startle and control conditions (P = 0.498).
a Simple RT task. Agonist duration means (and standard deviations) following either control or startle stimuli for both short (black) and long (white) movements. Note that a significant difference* occurred between the short and long movement on both control and startle trials. b Choice RT task. Note that a significant difference* occurred between the short and long control movements. No significant difference (NS) was found between the short and long startle movements
Kinematic measures
For peak displacement a main effect was found for movement type, F(1,9) = 956.473, P < 0.001, \( \eta_{\rho }^{2} \) = 0.991, which was due to the longer movements having a higher peak displacement compared to the short movements (as expected). A stimulus × movement interaction was also found, F(1,9) = 8.827, P = 0.016, \( \eta_{\rho }^{2} \) = 0.495. Simple main effects analysis revealed a significant difference between the control and startle trials for the short movement (32.9° vs. 36.2°), but no significant difference for the long movement. Analysis was also performed on the within-subject movement variability for the first (Var1) and second 100 ms (Var2) of the movement following displacement onset. For Var1, no significant differences occurred between the stimulus or movement conditions, confirming that the variability of the first 100 ms of movement was similar when triggered by startle. When Var2 was analyzed, a main effect was found for movement type, F(1,9) = 13.932, P = 0.005, \( \eta_{\rho }^{2} \) = 0.608, indicating that movements tended to be more variable in the second 100 ms for the long target (±5.1°) compared to the short target (±3.9°). Variability scores are represented in Fig. 3 by showing a group mean displacement plot with a 1 standard deviation of within-subject variability. Note the low variability in the simple RT condition (panels A and B) associated with both short and long movements under both stimulus conditions. Analysis of movement time revealed main effects for stimulus type, F(1,9) = 14.718, P = 0.004, \( \eta_{\rho }^{2} \) = 0.621, as well as movement type, F(1,9) = 35.996, P < 0.001, \( \eta_{\rho }^{2} \) = 0.800. This result indicates that movements to the 60° target took longer than movements to the 30° target and the startle stimulus reduced movement time to both targets. When TAPV was analyzed, a main effect was present for movement type, F(1,9) = 22.419, P = 0.001, \( \eta_{\rho }^{2} \) = 0.714, indicating that long movements take more time to reach the end of movement following peak velocity. This relationship was present following presentation of both control and startle stimuli. To explore the efficiency of online corrections, analysis was also performed on constant error (CE) and variable error (VE) of the final limb position. Analysis of CE revealed a main effect of movement type, F(1,9) = 5.70, P = 0.041, \( \eta_{\rho }^{2} \) = 0.388, and a stimulus × movement interaction, F(1,9) = 5.695, P = 0.041, \( \eta_{\rho }^{2} \) = 0.388. Simple main effects analysis uncovered a significant difference between the short (−0.2°) and long (−2.9°) startle trials, but no significant difference between control trials (−0.7° and −1.5°, respectively). Analysis of VE revealed no significant main effects between the movement and/or stimulus conditions.
Average displacement profiles with a ±1 averaged within-subject standard deviation window for each condition (see text for details). Long movement plot shown as white line with black window. Short movement plot shown as black line with gray window. a Simple RT control trials. b Simple RT startle trials. c Choice RT control trials. d Choice RT startle trials. In (d), note the increased within-subject variability after 100 ms
Choice RT
When the amplitude parameter of the movement was unknown in advance, presentation of a startling acoustic stimulus resulted in the early trigger of a default movement whose initial amplitude fell between the two potential targets, to be corrected later. See Fig. 1b (solid lines) and note the nearly identical displacement plots for the two movement types for the first 130 ms on startle trials. On control trials (dashed lines), participants performed movements to the correct target with clear early separation of the two movement types.
EMG measures
For premotor RT, a main effect was found only for stimulus type, F(1,9) = 42.390, P < 0.001, \( \eta_{\rho }^{2} \) = 0.825, indicating that RT was significantly shorter on startle trials (88.9 ms) compared to control (172.8 ms), and similar to values reported for the startle trials in the simple RT part of the experiment (80.2 ms; see Table 1). Agonist duration (Fig. 2b) showed a main effect for movement type, F(1,9) = 10.782, P = 0.009, \( \eta_{\rho }^{2} \) = 0.545. However, a significant stimulus × movement interaction, F(1,9) = 7.034, P = 0.026, \( \eta_{\rho }^{2} \) = 0.439, superseded this result. Simple main effects analysis uncovered a significant difference in agonist duration between the short and long movements on control trials (103.3 vs. 125.3 ms), but importantly, this significant difference was absent for startle trials (114.3 vs. 117.7 ms).
Kinematic measures
For peak displacement, main effects were found for movement type, F(1,9) = 54.140, P < 0.001, \( \eta_{\rho }^{2} \) = 0.857, as well as for stimulus type, F(1,9) = 11.662, P = 0.008, \( \eta_{\rho }^{2} \) = 0.564. A stimulus × movement interaction was also found, F(1,9) = 16.418, P = 0.003, \( \eta_{\rho }^{2} \) = 0.646, and simple main effects analysis revealed a significant difference on the short movement trials between the startle (46.5°) and control (33.5°) stimulus conditions. No significant difference was found between stimulus conditions for the long movement. Analysis was also performed on the within-subject movement variability of the first (Var1) and second 100 ms (Var2) of movement following displacement onset. For Var1, no significant differences were found between the stimulus or movement conditions, confirming that the variability of the first 100 ms of the movement was similar when triggered by startle compared to control. For Var2, a main effect was found for stimulus type, F(1,9) = 15.770, P = 0.003, \( \eta_{\rho }^{2} \) = 0.637, indicating that variability was much greater on startle trials for the second 100 ms of movement, which we attributed to online corrections. A stimulus × movement interaction was also found for Var2, F(1,9) = 8.388, P = 0.018, \( \eta_{\rho }^{2} \) = 0.482, and simple main effects analysis revealed greater variability occurring on long movement control trials, compared to short. This difference was not present on startle trials. See Fig. 3 (panels C and D) and note how the first 100 ms of movement on startle trials has a very low within-subject standard deviation. After 100 ms the variability begins to increase greatly, similar to the finding of Var2 increasing on startle trials. When movement time was analyzed, a main effect was found only for movement type, F(1,9) = 19.350, P = 0.002, \( \eta_{\rho }^{2} \) = 0.683, indicating that more time was needed to reach the 60° target compared to the 30° target. When the TAPV was analyzed, main effects were found for both stimulus type, F(1,9) = 5.608, P = 0.042, \( \eta_{\rho }^{2} \) = 0.384, and movement type, F(1,9) = 11.534, P = 0.008, \( \eta_{\rho }^{2} \) = 0.562. The main effect for movement type is similar to that found in the simple RT paradigm and indicates that on long movement trials, participants took longer to end the movement. More importantly, the main effect for stimulus type provides further evidence for the occurrence of online corrections following presentation of the startle stimulus. We attribute this increased time on startle trials to be a result of processing feedback. Finally, analysis was performed on CE and VE of the final limb position. For CE, an interaction was found, F(1,9) = 14.638, P = 0.004, \( \eta_{\rho }^{2} \) = 0.619. Simple main effects analysis revealed a significant difference between the short and long movements on startle trials (4.1° and −3.1°, respectively); however, no significant difference was found between the two movement amplitudes on control trials (−0.5° and −1.7°, respectively). Upon examination of VE, a main effect was found only for stimulus type, F(1,9) = 9.160, P = 0.014, \( \eta_{\rho }^{2} \) = 0.504, indicating greater variability on startle trials (6.9°), compared to control (4.5°).
Discussion
The purpose of present study was to examine whether preparation of a “default” movement amplitude could be behaviorally shown through the use of a startling acoustic stimulus. Although the present paper was not specifically designed to examine the effects of startle in a simple RT task, it was necessary to first replicate previous findings in the current experimental setup and use the simple RT as a form of control for the precue choice RT task.
In the simple RT condition, presentation of a startling stimulus reduced premotor RT from 119.8 to 80.2 ms. The particularly short latency to EMG onset suggests that a prepared movement was triggered early by the startling stimulus as shown previously (see Carlsen et al. in press; Valls-Solé et al. 2008 for reviews). We found no significant premotor RT differences between the short and long movements under both stimulus conditions. Further evidence of advance preparation of the specific movement was shown by the duration of the agonist burst which was significantly shorter for short movements (99.2 ms) than long movements (119.7 ms). As Gottlieb et al. (1989) suggested, movements of different amplitudes performed as fast and accurately as possible appear to be modulated by changes in the agonist duration. Although participants slightly overshot the 30° target following presentation of the startling stimulus, peak displacement of the short and long movements remained significantly different under both stimulus conditions. For Var1, the lack of significant difference between control and startle trials replicates previous findings (Maslovat et al. 2011) and confirms the prediction that what is prepared and initiated is consistent across stimulus conditions in a simple RT paradigm. This is illustrated in Fig. 3a, b by the low within-subject variability associated with the average displacement plots of this condition. In summary, when the required movement was known in advance, participants successfully preprogrammed the required movement parameters and presentation of an auditory startle resulted in the speeding of reaction time without modification to either EMG or kinematics.
Employing an auditory startling stimulus in the choice RT paradigm resulted in a speeding of premotor RT from 172.8 to 88.9 ms. This result is in stark contrast to previous findings in which startle had limited facilitating effects in a different choice RT paradigm, presumably because little-to-no preprogramming occurred in advance of the imperative stimulus (Carlsen et al. 2004a; Kumru et al. 2006; Maslovat et al. 2011; Nijhuis et al. 2007; Reynolds and Day 2007). This was because neither arm nor direction was provided. In the present experiment, arm and direction were known parameters, with amplitude being the sole parameter remaining to be specified by the imperative “go” stimulus, which appears to have allowed for much more advance preparation of the motor system. We believe that the present data suggest that a default intermediate-amplitude movement was prepared in advance, which was either adjusted prior to movement initiation on control trials, or triggered by the startle stimulus and then corrected online.
While the short latency to movement onset seen in startle trials suggests that some degree of advance programming occurred, agonist duration provides insight into the strategy of preparation used by participants. In control trials, the agonist duration was modulated to reach targets of different amplitudes (Gottlieb et al. 1989) as would be expected if the default parameters were modified prior to movement initiation. Importantly, on startle trials, no significant differences in agonist duration were observed between the short and long movements. Thus, our results indicate that for both amplitudes of movement, a similar agonist duration was triggered following unexpected presentation of a startle stimulus. This suggests that the early involuntary triggering did not allow for adjustment of the default agonist duration to be compatible with the correct target, and thus corrections occurred online.
Further support of default motor preparation was provided by analysis of the peak displacement. On short movement startle trials, participants overshot the 30° target reaching an average displacement of 46.5° (approximately the midpoint between the targets). By the time online corrections could be programmed and implemented, participants had already passed the short target and would have needed to make late corrections in order to reach the intended target. Online corrections have been shown to begin 100 ms after the onset of movement (Latash and Gottlieb 1991; Wadman et al. 1979), but in the present experiment the default movement triggered by startle had already passed 30° at the 100 ms point (e.g., see Fig. 1b). In the long movement trials, startle did not result in an undershoot of peak displacement, presumably because the triggered default movement could continue on to the long target prior to termination. In control trials the peak displacement for both short and long movements was appropriate for the respective target, consistent with the prediction that the amplitude parameter was adjusted prior to movement initiation. This adjustment of the amplitude parameter also accounts for the increased RT in both short and long movements compared to the simple RT conditions.
Previous research has shown that when there is little advance movement preparation, a startling stimulus results in an increase in the variability of movement for the first 100 ms (Maslovat et al. 2011). In contrast, the present study found no significant differences in movement variability for the first 100 ms (Var1) between the control and startle trials for each of the targets. We attribute this difference in findings to the fact that in the previous work (Maslovat et al. 2011) the choice paradigm did not precue direction and thus no advance preparation was possible, whereas in the present study the advance knowledge of direction and arm allowed for participants to preprogram a default movement. However, analysis of the second 100 ms of movement (Var2) revealed much larger variability on startle trials compared to control (see Fig. 3d). As startle caused the early triggering of a default movement between the targets, subsequent large adjustments were necessary for participants to reach the correct final target. We attribute the increased Var2 in startle trials to result from the implementation of online adjustments, which have been shown to begin after 100 ms (Latash and Gottlieb 1991; Wadman et al. 1979). Var2 was smaller in control trials (compared to startle—see Fig. 3c vs. d) likely because default parameters had been adjusted to the appropriate target, thus minimizing the need for large online corrections. As an additional measure of online corrections, we examined movement time and TAPV (see Table 1). As expected, on startle trials, participants spent more time ending the movement following peak velocity. Elliott et al. (1991) attributed any added time to this interval the result of correcting movement error. Therefore in the present study, we suggest that the increased time after peak velocity following presentation of the startling stimulus provides additional evidence for online corrections to the triggered default movement.
Other considerations
As an alternative to preprogramming default movements, it can also be argued that participants may have employed a guessing strategy whereby preparation of either the short or the long movement occurred at random. Presentation of the startling stimulus would have triggered the randomly prepared movement. In other words, half of the startle presentations would have triggered the short movement and the other half would have triggered the long. Consequently an average movement would appear in between the targets, similar to a triggered default movement. We discounted this guessing strategy, as variability of the first 100 ms of movement remained small, indicating a similar movement was being initiated following each startle presentation.
A default preparation strategy contrasts previous work where the preparation of movement endpoints initially occurred short of the target (e.g., Elliott et al. 1991). Elliott et al. (1991) found that preparing movements short of the target maximized speed and efficiency by avoiding time-consuming reversal movements (as seen with target overshoots). Elliott’s work was more concerned with corrections being made during a movement, whereas in the present study (in control trials), we are arguing for programming corrections made prior to initiation. It could be argued that participants initially prepared a short movement and made adjustments to reach the long target. However, our results clearly indicate that the startling stimulus triggered a movement past the short target. Therefore from the choice RT paradigm results in the present experiment, it appears that the most efficient preparation strategy was to prepare a single default movement in between response alternatives requiring only the specification of the response amplitude following the imperative stimulus.
Although the present study was not specifically designed to explore the efficiency of online corrections, error about the final endpoint provides further support for a default preparation strategy. On short startle trials, online corrective processes reduced constant error to 4.1 (from a peak displacement of 46.5°). The final limb position, however, was still beyond the 30° target. The monetary bonus provided to participants was based upon peak displacement, not final position, thus we attribute the overshoot of final position to a lack of incentive to correct movement error. On long startle trials, constant error was −3.1°, revealing a slight undershoot of the final limb position. The variable error data indicate greater variability on startle trials (6.9°), compared to control (4.5°). We take the increased endpoint variability on startle trials as further evidence of online adjustments made to a triggered default movement.
Conclusion
In summary, it appears that under conditions of movement amplitude uncertainty, participants adopted a strategy where they prepared a default movement whose amplitude fell between the potential response alternatives. For control trials, following presentation of the correct target, the default intermediate-amplitude parameter was adjusted during the RT interval to be compatible with the appropriate target. When the imperative stimulus was unexpectedly replaced with a startling stimulus, the default preprogrammed movement was triggered early without sufficient time during the RT interval to adjust the amplitude parameter to be compatible with the appropriate target. Online adjustments to the triggered default movement were necessary to reach the correct target.
References
Bock O, Arnold K (1992) Motor control prior to movement onset: preparatory mechanisms for pointing at visual targets. Exp Brain Res 90(1):209–216. doi:10.1007/bf00229273
Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM (2004a) Can prepared responses be stored subcortically? Exp Brain Res 159(3):301–309
Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM (2004b) Prepared movements are elicited early by startle. J Mot Behav 36(3):253–264
Carlsen AN, Chua R, Summers JJ, Inglis JT, Sanderson DJ, Franks IM (2009) Precues enable multiple response preprogramming: evidence from startle. Psychophysiology 46(2):241–251. doi:10.1111/j.1469-8986.2008.00764.x
Carlsen AN, Maslovat D, Lam MY, Chua R, Franks IM (2011) Considerations for the use of a startling acoustic stimulus in studies of motor preparation in humans. Neurosci Biobehav Rev 35(3):366–376. doi:10.1016/j.neubiorev.2010.04.009
Carlsen AN, Maslovat D, Franks IM (in press) Preparation for voluntary movement in healthy and clinical populations: evidence from startle. Clin Neurophysiol
Elliott D, Carson RG, Goodman D, Chua R (1991) Discrete vs. continuous visual control of manual aiming. Hum Mov Sci 10(4):393–418. doi:10.1016/0167-9457(91)90013-n
Favilla M, Hening W, Ghez C (1989) Trajectory control in targeted force impulses. Exp Brain Res 75(2):280–294. doi:10.1007/bf00247934
Favilla M, Gordon J, Hening W, Ghez C (1990) Trajectory control in targeted force impulses. Exp Brain Res 79(3):530–538. doi:10.1007/bf00229322
Ghez C, Favilla M, Ghilardi MF, Gordon J, Bermejo R, Pullman S (1997) Discrete and continuous planning of hand movements and isometric force trajectories. Exp Brain Res 115(2):217–233. doi:10.1007/pl00005692
Goodman D, Kelso JS (1980) Are movements prepared in parts? Not under compatible (naturalized) conditions. J Exp Psychol Gen 109(4):475–495. doi:10.1037/0096-3445.109.4.475
Gottlieb GL, Corcos DM, Agarwal GC (1989) Strategies for the control of voluntary movements with one mechanical degree of freedom. Behav Brain Sci 12:189–250
Klapp ST (1996) Reaction time analysis of central motor control. In: Zelaznik HN (ed) Advances in motor learning and control. Human Kinetics, Champaign, pp 13–35
Kumru H, Urra X, Compta Y, Castellote JM, Turbau J, Valls-Solé J (2006) Excitability of subcortical motor circuits in Go/no Go and forced choice reaction time tasks. Neurosci Lett 406:66–70
Latash ML, Gottlieb GL (1991) An equilibrium-point model for fast, single-joint movement: similarity of single-joint isometric and isotonic descending commands. J Mot Behav 23(3):179–191
Maslovat D, Hodges NJ, Chua R, Franks IM (2011) Motor preparation and the effects of practice: evidence from startle. Behav Neurosci (Advance online publication). doi:10.1037/a0022567
Maslovat D, Hodges NJ, Chua R, Franks IM (in press) Motor preparation of spatially and temporally defined movements: evidence from startle. J Neurophysiol
Nijhuis LBO, Janssen L, Bloem BR, Van Dijk JG, Gielen SC, Borm GF, Overeem S (2007) Choice reaction times for human head rotations are shortened by startling acoustic stimuli, irrespective of stimulus direction. J Physiol 584(1):97–109. doi:10.1113/jphysiol.2007.136291
Reynolds RF, Day BL (2007) Fast visuomotor processing made faster by sound. J Physiol 583(3):1107–1115. doi:10.1113/jphysiol.2007.136192
Rosenbaum DA (1980) Human movement initiation: specification of arm, direction, and extent. J Exp Psychol Gen 109(4):444–474
Valls-Solé J, Rothwell JC, Goulart F, Cossu G, Muñoz E (1999) Patterned ballistic movements triggered by a startle in healthy humans. J Physiol 516(3):931–938. doi:10.1111/j.1469-7793.1999.0931u.x
Valls-Solé J, Kumru H, Kofler M (2008) Interaction between startle and voluntary reactions in humans. Exp Brain Res 187(4):497–507
Wadman WJ, Dernier van der Gon JJ, Geuze RH, Mol CR (1979) Control of fast goal-directed arm movements. J Hum Mov Stud 5:3–17
Acknowledgments
This study was supported by a grant from the Natural Sciences and Engineering Research Council of Canada (NSERC) awarded to I.M.F. and an NSERC Undergraduate Student Research Award (USRA) conferred to C.J.F. We would like to thank Dr. Digby Elliott and two anonymous reviewers for comments on an earlier draft of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Forgaard, C.J., Maslovat, D., Carlsen, A.N. et al. Default motor preparation under conditions of response uncertainty. Exp Brain Res 215, 235–245 (2011). https://doi.org/10.1007/s00221-011-2893-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2893-7