Abstract
In selection tasks where target stimuli are accompanied by distractors, responses to target stimuli, target stimuli and the distractor stimuli can be encoded together as one episode in memory. Subsequent repetition of any aspect of such an episode can lead to the retrieval of the whole episode including the response. Thus, repeating a distractor can retrieve responses given to previous targets; this mechanism was labeled distractor-response binding and has been evidenced in vision and audition. Yet, previous research suggests possibly different distractor processing in the tactile as compared to the visual modality. In the present study, we therefore used a selection task in which participants always responded to one tactile stimulus while ignoring another. Evidence for the integration of tactile distractors with target responses was found in response times and errors. Our results indicate that binding of responses to distractors is a cognitive process that is independent of the stimulus’ modality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Imagine seeing a red ball rolling across the street. It is accepted that the single features (like color or shape, for example) of this ball are coded in a distributed fashion by the human brain (e.g., DeYoe and Van Essen 1988; Mishkin et al. 1983; Treisman 1996). Yet, we do not have the impression of perceiving the color red, a certain movement, and a spheric shape, but the impression of seeing a moving, red ball. The process responsible for the integration of distributed representations of object features into coherent objects is called binding. For example, Kahneman and Treisman (1984) argued that the different features of one object are bound together into one “object file.”
Yet, in the meanwhile, it is accepted that not only object features are subject to binding processes, but that responses and their features can be integrated with object features, as well. The theory of event coding (Hommel 2004; Hommel et al. 2001) proposes that a stimulus and the response to it are represented in the same stimulus-response (SR) episode or “event file,” which can be retrieved on subsequent presentation of any part of this SR episode. That is, if a stimulus is presented for a second time, the entire episode, including the stimulus and the previous response to it, is retrieved from memory. Thus, if the required response is the one given in the preceding encounter, responding is facilitated due to the retrieval of a compatible response.
However, response binding is not restricted to stimuli, to which participants respond. In fact, it has repeatedly been shown that even irrelevant stimuli or stimuli that have to be ignored can be integrated into event files and that these distractor stimuli can retrieve the event file containing the response to the target stimulus (Frings et al. 2007; Rothermund et al. 2005; see also Frings 2011; Frings and Moeller 2010; Giesen and Rothermund 2011; Mayr and Buchner 2006).
In a sequential prime–probe paradigm with a target and distractor stimulus on each display, such distractor-response bindings are evidenced by an interaction between response repetition and distractor repetition. If a prime distractor is repeated as the probe distractor, it retrieves the complete prime episode, which includes the response. Thus, the prime response is activated by the repetition of the distractor. Therefore, in a trial in which the response repeats from prime to probe, the repetition of the prime distractor as the probe distractor leads to faster and more accurate responses as compared to trials in which different distractors are presented on prime and probe displays. On the other hand, if the response changes from prime to probe, repeating the distractor from the prime to the probe leads to the retrieval of an incompatible response and hence causes interference.
Understanding the binding of stimulus and action features is crucial for understanding human behavior because binding of stimulus and action features helps to translate intentional actions, resulting from controlled and resource-demanding processing of information, into efficient behavioral routines and habits. The retrieval of previous SR episodes thus plays an important role in the automatization of behavior (Logan 1988; Hommel 2004). In turn, it seems worthwhile to analyze whether distractor-response binding can be observed in touch as it has been observed in vision and audition. More specifically to tactile distractor-response binding, this phenomenon could have clear implications for human action in everyday life. For example, current in-car-warning systems increasingly use tactile stimuli to trigger reactions by the driver (e.g., a vibration of the steering wheel which alerts the driver to an imminent lane departure). Yet, given that in next-generation interfaces for mobile phones, touch screens, MP3 players, etc., vibrotactile stimulation will often be used, it becomes quite likely that another tactile stimulus (i.e., a distractor stimulus, for example, the vibrating cell phone of the driver) occurs at the same time as the steering wheel vibrates. If tactile distractors can be bound to responses, an ignored cell phone vibration could then be integrated with the response to the tactile warning of the driver assistance system and retrieve the same response later on, even if it is not appropriate for the situation. Especially in a driving situation, fast and accurate responses are crucial and we need to understand the binding between tactile (distractor-) stimuli and responses as the re-encountering of the tactile distractor might retrieve the former response. Yet, generally speaking, as feedback from tactile interfaces will very probably increase in the years to come, it is in general worthwhile to understand the processes that might be involved when the cognitive system faces two tactile stimuli simultaneously. Therefore, the aim of the present study is to investigate whether distractor-response bindings can be obtained within the tactile modality.
On the one hand, it seems very plausible that tactile distractors can be bound to target responses. First, prior studies found evidence for distractor-response binding effects in vision (e.g., Frings et al. 2007), in audition, and even across these modalities (Frings et al. 2011). Thus, one may speculate that distractor-response bindings reflect modality-independent binding processes. Second, various studies found binding of target and response features in the visual (e.g., Hommel 1998, 2004), the auditory (e.g., Mondor et al. 2003; Mondor and Leboe 2008; Zmigrod and Hommel 2010), and the tactile modality (Zmigrod et al. 2009). In addition, some recent studies suggested that there is a common cortical representation of visual and tactual objects in the nervous system (e.g., James et al. 2006). Thus, if visual objects are bound together with response features and additionally if visual and tactile objects share a common representation, why should we not assume that tactile distractor features can be bound to response features as well?
However, on the other hand, the perception of tactile stimuli differs from visual and auditory stimuli in several aspects. First, the perception of tactile stimuli requires an integration of spatial somatosensory information over time, which is reflected in the involvement of the inferior parietal cortex (Saetti et al. 1999); second, somatosensory stimuli are typically perceived haptically, which includes exploratory finger movements; and third, location is coded by the location of the stimulus in external space, as well as by the perceived location of the stimulated body site. Even more important for the investigation of tactile versus visual distractor-response bindings are differences in the cortical organization of the somatosensory and visual system concerning the processing of perception and action (for a review, see Dijkerman and de Haan 2007). Several studies support the existence of separate “what” and “where” pathways in the visual systems (e.g., Mishkin et al. 1983; DeYoe and Van Essen 1988; Treisman 1996). A notion, which was further dissociated as “what” and “how” pathways (Goodale and Milner 1992; Milner and Goodale 1995; but see Franz et al. 2001). In particular, two separate but interacting cortical streams have been proposed for the processing of action and perception in the visual system: the dorsal stream is involved in the visual guidance of immediate goal-directed movements, whereas the ventral stream is primarily associated with visual perception and recognition (Milner and Goodale 1995). In contrast, in the tactile modality, action- and perception-related pathways are less distinct. The tactile dorsal pathway projects from the anterior parietal cortex (APC) to the posterior parietal cortex (PPC), either directly or via the secondary somatosensory cortex (SII). The tactile ventral pathway includes projections from the APC via the SII to the posterior insula and the PPC. Thus, for the somatosensory system, several cortical areas are involved in perception as well as in action-related processes (Dijkerman and de Haan 2007). This is not surprising, because most somatosensory perception of objects occurs haptically and therefore is a process that involves action. Further to the findings reported by Dijkerman and de Haan (2007), and Drewing and Schneider (2007) in fact suggest that the somatosensory system should not be divided into two structural streams (e.g., dorsal vs. ventral) but merely into functional streams of “how” and “what”; however, it should be noted that the dissociation into “what” and “how” pathways is controversially discussed not only for touch (e.g., Drewing and Schneider 2007).
Yet, concerning feature bindings, one can assume that the features of a distractor might be integrated into an event-file more readily, if object codes of the distractor and the response codes are less distinct. In particular, if the representation of the distractor and the response to the target partially include the same cortical regions, a repetition of the distractor would partially activate cognitive codes of the accompanying response. Consequently, effects of distractor-response bindings might be influenced by the extent to which perception- and action-related cortical pathways overlap. Regarding the possible differences in cortical representations of perception and action between vision and the somatosensory system, it seems possible that the tactile modality differs from the visual in terms of distractor-response bindings. One might argue that tactile distractor-response binding effects have the potential to emerge even under conditions in which visual binding effects diminish.
Additionally, several studies have found behavioral evidence for modality-specific processing of distractor stimuli (e.g., Frings et al. 2011; Spence 2010; Spence et al. 2004). For example, in spatial attention tasks, visual distractors lead to stronger interference as compared to tactile distractors (Soto-Faraco et al. 2002). Furthermore, in identification tasks, the effects of visual and tactile distractors seem to differ, as well. Sometimes vision seems to dominate touch and as a result tactile distractors are then irrelevant (e.g., Rock and Harris 1967; Pettypiece et al. 2010), whereas sometimes the effects of tactile distractors can even be stronger than the effects of visual distractors (Frings et al. 2011). In fact, in a negative priming task (for a review see, e.g., Fox 1995), repetitions of previously ignored stimuli as target stimuli led to stronger performance costs when the stimuli were tactile as compared to visual—even when stimulus factors (i.e., differences in the processing difficulty between tactile and visual stimuli) were controlled for.
To investigate whether tactile distractor-response bindings can be obtained, we transferred the visual task in which we usually observe distractor-response bindings to the tactile modality. We implemented a tactile selection paradigm (cf. Frings et al. 2008; see also Frings et al. 2011) with two orthogonally varied factors, namely response relation (repetition vs. change) and distractor relation (repetition vs. change). Four different tactile rhythms were used as stimuli. On each prime and each probe, two different rhythms, one target rhythm and one distractor rhythm, were presented simultaneously to the participants’ left and the participants’ right hand. The selection criterion was stimulus location (right vs. left). Tactile distractor-response bindings would be revealed by an interaction between response relation and distractor relation, that is, repeating a tactile distractor should enhance response repetition effects when the response repeats from the prime to the probe, whereas repeating a tactile distractor should decrease performance when the response changes between the prime and the probe.
Experiment
Method
Participants
Eighteen students (15 women) from Saarland University participated in the experiment; they were paid 8 € for participation. None of the participants reported any visual, auditory, or somatosensory perceptual impairment. The data of one participant were discarded due to the extreme error rate (27.08% probe errors, as compared with a mean probe error rate of 8.49% in the remaining sample). The median age of the remaining sample was 22 years with a range from 19 to 27 years.
Design
Essentially, the design comprised two within-subject factors, namely response relation (repetition vs. change) and distractor relation (repetition vs. change).
Materials
The experiment was conducted using the E-prime software (E-prime 1.1). Instructions and the fixation mark were presented in white on black background on a 17-in. standard cathode-ray tube screen (refresh rate was 75 Hz). Vibrotactile stimuli were presented via two skin transducers (Model VBW32, Audiological Engineering Corp.) to the left and right hand. The transient response of each transducer was measured at 5 ms (attack and decay). The amplitude range extended to 50 dB above the sensory threshold. The sound files used to generate the vibrotactile stimuli were amplified by a Sony hi-fi system before being presented via the transducers. Each transducer had a mass of 6.5 g (including the Velcro strap used to fasten it to the participant’s hand) and was 2.54 × 1.85 × 1.06 cm in size. A white- or blue-colored square with a visual angle of .8° was presented at the CRT screen to cue the target position. A white square indicated that the target would be presented on the right hand, and a blue square indicated that the target would be presented on the left hand. The straps on the subjects’ hands holding the skin transducers in place were colored accordingly (i.e., a white strap on the right and a blue strap on the left hand). The stimuli were four different vibrotactile rhythms that were used in a previous study and are known to be easily identifiable by participants (cf. Frings et al. 2011). All rhythms were presented repeatedly until participants responded. Rhythm 1 consisted of a single long vibration (340 ms) followed by three short vibrations (decreasing in intensity over 180 ms each). There were two pauses of 60 ms each, one after the long vibration and the other after the last of the three short vibrations. The whole sequence lasted 1,000 ms. Rhythm 2 was comprised of four vibrations of equal length (192 ms each), with each vibration being followed by a 58-ms empty interval. Rhythm 3 was composed of one short vibration (110 ms) followed by an 890-ms pause. Rhythm 4 consisted of a continuous vibration presented until participants responded. All vibrations had a frequency of 250 Hz.Footnote 1
Procedure
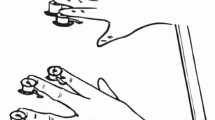
The experiment was conducted on a standard computer in a light-dimmed room. Instructions were given on the screen and summarized by the experimenter. White noise was presented to the participants over headphones throughout the experiment to mask all possible sounds made by the transducers. Participants were instructed to place the middle and index fingers of both hands on the keys D, F, J, and K of the computer keyboard. Each of the four rhythms was mapped to one particular finger. On every prime and every probe, one rhythm was presented as the target to one hand and a different rhythm was presented as the distractor to the other hand. Roughly following the procedure used by Frings et al. (2008), a colored square was presented in the center of the screen simultaneously to the rhythms, indicating whether the target was presented on the left or on the right hand. If the square was white, participants identified the rhythm on the right hand, and if the square was blue, participant reacted to the rhythm on the left hand. Rhythms were presented until participants’ response. Participant’s task was to identify the target rhythm by pressing the corresponding key as quickly and correctly as possible. Distractor rhythms had to be ignored. A single prime–probe sequence included the following events (cf. Fig. 1). The participant started the sequence by pressing the space bar and a white plus sign appeared as a fixation mark for 1,000 ms. Then, a white- or blue-colored square, indicating the target side, appeared in the center of the screen. Simultaneously, target and distractor rhythms were presented to the left and the right hand until participants reacted to the prime target rhythm by pressing the corresponding key. After the response to the prime, the fixation mark appeared for 1,000 ms, followed by a colored square indicating the target side for the probe. Simultaneously, the probe rhythms were presented until participants reacted to the probe target rhythm. Finally, an asterisk appeared in the middle of the screen, signaling to the participant that the next trial could be started.
Sequence of events in one trial. A visual cue presented simultaneously with each prime and probe indicated the hand on which the target was delivered. Participants reacted to the identity of the target rhythm by pressing the corresponding key. The letters A, B, and C depict different vibrotactile rhythms (bold letters indicate the target rhythm, whereas italic letters indicate the distractor rhythm). White is depicted in black, whereas blue is depicted in gray. Stimuli are not drawn to scale
In response repetition trials (RR), the same rhythm was presented as the target on the prime and the probe, respectively. In response change trials (RC), the target rhythm varied between prime and probe. Orthogonally to the response relation, the distractor relation was varied. In distractor repetition trials (DR), the rhythm presented as the distractor was the same on the prime and the probe, whereas in distractor change trials (DC), different rhythms were presented as distractor on the prime and the probe. In turn, four different conditions were conducted. In RRDR trials, the prime target and the prime distractor were repeated on the probe. In RRDC trials, the prime target rhythm was again presented as the probe target, while the distractor rhythm changed from prime to probe. In RCDR trials, the probe target differed from the prime target, while the prime distractor was repeated as the probe distractor. Finally, in RCDC trials, no rhythm was repeatedly presented on prime and probe.
Target rhythms were presented to the right hand in half of the trials and to the left hand in the other half. This holds true for primes and probes. With the orthogonal variation of response repetition, distractor repetition, side of the probe target, and side of the prime target, 16 different combinations resulted. In addition, we varied target identity orthogonal to these factors, i.e., every one of these 16 combinations was conducted equally often with each rhythm as the probe target. For each particular trial, the roles of the prime target, the prime distractor, and the probe distractor were randomly assigned to the three remaining rhythms. If the trial type demanded it, the prime stimuli were then changed as to realize the particular condition for this trial. For example, in a RRDC trial, the prime target rhythm was changed to the probe target rhythm. Participants worked through 192 trials that were presented in random order. The four trial types (RRDR, RRDC, RCDR, and RCDC) were realized in 48 trials each.
Before the experimental trials, participants worked through four learning and practice phases, gradually introducing the experimental task. During the first two phases, a photograph of two hands, indicating the correct response finger, was shown on the CRT screen. First, only one rhythm was presented to one hand and participants identified the rhythm. Simultaneously, a colored square, indicating the stimulated hand, was presented in the middle of the screen. The first phase consisted of eight prime–probe sequences. Before the second phase started, participants were instructed to determine the correct response without the help of the photograph. Everything else remained the same as in the first phase. The second phase included 16 prime–probe sequences. During the third part of the practice, no photograph indicating the correct response was shown. Each of the four rhythms had to be correctly identified for four consecutive times, before the last practice phase could be started. In the last phase, the distractor stimulus was introduced. Participants received one rhythm on each hand and identified the rhythm that was presented to the hand indicated by the colored square while ignoring the other rhythm. This last phase consisted of 48 prime–probe sequences. During all practice phases, feedback was presented after each response. On average, participants finished practice within 20–25 min.
Results
Only trials with correct answers to the prime and the probe were considered. Reaction times that were more than 1.5 interquartile ranges above the third quartile of the individual RT distribution of each participant (Tukey 1977) and those that were shorter than 200 ms were excluded from the analysis. Due to these constraints, 21.05% of all trials were discarded (probe error rate was 8.49%; prime error rate was 9.68%). Mean RTs and error rates for probe displays are depicted in Table 1.
In a 2 (response relation: repetition vs. change) × 2 (distractor relation: repetition vs. change) MANOVA with Pillai’s trace as the criterion, the main effect of distractor relation was significant, F(1,16) = 15.56, P = .001, η 2 p = .49. Reactions were faster if the distractor was repeated from prime to probe (1,882 ms) than if the distractor differed on prime and probe (1,998 ms). The main effect of response relation did not reach significance, F(1,16) < 1, η 2 p = .05. Importantly, the interaction between response relation and distractor relation was also significant, F(1,16) = 5.75, P = .029, η 2 p = .26, indicating that distractor-response bindings occurred (see Fig. 2, left panel). The same MANOVA on the error rates yielded an analogous pattern. The main effect of distractor relation reached significance, F(1,16) = 25.97, P < .001, η 2 p = .62, indicating more errors for trials with distractor change than for trials with distractor repetition. The interaction between response relation and distractor relation reached significance as well, F(1,16) = 13.57, P = .002, η 2 p = .46, again indicating distractor-response bindings (see Fig. 2, right panel). Participants made fewer errors if the distractor was repeated than if the distractor was changed, and this difference was significantly larger for trials with response repetition than for trials with response change. The main effect of response relation was not significant, F(1,16) = 2.25, P = .153, η 2 p = .12.
To further corroborate the robustness of tactile distractor-response binding effects, two control analyses were run. First, in most visual experiments, the target was always presented in the same location in the prime and the probe, whereas in our experiment, the probe target stimulus was presented to the same hand as on the prime only in half of the prime–probe sequences. In the other half, the prime and the probe target were presented to different hands. Depending on whether or not the target presentation side changed between prime and probe, a larger or smaller effect of distractor-response bindings might have revealed. To determine whether the relation of target presentation side influenced distractor-response bindings, a 2 (response relation) × 2 (distractor relation) × 2 (target presentation side relation: target location changed vs. target location repeated) MANOVA with Pillai’s trace as the criterion was conducted. The main effect of target presentation side relation was significant, F(1,16) = 85.33, P < .001, η 2 p = .84, indicating that response times were significantly faster if prime and probe targets were presented to the same hand (1,783 ms) as compared to trials in which prime and probe targets were presented to different hands (2,096 ms). However, the interaction between response relation and distractor relation (i.e., the effect of distractor-response bindings) was not significantly modulated by the relation of target presentation side between prime and probe, F(1,16) = 1.30, P = .271, η 2 p = .08. However, the main effect of distractor relation (i.e., distractor inhibition effect: an average benefit when the distractor repeats from the prime to the probe) was marginally modulated by target presentation side relation, F(1,16) = 4.04, P = .06, η 2 p = .20: if the distractor was presented to the same hand on the prime and the probe (i.e., target presentation side repeated), response times were significantly faster for distractor repetition than for distractor change trials, t(16) = 4.16, P = .001, whereas if the distractor was presented to different hands on the prime and the probe (i.e., target presentation side changed), response times for distractor repetition and for distractor change trials did not differ, t(16) = 1.44, P = .170.
Past research found evidence for spatial compatibility effects, i.e., Simon effects, within the tactile modality (e.g., Frings et al. 2008; Hasbroucq and Guiard 1992). In the present study, participants perceived the stimuli at their right and left hands while they also reacted with either their right or their left hand. As a consequence, the compatibility of presentation- and response hand could have had an influence on our results. To determine whether compatibility of presentation side and response side influenced distractor-response bindings, we conducted a 2 (response relation) × 2 (distractor relation) × 2 (presentation side/response side compatibility: compatible vs. incompatible) MANOVA with Pillai’s trace as the criterion. The main effect of compatibility was marginally significant, F(1,16) = 4.27, P = .055, η 2 p = .21, indicating faster responses if the presentation hand of the probe target was compatible with the response hand (1,904 ms) than in trials with incompatible presentation- and response hands (1,967 ms). However, the compatibility of presentation- and response hand did not significantly moderate the effect of distractor-response bindings, F(1,16) < 1, η 2 p = .03.
Discussion
We investigated whether tactile distractors can be bound to, and subsequently retrieve, responses to tactile target stimuli. In a prime–probe paradigm with orthogonally varying response and distractor relation, the interaction between response relation and distractor relation was significant, indicating a larger advantage in response times and error rates due to distractor repetition if the same response was required than if the response had to be changed. Note that participants were instructed to ignore distractor stimuli; nevertheless, we found clear evidence for distractor-response bindings both in probe reaction times and in probe errors (cf. Fig. 2). This result supports the idea that even an actively ignored tactile stimulus can be integrated with, and subsequently retrieve, the response given to a simultaneously presented relevant stimulus (Rothermund et al. 2005). In addition, our findings corroborate that distractor binding is a process that works in all three modalities tested, as now evidence for distractor-response binding has been yielded in vision, audition, and touch. That is, the differences in the neural processing of perception and action in the somatosensory as compared to the visual system did not modulate distractor-response bindings. In contrast, regarding the present results, one might speculate that the modality does not play a role in distractor-response bindings at all. Particularly, the effect size of η 2 p = .26, which we found with tactile stimuli, is comparable to the effects sizes of visual and auditory distractor-response binding effects in experiments in our laboratory using the same design and roughly the same number of trials and participants (mean effect of η 2 p = .29; Frings 2011; Frings and Moeller 2010; Frings and Rothermund 2011). This might be an indication that binding functions in a similar way in the different modalities, regardless of differences in the cortical processing of action and perception. It might also indicate that distractor-response bindings involve the integration of conceptual distractor features with the response rather than perceptual features (see Frings et al. 2011; Spapé and Hommel 2008).
However, the story might be more complex. Note that the effect sizes of distractor-response binding effects in previous visual and auditory experiments had a relatively large range (auditory range was from η 2 p = .13 to η 2 p = .58; visual range was from η 2 p = .08 to η 2 p = .55). Despite the influence of stimulus factors, the grouping of target and distractor stimuli enlarged the distractor-response binding effects. In particular, effect sizes were large if target and distractor were presented in a grouped fashion, whereas effect sizes were small if target and distractor were presented in a non-grouped fashion; this pattern was observed in vision (Frings and Rothermund 2011; Giesen and Rothermund 2011; van Dam and Hommel 2010) and audition.
In the current experiment, target and distractor stimuli were always presented at different hands, which could be interpreted as a non-grouped presentation (in contrast to presenting targets and distractors to the same hand, for example). In fact, it has been shown that participants are better able to focus their attention on a tactile target and ignore a tactile distractor, if target and distractor are presented at different hands as compared to the same hand (e.g., Evans and Craig 1991; Evans et al. 1992). Thus, with respect to distractor-response bindings in vision and audition, one might have expected to find a small or no effect of distractor-response binding in a tactile task with a non-grouped presentation of stimuli. The result that we still observed a distractor-response binding effect might be due to the fact that cortical processing pathways for perception and action features are less distinct in the somatosensory system.
However, in contrast to visual and auditory stimuli, the location of a tactile stimulus depends not only on the body site that is stimulated (the somatotopic frame of reference). As pointed out by Craig and Johnson (2000), another factor influencing tactile spatial perception is proprioception (e.g., Overvliet et al. 2011), that is, the perceived position of the stimulated body site in space (the external frame of reference). Thus, tactile stimulus locations can differ in their somatotopic distance and their distance in external space, which complicates the possible influence of spatial grouping on distractor-response binding. For example, if the right hand is positioned close to the left hand and one tactile stimulus is presented to each hand, the somatotopic distance of the stimulated areas is relatively large, while the distance in external space is rather small. A number of studies indicate that the distribution of tactile stimuli in external space plays an important role in tactile location perception (Spence et al. 2004; Craig 2003; Kennett et al. 2001, 2002; Rinker and Craig 1994; Shore et al. 2002; Spence et al. 2000). In contrast, Evans et al. (1992) found that the variation of hand position in external space did not influence the amount of interference induced by a tactile distractor stimulus. Yet, participants in the Evan et al.’s study received tactile stimuli at their individual fingers. Haggard et al. (2006) provide evidence suggesting a difference between the neural representation of fingers and that of hands. They argued that finger identification is achieved using somatotopic information and is insensitive to spatial and postural factors. In contrast, hand identification is strongly dependent on body posture and location in egocentric external space. In the present study, participants’ palms were stimulated and their hands were always placed close together. With regard to the findings cited above, it is likely that target and distractor were perceived to be at the same position (they were perceived as being grouped) rather than two different positions. In addition, all tactile rhythms used in the present study had a synchronized one-second interval. Compared to visual stimuli, which typically share merely the onset and the offset, this synchronization might have enhanced grouping even further. Taken together, whether the stimulus location in external space (or grouping in general) can modulate tactile distractor-response binding in a similar way as stimulus location modulates the effect in vision and audition remains an interesting question for future research. That is, whether grouped presentation of tactile target and distractor stimuli would result in a larger distractor-response binding effect as compared with non-grouped target and distractor presentation.
In contrast to the current indications regarding distractor-response bindings, the aftereffect of ignoring distractors seems to be influenced by the stimulus modality. The ignoring of tactile distractors elicited larger negative priming effects than the ignoring of visual distractors (Frings et al. 2011). Negative priming has been explained in terms of distractor inhibition (e.g., Houghton and Tipper 1994; Tipper 1985) as well as with retrieval processes (e.g., Neill 1997). Yet, if we assume that tactile negative priming reflected at least partially inhibition and further that tactile negative priming was larger than visual negative priming, one might conclude that distractor inhibition is indeed dependent on the distractor modality.
Notably, the current results also indicated effects of distractor inhibition. An average benefit in probe reaction times and probe errors for distractor repetition was found even in conditions with response change. This positive priming effect of distractor-to-distractor repetitions is exactly what an inhibition account would predict (Frings and Wühr 2007; Houghton and Tipper 1994): on probe presentation, the repeated distractor stimulus still suffers from inhibition; consequently, a repeated distractor interferes less with the processing of the target stimulus than a new distractor. The benefit for repeated distractors on both response repetition and response change trials indicates a general inhibition of distractor stimuli in the present study. However, an inhibition of the distractor stimulus cannot account for the finding that distractor repetition benefits (i.e., faster response times and smaller error rates if the distractor was repeated than if the distractor was changed) were larger in response repetition trials than in response change trials. Instead, an inhibition theory assumes distractor repetition benefits to be independent of the response relation between prime and probe. The difference in distractor repetition effects for response repetition and response change trials clearly indicates distractor-response bindings. Furthermore, the effect of distractor-response binding was neither modulated by the relation of target presentation side between prime and probe nor by the compatibility of response hand and target presentation side. In contrast, distractor inhibition tended to be influenced by target presentation side relation. Note that in the experiment presented here, a change in target presentation side automatically entailed a change in distractor presentation side. Apparently, a distractor inhibition effect only reveals for repeated distractor location, whereas repetition of distractor location seems not to be crucial for distractor-response bindings. Taken together, these results may suggest that both processes—distractor inhibition and distractor-response binding—work in parallel (cf. Frings 2011). In addition, one might speculate that the former process is dependent on the distractor-modality, whereas the latter is not.
Finally, we should discuss our findings with respect to previous studies on tactile response competition (e.g., Evans and Craig 1992; Evans et al. 1992). In fact, two levels of interference from a tactile distractor have been discussed. First, the interference caused by the distractor because the stimulus is physically different from the target and thus interferes with the identification of the target on a perceptual level (due to masking). Second, it has been suggested that both target and distractor stimuli are processed up to the representation of the response. Consequently, participants have two response representations available—the one of the target and the one of the distractor. The interference of the distractor is at least in part due to the incipient response activation of the distractor, which interferes with the response activation of the target (i.e., response competition). In the task we used in the present study, the distractor always interfered with the target at the level of response competition and at a perceptual level. Thus, response competition and masking have influenced all conditions to an equal amount and cannot explain our main finding of distractor-response binding.
In conclusion, our findings provide the first evidence for binding of distractor-features and responses-features when stimuli are delivered to the tactile modality. Given that similar effect sizes occurred in tactile, visual, and auditory modality, a general principle for the binding of distractor-features seems likely. In addition, our results point to possibly important differences between distractor inhibition and binding of distractors and responses regarding the dependence of the distractor modality.
Notes
Note that an interesting feature of our experiment was that we used quite complex tactile patterns as stimuli whereas in most published experiments on tactile processing short pulses were used as stimuli (e.g., Soto-Faraco et al. 2004). Our stimuli develop over time and could be identified not earlier than about 500 ms after stimulus onset. However, participants needed on average about 1,250 ms for identifying these patterns even when they were presented without distractors in a learning phase before the experiment. Note, that the average RTs in our experiment are hence much higher as compared to the RTs in studies using simpler stimuli. Yet, the tactile patterns used here might tap tactile processing as it happens in real life, as most tactile information in real life is generally more complex than a brief pulse (e.g. a specific vibration of a cell phone).
References
Craig J (2003) The effect of hand position and pattern motion on temporal order judgments. Percept Psychophys 65:779–788
Craig J, Johnson K (2000) The two-point threshold: not a measure of tactile spatial resolution. Curr Dir Psychol Sci 9:29–32
DeYoe EA, Van Essen DC (1988) Concurrent processing streams in monkey visual cortex. Trends Neurosci 11:219–226
Dijkerman HC, de Haan EHF (2007) Somatosensory processes subserving perception and action. Behav Brain Sci 30:189–239
Drewing K, Schneider WX (2007) Disentangling functional from structural descriptions, and the coordinating role of attention. Behav Brain Sci 30:205–206
Evans P, Craig J (1991) Tactile attention and the perception of moving tactile stimuli. Percept Psychophys 49:355–364
Evans P, Craig J (1992) Response competition: a major source of interference in a tactile identification task. Percept Psychophys 51:199–206
Evans P, Craig J, Rinker M (1992) Perceptual processing of adjacent and nonadjacent tactile nontargets. Percept Psychophys 52:571–581
Fox E (1995) Negative priming from ignored distractors in visual selection: a review. Psychon Bull Rev 2:145–173
Franz VH, Fahle M, Bülthoff HH, Gegenfurtner KR (2001) Effects of visual illusions on grasping. J Exp Psychol Hum Percept Perform 27:1124–1144
Frings C (2011) On the decay of distractor-response episodes. Exp Psychol 58:125–131
Frings C, Moeller B (2010) Binding targets’ responses to distractors’ locations: distractor response bindings in a location priming task. Atten Percept Psychophys 72:2176–2183
Frings C, Rothermund K (2011) To be, or not to be…included in an event file: When are distractors integrated into S-R episodes and used for response retrieval? J Exp Psychol Learn Mem Cogn. doi:10.1037/a0023915
Frings C, Wühr P (2007) On distractor repetition benefits in the negative-priming paradigm. Vis Cogn 15:166–178
Frings C, Rothermund K, Wentura D (2007) Distractor repetitions retrieve previous responses to targets. Q J Exp Psychol 60:1367–1377
Frings C, Bader R, Spence C (2008) Selection in touch: negative priming with tactile stimuli. Percept Psychophys 70:516–523
Frings C, Amendt A, Spence C (2011) When seeing doesn’t matter: assessing the after-effects of tactile distractor processing in the blind and the sighted. J Exp Psychol Hum Percept Perform. doi:10.1037/a0022336
Giesen C, Rothermund K (2011) Affective matching moderates S-R binding. Cogn Emot 25:342–350
Goodale M, Milner A (1992) Separate visual pathways for perception and action. Trends Neurosci 15:20–25
Haggard P, Kitadono K, Press C, Taylor-Clarke M (2006) The brain’s fingers and hands. Exp Brain Res 172:94–102
Hasbroucq Y, Guiard T (1992) The effects of intensity and irrelevant location of a tactile stimulation in a choice reaction time task. Neuropsychologia 30:91–94
Hommel B (1998) Event files: evidence for automatic integration of stimulus-response episodes. Vis Cogn 5:183–216
Hommel B (2004) Event files: feature binding in and across perception and action. Trends Cogn Sci 8:494–500
Hommel B, Müsseler J, Aschersleben G, Prinz W (2001) The theory of event coding (TEC): a framework for perception and action planning. Behav Brain Sci 24:849–937
Houghton G, Tipper SP (1994) A model of inhibitory mechanisms in selective attention. In: Dagenbach D, Carr TH (eds) Inhibitory processes in attention, memory, and language. Academic Press, San Diego, CA, pp 53–112
James T, James K, Humphrey G, Goodale M (2006) Do visual and tactile object representations share the same neural substrate? In: Heller M, Ballesteros S (eds) Touch and blindness: psychology and neuroscience. Lawrence Erlbaum Associates Publishers, Mahwah, NY, pp 139–155
Kahneman D, Treisman A (1984) Changing views of attention and automaticity. In: Parasuraman R, Davies R (eds) Varieties of attention. Academic Press, Orlando, FL, pp 29–61
Kennett S, Eimer M, Spence C, Driver J (2001) Tactile-visual links in exogenous spatial attention under different postures: convergent evidence from psychophysics and ERPs. J Cogn Neurosci 13:462–478
Kennett S, Spence C, Driver J (2002) Visuo-tactile links in covert exogenous spatial attention remap across changes in unseen hand posture. Percept Psychophys 64:1083–1094
Logan GD (1988) Toward an instance theory of automatization. Psychol Rev 95:492–527
Mayr S, Buchner A (2006) Evidence for episodic retrieval of inadequate prime responses in auditory negative priming. J Exp Psychol Hum Percept Perform 32:932–943
Milner AD, Goodale MA (1995) The visual brain in action. Oxford University Press, Oxford
Mishkin M, Ungerleider LG, Macko KA (1983) Object vision and spatial vision: two cortical pathways. Trends Neurosci 6:414–417
Mondor TA, Leboe LC (2008) Stimulus and response repetition effects in the detection of sounds: evidence of obligatory retrieval and use of a prior event. Psychol Res 72:183–191
Mondor TA, Hurlburt J, Thorne L (2003) Categorizing sounds by pitch: effects of stimulus similarity and response repetition. Percept Psychophys 65:107–114
Neill WT (1997) Episodic retrieval in negative priming and repetition priming. J Exp Psychol Learn Mem Cogn 23:1291–1305
Overvliet K, Anema H, Brenner E, Dijkerman H, Smeets J (2011) Relative finger position influences whether you can localize tactile stimuli. Exp Brain Res 208:245–255
Pettypiece C, Goodale M, Culham J (2010) Integration of haptic and visual cues in perception and action revealed through cross-modal conflict. Exp Brain Res 201:863–873
Rinker M, Craig J (1994) The effect of spatial orientation on the perception of moving tactile stimuli. Percept Psychophys 56:356–362
Rock I, Harris CS (1967) Vision and touch. Sci Am 216:96–104
Rothermund K, Wentura D, De Houwer J (2005) Retrieval of incidental stimulus-response associations as a source of negative priming. J Exp Psychol Learn Mem Cogn 31:482–495
Saetti MC, De Renzi E, Comper M (1999) Tactile morphagnosia secondary to spatial deficits. Neuropsychologia 37:1087–1100
Shore D, Spry E, Spence C (2002) Confusing the mind by crossing the hands. Cogn Brain Res 14:153–163
Soto-Faraco S, Spence C, Fairbank K, Kingstone A, Hillstrom AP, Shapiro K (2002) A crossmodal attentional blink between vision and touch. Psychon Bull Rev 9:731–738
Soto-Faraco S, Ronald A, Spence C (2004) Tactile selective attention and body posture: assessing the multisensory contributions of vision and proprioception. Percept Psychophys 66:1077–1094
Spapé MM, Hommel B (2008) He said, she said: episodic retrieval induces conflict adaptation in an auditory stroop task. Psychon Bull Rev 15:1117–1121
Spence C (2010) Crossmodal spatial attention. Ann N Y Acad Sci 1191:182–200
Spence C, Pavani F, Driver J (2000) Crossmodal links between vision and touch in covert endogenous spatial attention. J Exp Psychol Hum Percept Perform 4:1298–1319
Spence C, Pavani F, Driver J (2004) Spatial constraints on visual-tactile cross-modal distractor congruency effects. Cogn Affect Behav Neurosci 4:148–169
Tipper SP (1985) The negative priming effect: inhibitory effects of ignored primes. Q J Exp Psychol 37A:571–590
Treisman A (1996) The binding problem. Curr Opin Neurobiol 6:171–178
Tukey J (1977) Exploratory data analysis. Addison-Wesley, Reading, MA
Van Dam W, Hommel B (2010) How object-specific are object files? Evidence for integration by location. J Exp Psychol Hum Percept Perform 36:1184–1192
Zmigrod S, Hommel B (2010) Temporal dynamics of unimodal and multimodal feature binding. Atten Percept Psychophys 72:142–152
Zmigrod S, Spapé M, Hommel B (2009) Intermodal event files: integrating features across vision, audition, taction, and action. Psychol Res 73:674–684
Acknowledgments
The research reported in this article was supported by a grant of the Deutsche Forschungsgemeinschaft to Christian Frings (FR 2133/1-2). We thank Charlotte Schwedes, Michaela Wanke and Manfred Neumann for conducting the experiment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moeller, B., Frings, C. Remember the touch: tactile distractors retrieve previous responses to targets. Exp Brain Res 214, 121–130 (2011). https://doi.org/10.1007/s00221-011-2814-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2814-9