Abstract
Evidence from animal, clinical, and imaging studies suggests that the basal ganglia and their frontal connections mediate motor inhibition, but the role of dopamine remains unclear. The aim of our study was to investigate, for the first time, whether levodopa medication influences motor inhibition and conflict resolution on the conditional stop-signal reaction time task in patients with Parkinson’s disease (PD) tested on or off their medication. Sixteen PD patients and 17 healthy controls performed the conditional stop-signal reaction time (SSRT) task, which requires inhibition of responses when a stop signal is presented on “critical” trials. Additionally, on “non-critical” trials, participants are instructed to ignore the stop signal and respond, thus generating conflict between motor inhibition and initiation; and conflict-induced slowing (CIS) on these “non-critical” trials. Levodopa medication did not significantly influence response initiation, inhibition (SSRT) or the measure of conflict resolution (CIS). Compared to healthy controls, PD patients showed significantly worse response initiation and inhibition both on and off their levodopa medication. Our results suggest that motor inhibition or conflict-induced slowing on the conditional stop-signal RT task are not altered by dopamine replacement in PD. This conclusion is consistent with evidence from animal studies and clinical pharmacological investigations suggesting a role for noradrenaline in motor inhibition and impulsivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ability to inhibit prepotent or habitual responses and to engage in alternative behaviors more appropriate to the context or to goal achievement is an important component of executive control. Conversely, impulsivity, the tendency to respond quickly without full consideration of the consequences, represents a lack of executive control over behavior. Impulsivity is a feature of a range of psychiatric disorders such as attention deficit hyperactivity disorder (ADHD), addiction and substance abuse, impulse control disorders such as pathological gambling or shopping and is also observed in patients with Parkinson’s disease (PD) with these impulse control disorders (ICD). In PD, occurrence of such ICDs is associated with use of dopamine agonist medication for the treatment of the motor symptoms of the disorder (Weintraub et al. 2006; Evans et al. 2009).
Pharmacological studies in man (de Wit et al. 2002; Friedel 2004) and animal studies (Rubinstein et al. 1997; Puumala and Sirvio 1998; Cardinal et al. 2000; Winstanley et al. 2006) suggest that dopamine plays a role in mediating impulsive behavior, which represents delay aversion or failure to reflect and consider options and their consequences before acting or an inability to inhibit or withhold prepotent action. In healthy adults, individual differences in genetic polymorphisms that affect dopamine binding or reuptake have been shown to affect motor inhibition during the stop-signal reaction time task (Congdon et al. 2008). Most relevant to the relative participation of the direct and indirect fronto-striatal pathways in inhibition, recent evidence demonstrates that while direct infusions of a D1 receptor antagonist in the dorsomedial striatum of the rat shortens stop-signal reaction times (SSRTs) (an estimate of the time taken to stop a movement when a stop signal is presented), following D2 receptor antagonist infusions SSRT is prolonged (Eagle et al. 2008a, 2011). Others have examined the influence of dopamine regulation on inhibition through investigating medication effects in ADHD and shown that levodopa had no effect on SSRTs (Overtoom et al. 2009). In studies on rats, administration of a mixed D1/D2 receptor antagonist cis-flupenthixol (Eagle et al. 2007) or blocking dopamine transporter (Bari et al. 2009) did not affect SSRTs on a modified version of the stop-signal task. As a result, it has been proposed that dopamine is involved in the go but not the stop process (Eagle et al. 2007).
Therefore, at present, the evidence relating to the influence of dopamine on inhibitory processing is inconsistent. The stop-signal reaction time task allows assessment of motor inhibition. When faced with conflicting options, choice of the appropriate response often requires inhibition of the alternative competing response. Thus, investigation of reaction times in situations of conflict offers another approach to studying inhibition in addition to conditions where outright motor inhibition of a prepared action is required. The aim of our study was to examine whether levodopa medication affects speed of movement initiation, motor inhibition, and conflict resolution in a conditional stop-signal RT task in PD, and if so, whether it has similar or differential effects on these processes. Based on previous evidence on the lack of effect of dopamine manipulations on SSRTs in animal studies (Eagle et al. 2007; Bari et al. 2009) or of levodopa on SSRTs in ADHD (Overtoom et al. 2003), we predicted that dopaminergic medication state would not produce a significant effect on motor inhibition indexed by SSRTs in PD patients.
Methods
Participants
Seventeen individuals with PD (12 men, 11 right handed) were recruited from The National Hospital for Neurology and Neurosurgery. All met UK Brain Bank diagnostic criteria for PD (Hughes et al. 1992). The Unified Parkinson’s disease rating scale motor part (UPDRS-III; Fahn and Elton 1987) was used to obtain a measure of disease severity when patients were on and off their usual medication. All patients were non-demented with scores >26 on the mini-mental state examination (MMSE; Folstein et al. 1975). None were clinically depressed (Beck Depression Inventory, BDI; score <18; Beck et al. 1961). All patients were treated with levodopa. The mean levodopa equivalent daily dose (LEDD; Williams-Gray et al. 2007) was 915.94 milligrams (SD = 698.9). In addition, three patients were receiving dopamine agonists (pergolide). On medication, patients were assessed within 1 h of taking their last dose. In the “off” state, patients were assessed after overnight withdrawal of medication, after an average of 13.26 (SD = 2.5) h off medication. This withdrawal time is standard practice in Parkinson’s research (Defer et al. 1999) and is intended to standardize and minimize the effect of medication while patients are still functional.
Sixteen healthy volunteers (7 men, 13 right handed) participated. None had any neurological disorder, psychiatric illness, head injury, or history of alcohol or drug abuse. Information about the controls and PD patients is presented in Table 1.
The study was approved by the Joint Ethics Committee of the Institute of Neurology and The National Hospital for Neurology and Neurosurgery. Informed consent was obtained from all participants
Assessment of motor inhibition and conflict resolution
Conditional stop-signal task
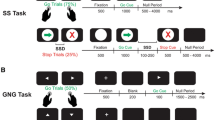
We employed the conditional version of the stop-signal task (Aron et al. 2007), which has the advantage that it allows concurrent measurement of speed of initiating a response (go RTs) inhibiting a response (SSRT), and speed of initiating a response under conditions of conflict (conflict-induced slowing, CIS).
The conditional stop-signal task involved a series of Go and Stop trials. On Go trials (75% of all trials), a go signal (either a left or right pointing green arrow) was presented on the computer screen and participants had to respond as fast as possible using the index and middle fingers of their dominant hand to press either a left or right key. On Stop trials (25% of all trials), a stop signal (red cross) was presented following the go signal, after a variable stop-signal delay (SSD). For each participant, either the left or right pointing arrows were designated as the “critical” direction. When a stop signal was presented following a go signal in the “critical” direction, participants had to stop their response to the go signal. In contrast, if a stop signal was presented following a go signal in the “non-critical” direction, participants had to ignore the stop signal and respond to the go signal. There were three blocks of trials, consisting of 32 Stop and 96 Go trials per block (128 trials per block, 384 trials in total). In each block, the number of left and right pointing arrows was equal, and in every four trials, there was 1 Stop and 3 Go trials. Durations from 5 to 4 s were inserted as null events between the Stop and Go trials.
The SSD value for the Stop trials was sampled from one of four staircases, changing dynamically throughout the task based on the participant’s response. Initially, the four staircases started with SSD values of 100, 150, 200, and 250 ms, respectively. Successful inhibition of a response on a Stop trial made inhibition more difficult on the next Stop trial by increasing the SSD by 50 ms. In contrast, if a response was not successfully inhibited, inhibition became easier by decreasing the SSD by 50 ms. Staircases of four step-up and step-down algorithms were used in this way to ensure convergence to P(inhibit) of 50% by the end of the three blocks. Each staircase (100, 150, 200, and 250 ms) moved four times within each block for Stop trials of the “critical” direction. SSDs for Stop trials of the “non-critical” direction were yoked to the “critical” direction values. The staircases were independent but were randomly mixed in a block of trials.
The RT and accuracy measures obtained are listed in Table 2. The measure of “conflict-induced slowing” (CIS) was obtained by subtracting mean RTs for “non-critical” Go trials from mean RTs for “non-critical” Stop trials. Using the standard Race Model (Logan and Cowan 1984), we estimated the stop-signal reaction time (SSRT) by subtracting the average SSD from the mean correct “critical” Go RT. Due to the dynamic adjusting of the SSD, we computed the average SSD for each participant, using the values of the four staircases after the participant had converged on 50% P(inhibit). The SSD estimation was averaged from the mean values for the last six moves in each of the four staircases.
For most comparisons of patients’ performance on and off medication, we employed paired t tests, and for most comparisons of patients’ performance with controls, we used independent t tests. However, for the within subject comparisons of errors, we used Wilcoxon tests, and for the between group comparisons of errors, we employed Mann–Whitney tests.
Procedure
PD patients performed the conditional stop-signal RT task twice, on 2 days with a mean interval of 7.2 days (SD = .9). Nine of seventeen PD patients were tested off medication first and 8 on medication first. Controls completed all tests once. Assignment of left or right arrows as the “critical” direction was counterbalanced in each group and on each occasion.
Participants were instructed to “respond to the green arrows by pressing the correct response key as fast and as accurately as possible, while at the same time, also look out for the appearance of the red cross and try to withhold the response when this followed a green arrow pointing in the ‘critical’ direction.” Participants were specifically instructed not to “let their performance on the stopping task interfere with their performance on the Go task”. Twenty practice trials were completed.
Results
One patient was excluded from the study as the disease severity while “off” medication led to altered performance on the stop-signal task that did not result in inhibition of 50%, which is required for accurate calculation of SSRT, thus resulting in 17 PD patients overall. The groups were matched in terms of age [t (31) = 1.93, p = .08], sex distribution [χ (1) = 1.48, p = .22], education [t (31) = −1.88, p = .07], and handedness [t (31) = .58, p = .56]. PD patients did not have dementia or clinical depression, and there was no significant difference relative to controls on the MMSE [t (31) = −1.21, p = .23] or BDI [t (31) = 1.37, p = .17] (see Table 1).
Effects of levodopa medication on inhibition of a motor response
Conditional stop-signal task
Table 2 summarizes the main measures of the conditional stop-signal task. The dynamic adjustment of SSD resulted in successful inhibition of around 50% for all participants (50% for patients off medication, 51% for patients on medication, and 54% for controls), and these percentages did not differ significantly on versus off medication [Wilcoxon z = −.54, p = .58] or between groups [PD off vs. controls: Mann–Whitney U = 107.00, p = .29; PD on vs. controls: Mann–Whitney U = 114.00, p = .42]. This equivalence across groups and medication states is essential for correct interpretation of the results. A longer SSD reflects better response inhibition as it means that the participant reached 50% inhibition after longer stop-signal delays that make inhibition more difficult. The inhibition on 50% or so of trials was achieved in patients off medication with a mean SSD of 168.25 (SD = 89.9), and with a numerically longer (improved inhibition) mean SSD when the patients were on medication (M = 190.59, SD = 93.0), albeit not significantly so [t (16) = −.86, p = .40]. The control group had a numerically longer mean SSD (M = 216.17, SD = 104.8) than PD patients off or on medication, although the differences were not significant relative to patients either off [t (31) = −1.41, p = .16] or on medication [t (31) = −.74, p = .46].
“Critical” trials
“Critical” Go RTs were comparable for PD patients off and on medication [t (16) = .28, p = .77]. Controls had significantly faster “critical” Go RTs relative to patients off medication [t (31) = 1.95, p = .05] and marginally significantly faster “critical” Go RTs relative to patients on medication [t (31) = 1.96, p = .07]. Similarly, “critical” StopRespond RTs were comparable for PD patients off and on medication [t (16) = −.29, p = .77]. Controls had significantly faster “critical” StopRespond RTs relative to patients both off [t (31) = 2.73, p = .01] and on [t (31) = 3.20, p < .01] medication (see Fig. 1).
Mean RTs in milliseconds for Go and StopRespond trials in the “critical” and “non-critical” directions, plotted separately for patients with Parkinson’s disease (PD) tested on or off levodopa medication and healthy controls. For the within subject comparisons, we used paired t tests, and for between subjects comparisons, we employed independent t tests. Asterisks show significant values between groups (p < .05). Error bars show standard errors
PD patients’ response accuracy as indexed by fewer “critical” Go omission errors was significantly better off compared to on medication [Wilcoxon Z = −2.10, p = .03]. Moreover, when PD patients were tested off medication, the number of “critical” Go omission errors made was only marginally worse than controls [Mann–Whitney U = −.94, p = .09], whereas when patients were tested on medication, they made significantly more “critical” go omission than controls [Mann–Whitney U = 38.0, p < .001] (see Fig. 2).
Mean number of errors of omission on “critical” and “non-critical” trials and discrimination errors, plotted separately for patients with Parkinson’s disease (PD) tested on or off levodopa medication and healthy controls. For the within subject comparisons, we used Wilcoxon tests, and for between subjects comparisons, we employed Mann–Whitney tests. Asterisks show significant values between groups (p < .05). Error bars show standard errors
For PD patients tested off medication, motor inhibition (SSRT) was numerically (M = 389.34 ms, SD = 81.0) but not significantly worse than when they were tested on medication (M = 361.20 ms, SD = 83.7; t (16) = −1.32, p = .21). Controls had significantly faster/better SSRTs (M = 293.73, SD = 38.7) relative to patients both off [t (31) = 4.49, p < .001] and on [t (31) = 3.14, p < .01] medication (see Fig. 3).
Mean stop-signal reaction time (SSRT) in milliseconds plotted separately for patients with Parkinson’s disease (PD) tested on or off levodopa medication and healthy controls. For the within subject comparisons, we used paired t tests, and for between subjects comparisons, we employed independent t tests. Asterisks show significant values between groups (p < .05). Error bars show standard errors
“Non-critical” trials
“Non-critical” Go RTs were comparable for PD patients off and on medication [t (16) = .18, p = .85]. Controls had significantly faster “non-critical” Go RTs relative to patients off [t (31) = 3.55, p = .001] and on [t (31) = 3.97, p < .001] medication. “Non-critical” StopRespond RTs were comparable for PD patients off and on medication [t (16) = .24, p = .81]. Controls had significantly faster “non-critical” StopRespond RTs relative to patients both off [t (23.3) = 2.54, p = .01] and on [t (31) = 2.53, p = .01] medication (see Fig. 1).
For each participant, a difference score was computed by subtracting mean “non-critical” Go RTs from mean “non-critical” StopRespond RTs to obtain a measure of the conflict-induced slowing (CIS) effect. A positive CIS difference score is indicative of greater slowing under conflict and more difficulty in resolving it. For PD patients tested off medication, the CIS (M = 118.59, SD = 101.1) was larger/worse compared to when they were tested on medication (M = 112.46, SD = 75.9) although this difference was not significant [t (16) = −.21, p = .83]. Furthermore, for controls, the CIS (M = 104.60, SD = 51.7) was numerically smaller but not significantly different from patients both off [t (31) = .49, p = .62] and on [t (31) = .34, p = .73] medication (see Fig. 4).
The mean conflict-induced slowness (CIS) in milliseconds for healthy controls, patients with Parkinson’s disease (PD) tested on or off levodopa medication. For the within subject comparisons, we used paired t tests, and for between subjects comparisons, we employed independent t tests. Error bars show standard errors
The number of “non-critical” Go omission errors made by PD patients was comparable when they were off relative to on [Wilcoxon Z = −1.07, p = .28] medication. Controls made significantly fewer “non-critical” Go omission errors relative to patients off medication [Mann–Whitney U = 55.5, p < .01]. However, there was no difference between the number of “non-critical” Go omission errors made by controls and PD patients on [Mann–Whitney U = 90.5, p = .10] medication (see Fig. 3).
The percentage of incorrect inhibition of the response on “non-critical” trials was comparable for PD patients tested off and on [Wilcoxon Z = −.07, p = .93] medication. Controls made significantly fewer incorrect inhibitory responses than patients off medication [Mann–Whitney U = −2.08, p = .04]. However, the percentage of incorrect inhibitory responses was comparable for controls and patients tested on medication [Mann–Whitney U = −1.06, p = .28].
Discrimination errors were comparable for PD patients off and on medication [Mann–Whitney U = −.19, p = .84]. Controls made significantly fewer discrimination errors relative to patients off [Mann–Whitney U = 37.0, p < .001] and on [Mann–Whitney U = 41.0, p < .001] medication (see Fig. 2). This reveals a response selection bias in PD patients relative to controls that was not influenced or improved by medication state.
Effect of levodopa medication on SSD, SSRT, and CIS for the slower and faster PD patients
As shown in Table 3, no “baseline” effects biased the medication effects on SSRT, SSD, or CIS for the patients with slower or faster (median split) “critical” Go RTs off medication.
Effect of disease severity on and off medication
A median split of the UPDRS off medication was used to determine whether subgroups with different disease severity differed in inhibition of a motor response. Off medication, 9 patients had high UPDRS scores and 8 low scores. There were no significant differences between the subgroups in SSRT or CIS [p > .05]. However, the low UPDRS scorers had significantly [t (15) = 2.40, p = .03] higher (better) SSD (M = 215.62, SD = 70.9) compared to the high UPDRS scorers (M = 126.15, SD = 87.0). The same comparisons of subgroups based on UPDRS scores “on” medication produced no significant effects on SSRT, CIS, or SSD [p > .05].
Correlational analysis
When patients were “off” medication, a significant and negative correlation between off UPDRS scores with mean SSD [r = −.49, p = .04] and a significant positive correlation with SSRT [r = .54, p = .02] were found. None of the other correlations between clinical measures (duration of illness, UPDRS, LEDD, MMSE, and BDI) and RT measures (GoRTs, CIS, SSD, and SSRT) were noteworthy or significant either on or off medication.
Discussion
Our results show for the first time that levodopa medication significantly improved the motor symptoms of PD but did not influence the efficiency or speed of inhibition or conflict resolution in PD patients. The only significant medication effect observed in our study was that PD patients made significantly more errors of omission in the “critical” direction when tested “on” than “off” medication. Those with less severe PD off medication inhibited their motor responses with significantly higher (better) SSD values than patients with more severe PD, an effect that was not observed on medication. This was confirmed by correlational analysis showing that more severe PD off medication was significantly associated with shorter SSDs and longer SSRTs.
Relative to healthy controls, PD patients tested off or on medication showed significantly longer “non-critical” Go RTs, “critical” and “non-critical” Stop Respond RTs, SSRTs and discrimination errors. These findings in the medicated state are consistent with the findings of previous studies that assessed PD patients on medication either on the standard version of the stop-signal RT (Gauggel et al. 2004) or the conditional stop-signal task (Obeso et al. 2011). The current study further demonstrated, for the first time, that these deficits in response initiation, response initiation under conflict, and motor inhibition are also present when PD patients off medication are compared to healthy controls and that the observed deficits are not simply observed on dopamine medication.
The present “negative” results have nonetheless important implications for the role of dopamine in motor inhibition and impulsivity and conflict resolution. Consistent with past animal studies (Eagle et al. 2007; Bari et al. 2009) and medication studies in PD (Crucian et al. 2007) or ADHD (Overtoom et al. 2003), the present findings indicate that levodopa does not induce impulsivity as measured by the SSRT and confirm the lack of effect of dopaminergic medication on conflict resolution in PD (Frank et al. 2007). Further, as discussed below, restoration of dopamine levels may not be a crucial element of stopping a response and alternative neurotransmitters such as noradrenaline may exert greater control over motor inhibition.
Levodopa medication did not influence response initiation under conflict
The magnitude of the conflict-induced slowing/interference was not altered by the patients’ medication state. The effect of levodopa medication on CIS was not “baseline” dependent, and no medication effects were observed for either the patients with slow or fast Go RTs off medication. Similarly, those with more severe PD did not differ from the subgroup with less severe PD in terms of CIS. In contrast, instead of ignoring the stop signal and responding on all “non-critical” trials, when tested off medication, PD patients incorrectly inhibited their responses and failed to respond on a significantly greater proportion of the “non-critical” StopRespond trials than the controls, which indicates an inability to ignore the stop signal on these “non-critical” trials while “off” medication. When tested on medication, patients showed no differences from healthy controls on the “non-critical” StopRespond trials. Overall, the error rate was low. However, medicated PD patients made significantly more errors of omission in the “critical” direction than when tested “off” medication. This was the only significant medication effect observed in our study. These higher rates of erroneous inhibition and failure to respond on “non-critical” stop trials when a response is required when off medication, and the higher omission errors (failure to make a response on “critical” Go trials) when on medication, indicate a greater susceptibility of PD patients to errors compared to controls. This is consistent with the proposal that the BG together with the anterior cingulate contribute to error monitoring and the error-related negativity that is reduced in PD (Falkenstein et al. 2001).
Levodopa medication did not influence motor inhibition in PD
Our results demonstrating that levodopa medication did not influence speed or efficiency of motor inhibition in PD are consistent with the findings from studies showing that a D1/D2 receptor antagonist (Eagle et al. 2007) or a dopamine transporter blocker (Bari et al. 2009) had no effect on SSRTs in a modified version of the stop-signal RT task in rats. Our results are also in line with the demonstration that levodopa did not affect Go RTs, SSRTs or percent of inhibition on the stop-signal task in 16 children with ADHD (Overtoom et al. 2003) or affect speed or accuracy of patients with tic disorders on a go no RT task (Hershey et al. 2004). While the dopamine manipulations in rats were associated with significant effects on Go RTs but not SSRTs on the stop-signal task (Eagle et al. 2007; Bari et al. 2009), our results in PD and those of Overtoom et al. (2003) in children with ADHD concur that levodopa did not influence either the speed of inhibition or initiation on this task. Such lack of dopaminergic effects on response initiation in RT tasks in PD has been previously documented by us (Jahanshahi et al. 1992) and others (Girotti et al. 1986; Bloxham et al. 1987; Pullman et al. 1990). Our finding that levodopa medication had no effect on motor inhibition on the stop-signal RT task is also consistent with the only previous study that examined dopamine effects on another task assessing inhibition in PD. Crucian et al. (2007) found no differences in the performance of PD patients on a crossed motor inhibition task (with eyes shut, lift hand opposite to the one touched by examiner) on or off medication, indicating that inhibition on this task was not sensitive to dopamine manipulation either.
Some previous studies have found the effect of dopamine (Eagle et al. 2007) or deep brain stimulation (DBS) therapy (Ray et al. 2009) on SSRTs to be baseline dependent. Similarly, other studies found baseline dependent effects from methylphenidate and d-amphetamine stimulants on inhibition in ADHD patients (Tannock et al. 1989; Aron et al. 2003) and in rats (Eagle and Robbins 2003). We specifically examined whether the effects of levodopa medication on SSRT or CIS were baseline dependent and found no such effects. However, a median split in UPDRS scores revealed that disease severity off medication influenced the stopping “threshold” based on the mean SSD results, such that patients with less severe disease achieved motor inhibition with mean SSDs that were twice longer (better inhibition) than those for patients with more severe disease, an effect that was not observed on medication. This indicates that as PD progresses, patients with more severe PD are less able to inhibit a motor response when a stop signal is presented at longer SSDs after the go signal when assessed off medication. When tested on levodopa medication, there were no differences in inhibition between patients with more or less severe PD. These results suggest that medication state may interact with disease severity in influencing motor inhibition in PD. This proposal would require more direct investigation in future studies with PD samples with a range of disease severity tested on and off medication.
It has been noted that failure of inhibition and impulsivity may incorporate a number of dissociable components (Chamberlain and Sahakian 2007). One component labeled “reflection” relates to the ability to obtain and evaluate information prior to arriving at a decision. The second component of impulsivity, “deferment of reward,” refers to the ability to delay gratification and wait for delayed larger rewards in preference to more immediate but smaller rewards, usually assessed with the delay discounting task. In relation to the first and second components, impulsivity is manifested as delay aversion and an inability to take time to reflect/weigh evidence or wait for gratification. The final component, “motor inhibition” is the ability to inhibit prepotent responses. The latter component of motor inhibition is tapped by the conditional stop-signal RT task and was assessed here. It is possible that these different components of inhibition/impulsivity are differentially sensitive to dopamine. In fact, on the basis of a comprehensive review of the evidence, it has been suggested that impulsivity is multifactorial, with each factor having a different biological basis (Evenden 1999). For example, the “reflection” or the “hold your horses” component necessary for decision-making in high-conflict situations has been shown not to be sensitive to levodopa medication in PD, with patients showing longer RTs in high than low conflict situations both on and off medication in a probabilistic decision-making task (Frank et al. 2007). In contrast, in PD patients with deep brain stimulation of the subthalamic nucleus, patients were more impulsive and had significantly faster RTs in high conflict than low conflict situations with DBS on than with DBS off (Frank et al. 2007). Similarly, in terms of delay discounting, PD patients without ICDs assessed on levodopa medication performed similar to healthy controls, whereas those with PD and ICD showed increased delay discounting and impulsivity (Housden et al. 2010; Voon et al. 2010). From available evidence, it seems that levodopa therapy does not influence motor inhibition on the stop-signal task (present study) or “reflective” impulsivity on decision-making tasks (Frank et al. 2007). The effect of dopamine replacement therapy on delay discounting in PD remains to be examined in a study assessing patients on and off medication.
Neurochemical substrates of motor inhibition and impulsivity
At first sight, our results and those of others (Overtoom et al. 2003; Crucian et al. 2007; Eagle et al. 2007) seem to suggest that dopamine is not the key neurotransmitter relevant to motor inhibition and impulsivity on the stop-signal task. However, more recent evidence allows an alternative interpretation of these levodopa effects. Eagle et al. (2008a, 2011) found that direct infusion of D1 and D2 receptor antagonists into the dorsomedial striatum had opposing effects on SSRT in the rat, respectively, decreasing and increasing SSRTs and either having no effect (D1 antagonist) or prolonging (D2 antagonist) go RTs. The results of Eagle’s et al. (2011) study suggest that when acting on D1 receptors in the direct pathway dopamine slows down inhibition so that action is facilitated; whereas when acting on D2 receptors in the indirect pathway dopamine speeds up inhibition at the expense of action, to brake more quickly. As levodopa has non-specific D1/D2 effects, it is possible that our finding that levodopa did not significantly alter SSRT in PD is due to the opposing effect of dopamine replacement therapy on the direct and indirect pathways, the net effect of which would be no change in the speed of inhibitory processing.
Which other neurotransmitter systems are important to inhibitory control? Neuropharmacological studies in experimental animals, in healthy participants or in patients with ADHD show that noradrenaline is a key neurotransmitter with an impact on inhibitory control during the stop-signal task (Chamberlain et al. 2006, 2007, 2009; Robinson et al. 2008). For instance, atomoxetine, the selective noradrenaline reuptake inhibitor, has been shown to improve/speed up SSRT (i.e. to reduce impulsivity during the stop-signal RT task) in healthy participants (Chamberlain et al. 2006) and in adults with ADHD (Chamberlain et al. 2007), and to improve SSRT in a dose-dependent fashion in rats without affecting the speed of Go RTs (Robinson et al. 2008). More recently, imaging in healthy controls has demonstrated that 40 mg of atomoxetine increased activation of the right inferior frontal gyrus during successful stopping on the stop-signal RT task and this increased activation during successful inhibition was associated with plasma levels of the drug (Chamberlain et al. 2009).
Dopamine is considered to play a crucial role in mediating reward-related processing (Schultz 1997), reinforcement learning (Montague et al. 1996; Frank and Claus 2006; Frank et al. 2007), working memory (Kimberg et al. 1997; Cools et al. 2008), and temporal processing (Meck 1996; Rammsayer 2008; Jahanshahi et al. 2010; Wiener et al. 2011) and to determine the vigor of actions (Niv et al. 2007). According to the opponency model of the action of dopamine and serotonin, dopamine is associated with invigoration, reward, and positively valenced appetitive or approach behaviors. In contrast, inhibition, punishment, and negatively valenced or aversively motivated behaviors are mediated by serotonin (Boureau and Dayan 2011). In this regard, there is evidence implicating serotonin in some types of inhibitory control and impulsivity (Evenden 1999; Eagle et al. 2008b). For example, following 5-HT depletion, anticipatory and premature responses increase, reflecting an inability to inhibit and withhold a response until the appropriate stimulus presentation (Harrison et al. 1999; Winstanley et al. 2004). Imaging studies have established that manipulation of the serotonergic system alters orbitofrontal activation in no Go trials (e.g. Del-Ben et al. 2005). Furthermore, from a review of the literature, it was concluded that while serotonin mediates action restraint on the go no go RT task, it is not critical to action cancelation in the stop-signal RT task (Eagle et al. 2008b).
Our results indicate that levodopa medication does not influence either motor inhibition or conflict resolution on the conditional stop-signal RT task in PD. The more specific effects of dopamine action on D1 and D2 receptors in behavioral inhibition as measured by the SSRT can be examined in future human studies through investigation of the effect of D2 specific antagonists on SSRT in healthy participants or administration of dopamine agonists with specific affinity for D1 or D2 receptors in PD in further on/off medication studies.
References
Aron AR, Dowson JH, Sahakian BJ, Robbins TW (2003) Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 54:1465–1468
Aron AR, Behrens TE, Smith S, Frank MJ, Poldrack RA (2007) Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci 27(14):3743–3752
Bari A, Eagle DM, Mar AC, Robinson ES, Robbins TW (2009) Dissociable effects of noradrenaline, dopamine, and serotonin uptake blockade on stop task performance in rats. Psychopharmacology (Berl) 205:273–283. doi:10.1007/s00213-009-1537-0
Beck AT, Erbaugh J, Ward CH, Mock J, Mendelsohn M (1961) An inventory for measuring depression. Arch Gen Psychiatry 4:561–571
Bloxham CA, Dick DJ, Moore M (1987) Reaction times and attention in Parkinson’s disease. J Neurol Neurosurg Psychiatry 50:1178–1183
Boureau YL, Dayan P (2011) Opponency revisited: competition and cooperation between dopamine and serotonin. Neuropsychopharmacology 36:74–97. doi:10.1038/npp.2010.151
Cardinal RN, Robbins TW, Everitt BJ (2000) The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl) 152:362–375
Chamberlain SR, Sahakian BJ (2007) The neuropsychiatry of impulsivity. Curr Opin Psychiatry 20:255–261. doi:10.1097/YCO.0b013e3280ba4989
Chamberlain SR, Muller U, Blackwell AD, Robbins TW, Sahakian BJ (2006) Noradrenergic modulation of working memory and emotional memory in humans. Psychopharmacology (Berl) 188:397–407. doi:10.1007/s00213-006-0391-6
Chamberlain SR, Del Campo N, Dowson J, Muller U, Clark L, Robbins TW, Sahakian BJ (2007) Atomoxetine improved response inhibition in adults with attention deficit/hyperactivity disorder. Biol Psychiatry 62:977–984. doi:10.1016/j.biopsych.2007.03.003
Chamberlain SR, Hampshire A, Muller U et al (2009) Atomoxetine modulates right inferior frontal activation during inhibitory control: a pharmacological functional magnetic resonance imaging study. Biol Psychiatry 65:550–555. doi:10.1016/j.biopsych.2008.10.014
Congdon E, Lesch KP, Canli T (2008) Analysis of DRD4 and DAT polymorphisms and behavioral inhibition in healthy adults: implications for impulsivity. Am J Med Genet B Neuropsychiatr Genet 147B:27–32. doi:10.1002/ajmg.b.30557
Cools R, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M (2008) Working memory capacity predicts dopamine synthesis capacity in the human striatum. J Neurosci 28:1208–1212. doi:10.1523/JNEUROSCI.4475-07.2008
Crucian GP, Heilman K, Junco E, Maraist M, Owens WE, Foote KD, Okun MS (2007) The crossed response inhibition task in Parkinson’s disease: disinhibition hyperkinesia. Neurocase 13:158–164. doi:10.1080/13554790701448184
de Wit H, Enggasser JL, Richards JB (2002) Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology 27:813–825. doi:10.1016/S0893-133X(02)00343-3
Defer GL, Widner H, Marie RM, Remy P, Levivier M (1999) Core assessment program for surgical interventional therapies in Parkinson’s disease (CAPSIT-PD). Mov Disord 14:572–584
Del-Ben CM, Deakin JF, McKie S et al (2005) The effect of citalopram pretreatment on neuronal responses to neuropsychological tasks in normal volunteers: an FMRI study. Neuropsychopharmacology 30:1724–1734. doi:10.1038/sj.npp.1300728
Eagle DM, Robbins TW (2003) Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and d-amphetamine. Behav Neurosci 117(6):1302–1317
Eagle DM, Tufft MR, Goodchild HL, Robbins TW (2007) Differential effects of modafinil and methylphenidate on stop-signal reaction time task performance in the rat, and interactions with the dopamine receptor antagonist cis-flupenthixol. Psychopharmacology (Berl) 192:193–206. doi:10.1007/s00213-007-0701-7
Eagle DM, Allan ME, Wong JCK, Mar AC, Theobald DE, Robbins TW (2008a) Different effects of D1 and D2 receptor antagonists on the stop-signal task: comparison of effects in the dorsomedial striatum and nucleus accumbens core. In: Society for Neuroscience Annual Meeting, Washington, DC
Eagle DM, Bari A, Robbins TW (2008b) The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology (Berl) 199:439–456. doi:10.1007/s00213-008-1127-6
Eagle DM, Wong JC, Allan ME, Mar AC, Theobald DE, Robbins TW (2011) Contrasting roles for dopamine D1 and D2 receptor subtypes in the dorsomedial striatum but not the nucleus accumbens core during behavioral inhibition in the stop-signal task in rats. J Neurosci 31:7349–7356. doi:10.1523/JNEUROSCI.6182-10.2011
Evans AH, Strafella AP, Weintraub D, Stacy M (2009) Impulsive and compulsive behaviors in Parkinson’s disease. Mov Disord 24:1561–1570. doi:10.1002/mds.22505
Evenden JL (1999) Varieties of impulsivity. Psychopharmacology (Berl) 146:348–361
Fahn S, Elton RL, and members of the UPDRS Development Committee Unified Parkinson’s Disease Rating Scale (1987) In: Fahn SMC, Goldstein M, Clane DB (ed) Recent developments in Parkinson’s disease. Macmillan Healthcare Information, Florham Park (NJ), pp 153–163
Falkenstein M, Hielscher H, Dziobek I, Schwarzenau P, Hoormann J, Sunderman B, Hohnsbein J (2001) Action monitoring, error detection, and the basal ganglia: an ERP study. Neuroreport 12:157–161
Folstein MF, Folstein SE, McHugh PR (1975) Mini-mental state—practical method for grading cognitive state of patients for clinician. J Psychiatr Res 12:189–198
Frank MJ, Claus ED (2006) Anatomy of a decision: striato-orbitofrontal interactions in reinforcement learning, decision making, and reversal. Psychol Rev 113:300–326. doi:10.1037/0033-295X.113.2.300
Frank MJ, Samanta J, Moustafa AA, Sherman SJ (2007) Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science 318:1309–1312. doi:10.1126/science.1146157
Friedel RO (2004) Dopamine dysfunction in borderline personality disorder: a hypothesis. Neuropsychopharmacology 29:1029–1039. doi:10.1038/sj.npp.1300424
Gauggel S, Rieger M, Feghoff TA (2004) Inhibition of ongoing responses in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 75:539–544
Girotti F, Carella F, Grassi MP, Soliveri P, Marano R, Caraceni T (1986) Motor and cognitive performances of Parkinsonian patients in the on and off phases of the disease. J Neurol Neurosurg Psychiatry 49:657–660
Harrison AA, Everitt BJ, Robbins TW (1999) Central serotonin depletion impairs both the acquisition and performance of a symmetrically reinforced go/no-go conditional visual discrimination. Behav Brain Res 100:99–112
Hershey T, Black KJ, Hartlein J, Braver TS, Barch DM, Carl JL, Perlmutter JS (2004) Dopaminergic modulation of response inhibition: an fMRI study. Brain Res Cogn Brain Res 20:438–448. doi:10.1016/j.cogbrainres.2004.03.018
Housden CR, O’Sullivan SS, Joyce EM, Lees AJ, Roiser JP (2010) Intact reward learning but elevated delay discounting in Parkinson’s disease patients with impulsive-compulsive spectrum behaviors. Neuropsychopharmacology 35:2155–2164. doi:10.1038/npp.2010.84
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55:181–184
Jahanshahi M, Brown RG, Marsden CD (1992) The effect of withdrawal of dopaminergic medication on simple and choice reaction time and the use of advance information in Parkinson’s disease. J Neurol Neurosurg Psychiatry 55:1168–1176
Jahanshahi M, Jones CR, Zijlmans J et al (2010) Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson’s disease. Brain 133:727–745. doi:10.1093/brain/awq012
Kimberg DY, D’Esposito M, Farah MJ (1997) Effects of bromocriptine on human subjects depend on working memory capacity. Neuroreport 8:3581–3585
Logan GD, Cowan WB (1984) On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev 91:295–327
Meck WH (1996) Neuropharmacology of timing and time perception. Brain Res Cogn Brain Res 3:227–242
Montague PR, Dayan P, Sejnowski TJ (1996) A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci 16:1936–1947
Niv Y, Daw ND, Joel D, Dayan P (2007) Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology (Berl) 191:507–520. doi:10.1007/s00213-006-0502-4
Obeso I, Wilkinson L, Casabona E et al (2011) Deficits in inhibitory control and conflict resolution on cognitive and motor tasks in Parkinson’s disease. Exp Brain Res 212:371–384
Overtoom CC, Verbaten MN, Kemner C et al (2003) Effects of methylphenidate, desipramine, and L-dopa on attention and inhibition in children with attention deficit hyperactivity disorder. Behav Brain Res 145:7–15
Overtoom CC, Bekker EM, van der Molen MW, Verbaten MN, Kooij JJ, Buitelaar JK, Kenemans JL (2009) Methylphenidate restores link between stop-signal sensory impact and successful stopping in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 65:614–619. doi:10.1016/j.biopsych.2008.10.048
Pullman SL, Watts RL, Juncos JL, Sanes JN (1990) Movement amplitude choice reaction time performance in Parkinson’s disease may be independent of dopaminergic status. J Neurol Neurosurg Psychiatry 53:279–283
Puumala T, Sirvio J (1998) Changes in activities of dopamine and serotonin systems in the frontal cortex underlie poor choice accuracy and impulsivity of rats in an attention task. Neuroscience 83:489–499
Rammsayer TH (2008) Neuropharmacological approaches to human timing. In: Grondin S (ed) Psychology of time. Emerald, Bingley, UK, pp 295–320
Ray NJ, Jenkinson N, Brittain J et al (2009) The role of the subthalamic nucleus in response inhibition: evidence from deep brain stimulation for Parkinson’s disease. Neuropsychologia 47:2828–2834. doi:10.1016/j.neuropsychologia.2009.06.011
Robinson ES, Eagle DM, Mar AC et al (2008) Similar effects of the selective noradrenaline reuptake inhibitor atomoxetine on three distinct forms of impulsivity in the rat. Neuropsychopharmacology 33:1028–1037. doi:10.1038/sj.npp.1301487
Rubinstein M, Phillips TJ, Bunzow JR et al (1997) Mice lacking dopamine D4 receptors are supersensitive to ethanol, cocaine, and methamphetamine. Cell 90:991–1001
Schultz W (1997) Dopamine neurons and their role in reward mechanisms. Curr Opin Neurobiol 7:191–197
Tannock R, Schachar RJ, Carr RP, Chajczyk D, Logan GD (1989) Effects of methylphenidate on inhibitory control in hyperactive children. J Abnorm Child Psychol 17:473–491
Voon V, Reynolds B, Brezing C et al (2010) Impulsive choice and response in dopamine agonist-related impulse control behaviors. Psychopharmacology (Berl) 207:645–659. doi:10.1007/s00213-009-1697-y
Weintraub D, Siderowf AD, Potenza MN et al (2006) Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol 63:969–973. doi:10.1001/archneur.63.7.969
Wiener M, Lohoff FW, Coslett HB (2011) Double dissociation of dopamine genes and timing in humans. J Cogn Neurosci. doi:10.1162/jocn.2011.21626
Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA (2007) Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain 130:1787–1798. doi:10.1093/brain/awm111
Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW (2004) 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology (Berl) 176:376–385. doi:10.1007/s00213-004-1884-9
Winstanley CA, Theobald DE, Dalley JW, Cardinal RN, Robbins TW (2006) Double dissociation between serotonergic and dopaminergic modulation of medial prefrontal and orbitofrontal cortex during a test of impulsive choice. Cereb Cortex 16:106–114. doi:10.1093/cercor/bhi088
Acknowledgments
We would like to thank all the participants. This work was supported by a PhD studentship from Fundación Caja Madrid (IO) and a Career Development Fellowship from the Parkinson’s disease Society (LW).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Obeso, I., Wilkinson, L. & Jahanshahi, M. Levodopa medication does not influence motor inhibition or conflict resolution in a conditional stop-signal task in Parkinson’s disease. Exp Brain Res 213, 435–445 (2011). https://doi.org/10.1007/s00221-011-2793-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2793-x