Abstract
Electrical low-frequency stimulation (LFS) evokes long-term depression (LTD) of nociception. Human studies suggested a strictly homotopic organization. This study hypothesizes that even heterotopic LFS evokes LTD within the same receptive field (RF). In 33 healthy volunteers, painful electrical test stimulation and LFS were applied to the low back by a concentric electrode (ExpBack) and to the forearm by a multiarray electrode (ExpArm). Volunteers rated pain perception during test stimulation that was applied before and after LFS. In ExpBack, test stimuli were administered within the right T12 dermatome. LFS was applied heterotopically within the same RF or remote in dermatome T8. In ExpArm, test stimulation was carried out in the center of the RF whereas LFS was applied to the center, margin, or outside the RF. In ExpBack (n = 20), pain ratings decreased significantly stronger in T12 than in T8 dermatome (P < 0.01). In ExpArm (n = 20), LFS to the center of the RF induced a stronger pain reduction than LFS applied outside the RF (P < 0.001). This study demonstrates a heterosynaptic organization of LTD within the same RF. Profound knowledge about RF involvement on LTD seems crucial in order to judge the quality of LFS as a possible neuromodulatory treatment of pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Long-term potentiation (LTP) and long-term depression (LTD) describe long-lasting modifications in the efficiency of synaptic transmission. Repetitive electrical high-frequency stimulation (HFS) was shown to induce LTP (Bliss and Lomo 1973), whereas low-frequency stimulation (LFS) led to LTD (Dudek and Bear 1992; Randic et al. 1993).
There is evidence for a shift from LTD to LTP induction above a certain frequency threshold in rodents (Dudek and Bear 1992). Although the precise molecular mechanisms underlying LTP and LTD are not completely understood yet, both require influx of Ca2+ into the cell through N-methyI-D-aspartate receptors (Malenka et al. 1992; Mulkey and Malenka 1992). The different levels of postsynaptic Ca2+ influx achieved by different frequencies are thought to activate opposing changes in protein phosphorylation, with the higher frequencies activating protein kinases necessary for LTP (Malenka et al. 1989) and the lower frequencies activating protein phosphatases necessary for LTD (Mulkey et al. 1993).
LTD of nociception and pain has been investigated in trigeminal and spinal nociceptive system in healthy volunteers. Noxious LFS of cutaneous afferents induced sustained reduction in brainstem reflexes (Ellrich and Schorr 2002; Schorr and Ellrich 2002; Ellrich 2006), cortical potentials (Rottmann et al. 2008; Jung et al. 2009), and pain perception ratings (Rottmann et al. 2010a) for more than 1 h. LTD of cerebral pain-related activation was demonstrated by functional brain imaging (Rottmann et al. 2010b).
In vitro studies suggest a homosynaptic LTD that is input specific and confined to stimulated synapses. LFS of primary afferent Aδ fibers selectively reduced synaptic transmission in the dorsal horn of the conditioned pathway (Chen and Sandkuhler 2000). Convergent input from adjacent afferents to the same postsynaptic neuron was not affected.
Recent studies in human volunteers support the assumption of homotopic organization. Application of LFS to right-hand dorsum solely depressed pain perception rating on right hand, but had no effect on left-hand rating. Stimulation of radial side of right-hand dorsum exclusively decreased perception on radial but not on ulnar side of the same hand (Rottmann et al. 2008). Experiments carried out bilaterally on the forehead showed a decrease in pain perception solely after ipsilateral but not after contralateral LFS (Yekta et al. 2006). Furthermore, trigeminal pain perception was solely inhibited by homotopic LFS within all three sensory branches of the trigeminal nerve (Aymanns et al. 2009).
Various in vitro studies demonstrated homosynaptic organization of LTD whereas data from human experiments were not able to make similar conclusions due to methodological limitations. It is important to point out the differences in experimental setups between animal and human studies that complicate a direct comparison. Recording of neurons of spinal cord slices in vitro provides precise information regarding involved primary receptive fields (RFs). In contrast, non-invasive psychophysical experiments in man are restricted to conclusions about third-order neurons that transmit information to the cerebral cortex as a prerequisite of sensory perception.
Thus, previous human studies were restricted to conclusions about homotopic effects and could not provide information about involved RFs. Due to the small size of RFs on the hand dorsum and face region (Weinstein 1968), it was hardly possible to selectively stimulate a skin area that is exclusively innervated by terminals of only one primary sensory neuron. Defining central RFs illustrates a possibility to converge animal and human experimental setups in order to gain deeper insight into spatial mechanisms of LTD in man. This optimization of experimental setup allows for the first time a differentiation between homotopic and heterotopic LTD effects within the same central RF. Therefore, this study hypothesized that even heterotopic LFS evokes LTD within the same central RF. Parts of this study were presented as an abstract (Larsen et al. 2010).
Methods
This study consists of two experiments that were carried out on the low back (ExpBack) and forearm (ExpArm) in healthy volunteers. These body regions were selected due to their large receptive fields (RF) that allow multiple electrode placements within the same central RF. The forearm region was chosen over the low back for the second experiment due to reasons of practicality. The distribution of RFs on the forearm allowed a fixation of the multiarray electrode within different RFs. Due to the larger RFs and a clear distinction on the low back area, the size of this multiarray electrode is not suitable for distinguishing effects in different RFs in this body region.
Subjects gave their informed consent prior to their inclusion in the study according to the 1964 Declaration of Helsinki (as amended by the 59th General Assembly, Seoul, 2008; www.wma.net). The protocol was approved by the local ethics committee. All participants were without any prior or ongoing skin disease, no one was taking any analgesic medication. Volunteers were not given any information regarding the theoretical background of this investigation or possible outcomes.
Two-point discrimination test (2PD)
In order to determine RFs for electrode placement, a 2PD was performed at the beginning of both experiments. 2PD is the ability to identify separate regions of perception evoked from simultaneously stimulating two discrete regions of the body. This test measures the minimum distance (two-point threshold) at which two stimuli are perceived as separate (Lundborg and Rosen 2004). This threshold varies for different body regions and describes the spatial resolution of a specific skin region (Weinstein 1968). A commercially available compass-type instrument, commonly used in clinical investigation and specifically designed for this purpose, was used in the assessment of 2PD in the low back and forearm region. In contrast to the standard 2PD test protocol, this study did not focus on examining cutaneous mechanoreceptors but on nociceptors. Therefore, the two blunt points of the test instrument were replaced with pricking spikes (tip diameter: 0.5 mm) that elicited a pinprick-like sensation indicating activation of Aδ nociceptors. All subjects described perceived stimulation as a pinprick-like pain perception. Nerve block studies demonstrated that pricking pain to punctuate probes was predominantly mediated by A fiber nociceptors (Ziegler et al. 1999). When multiple primary sensory neurons converge on a single secondary sensory neuron, their individual RFs merge into a single, large secondary RF (Silverthorn 2007), referred to as central RF (Fig. 1). The present psychophysical study examined LFS effects on central RFs.
Many primary sensory neurons converge onto a single secondary neuron creating a large secondary receptive field. The two stimuli will be perceived as a single point when both stimuli fall within the same secondary receptive field. When fewer primary neurons converge, secondary receptive fields are smaller. The two stimuli activate separate pathways and are perceived as distinct stimuli (modified from Silverthorn 2007)
According to a standardized protocol, the two spikes of that specially designed compass were delivered simultaneously to the skin. The distance between the two spikes was adjustable, starting with a separation distance of 10 cm. 2PD was then performed according to a descending protocol, with decreasing separation distances from 10 down to 1 cm, in 1 cm steps. This procedure was repeated four times. If two points within the same central RF were stimulated, the subject perceived only one stimulus. Both stimuli were separately perceived if the stimulation points were located in different central RFs (Fig. 1).
Noxious mechanical stimuli were applied to both skin test areas with approximately 5 s between each application. Care was taken when touching the skin with two points to ensure that the stimuli were applied simultaneously and that both were of equal pressure causing a first “blanching” around the prong in the tested skin area. Several studies proved that the application force should be very light, suggesting 10–15 g, which corresponds to the force producing the very first small ‘‘blanching’’ around the prongs (ASHT 1992; Moberg 1990). These 2PD test re-test studies provided reliable and reproducible results (Dellon et al. 1987; Novak et al. 1993). The application of too much pressure can influence the result as more pressure will bring more receptors into the field of stimulation and cause more deformation on the skin (Moberg 1978).
To reduce the possibility of sensitization and adaptation to the test stimulus, the examiner avoided touching the exact same point on the skin more than once during the testing of that particular area. Care was taken to avoid touching or moving hair when the test instrument was applied to the skin. One examiner performed all 2PD tests in this study. Subjects had to decide immediately if they felt the sensation of one or two tips by answering “one” or “two.” Discrimination values for each area were considered to be accurate and reliable when the subject described perceived sensation as one stimulus in 3 out of 4 successive applications. These distance values were marked on the skin resulting in an area representing the central RF location. After 2PD, there was a 10 min break in order to allow the skin recovering from noxious mechanical stimulation. Results of mechanical noxious 2PD were confirmed by electrical nociceptive 2PD. Therefore, noxious electrical stimuli were delivered via two concentric electrodes simultaneously at constantly increasing distance intervals. One electrode was fixed at the center of the mechanically determined and marked RF area, whereas the second one was moved towards the RF border. The stimulation paradigm corresponded to the one performed during 2PD test. Stimulation intensity of the attached electrode was set to threefold pain threshold. In order to adjust perceived intensity of both electrodes, subjects were asked to rate applied electrical stimuli (VRS: 0 = no pain; 100 = maximum imaginable painful). Electrical 2PD was then performed with an adequate intensity that elicited the same rating for both electrodes on a VRS.
ExpBack
Forty sessions were performed on 20 healthy subjects, age 20–37 years (10 women). Subjects were comfortably lying on an examination coach in a prone position. Two concentric electrodes, master electrode (ME) and inner electrode (IE), were fixed within one RF in the area of right dermatome T12 according to the 2PD (Fig. 2). A third electrode was adjusted outside this RF (OE) within the area of right dermatome T8. In order to confirm the aimed electrode location within and outside the RF, a series of eight noxious paired electrical stimuli (ISI = 0.1 Hz) were applied to electrodes within dermatome T8 and T12 in a randomized order. Stimulation intensity was adjusted to an intensity that elicited the same rating on a VRS. Stimuli were simultaneously applied to both ME and IE within T12 or to ME and OE within T8 and T12, respectively. Subjects were asked to report if they perceived the stimulation as two separated stimuli or one single stimulus. Electrode location was considered as appropriate when error rate was less than 2 out of 8.
In order to avoid any bias all three electrodes remained in mechanical contact with the skin regardless of experimental session. Thus, subjects were aware of three fixed electrodes during all sessions despite changes in the area of skin receiving electrical stimulation.
Electrical rectangular pulses (2 ms duration) were applied by a custom-made concentric electrode. This electrode consists of a small central cathode (1 mm diameter) and a large ring anode (8 mm inner diameter, 24 mm outer diameter). Due to its special geometry, this electrode produces high current density at low current intensities, which allows preferential activation of cutaneous Aδ fibers. Previous studies indicated a necessity of Aδ fiber activation for LTD induction (Sandkuhler et al. 1997). LFS with higher intensities, resulting in additional recruiting of C fibers did not lead to a stronger decrease. LFS with lower intensities mainly activating large diameter fibers induced short-term depression but not LTD. A recent human study with varying LFS stimulation parameters supported these results by revealing sustained depression of somatosensory-evoked cortical potentials and pain perception only after clearly painful LFS intensities (Jung et al. 2009). Application of LFS with an intensity close to pain threshold did not induce LTD, emphasizing the importance of sufficient Aδ fiber activation compared with the negligible role of Aβ fiber involvement.
Human experimental studies applying local anesthesia, cortical potentials, and sensorimotor reflexes emphasize appropriate noxious stimulation by this kind of electrode (Bromm et al. 1983; Bromm and Meier 1984; Kaube et al. 2000; Katsarava et al. 2006). The electrical stimulation was performed with a constant current stimulator (Model DS7A, Digitimer Limited, Hertfordshire, UK). This stimulator can vary the potential difference dependent on the resistance in order to maintain a constant current output.
ExpArm
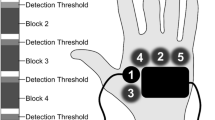
Sixty experiments were carried out on the left forearm of 20 healthy volunteers (10 women) that ranged in age from 20 to 36 years. At 1/2 of the measured distance between wrist joint and antecubital fossa, a square with the size of the multiarray electrode (5 × 10 cm) was drawn on the skin (Fig. 3), defined as region of interest (ROI). 2PD was performed in this ROI in order to characterize the location of a RF. This RF was subdivided into a central, marginal, and outlying area by mechanical 2PD and confirmed by electrical stimulation as described earlier (Fig. 3). A specially designed multiarray electrode was fixed on the forearm. This electrode consists of 6 rows with 4 pin electrodes each, resulting in 24 pin electrodes. In order to operate all 24 pin electrodes separately, each row is energized by a particular stimulator that powers 4 pin electrodes. The multiarray electrode was placed according to 2PD with four pin electrodes each in central (cRF), marginal (mRF), and outlying (oRF) RF.
Electrode positioning during ExpArm. At 1/2 of the measured distance between wrist joint and antecubital fossa, a square was drawn on the skin, defined as region of interest (ROI). 2PD was performed in this ROI as described earlier in order to characterize the location of a receptive field (RF). This RF was subdivided into a central, marginal, and outlying area. The multiarray electrode was placed according to 2PD with four pin electrodes each in central (cRF), marginal (mRF), and outlying (oRF) receptive field
Electrical stimulation
At first, individual thresholds for pain (IP) were determined for each electrode site. Therefore, two series of electrical pulses with decreasing and increasing stimulus intensity each were applied using increments of 50 μA according to the method of limits. In ExpBack, test stimulus intensity was adjusted to an intensity of about fourfold IP, resulting in a mean VRS rating of 23. Due to the special design of the multiarray electrode in ExpArm (4 pin electrodes), test stimulus intensity was set to threefold IP in order to obtain a comparable rating on the VRS for ExpBack. A series of five test stimuli (0.05 Hz) were applied (predummy) in order to familiarize subjects with the rating procedure. Two series of test stimuli (0.05 Hz) were then repeated before (preseries) and after (postseries) conditioning LFS (Fig. 4). Conditioning noxious LFS was applied with a frequency of 1 Hz for 20 min, i.e., 1,200 pulses, with the same intensity as test stimuli. This stimulation paradigm was recently demonstrated to be most effective for LTD induction of nociception in man (Jung et al. 2009).
Stimulation protocol for testing spatial organization of LTD on the low back a and on the left forearm b in human volunteers. Five test stimuli (0.05 Hz) represent a test stimulation series. The first series of five test stimuli (0.05 Hz) was defined as dummy series. Two series of test stimuli (0.05 Hz) were repeated before (preseries) and after (postseries) conditioning LFS. Exp Back a Test stimulation was always applied to master electrode (ME) whereas LFS (1,200 pulses, 1 Hz, fourfold IP) was applied to inner electrode (IE) or outer electrode (OE) during 2 separate sessions. ExpArm b Test stimulation was always applied to central receptive field (cRF), whereas LFS (1,200 pulses, 1 Hz, threefold IP) was applied to central (cRF), marginal (mRF), and outlying receptive field (oRF)
Test stimulation for ExpBack was always applied to ME, whereas LFS was either applied to IE within the same RF as ME (LFS IE) or OE (LFS OE) outside this RF (Fig. 2). Due to the modified electrode design, test and conditioning stimulation during ExpArm were applied via four pin electrodes of the multiarray electrode. Test stimulation for ExpArm was always applied to cRF electrodes, whereas LFS was applied to electrodes located in cRF, mRF, or oRF (Fig. 3). Each subject participated in all three LFS sessions in a randomized order. During both experiments, volunteers were asked to rate stimulus intensity according to a VRS.
Data analysis and statistics
Pain ratings recorded in all test stimulus series were expressed as absolute data. Data were described by arithmetic mean and standard error of mean (sem), by median, 5th, 25th, 75th, and 95th percentiles (box plot). Ratings were examined by 2-way repeated measures (RM) ANOVA (F, P value). Factor 1 was the time course comparing test stimulation series (pre and post); factor 2 was site of LFS application (IE vs. OE in ExpBack, and cRF vs mRF vs. oRF in ExpArm). Subsequently, Student–Newman–Keuls post hoc test was conducted (Difference of means = DM, P value).
Statistical analysis of IP pre at different electrode sites for ExpBack within the same experiment (Fig. 4) was performed by Friedman repeated measures ANOVA (Chi-square = Χ 2, P value). IP pre of the same electrode for different experiments was compared by Wilcoxon Signed Rank test (W and P value). Friedman repeated measures ANOVA (Chi-square = Χ 2, P value) was performed in order to compare IP pre of all electrodes for all three experimental sessions in ExpArm.
P values of < 0.05 were regarded to be significant. The SigmaStat® software 3.1 (SPSS Inc., Chicago, Illinois, USA) was applied.
Results
Effects of LFS application within different receptive fields on the low back and forearm were investigated. Thirty-three healthy volunteers participated in two different experiments defined as ExpBack and ExpArm. In all 100 experimental sessions, sensory thresholds and pain perception ratings were recorded. The central RF locations of the low back and forearm region obtained from mechanical 2PD were confirmed after electrical RF determination. Mechanically and electrical determined RF sizes showed an average length of around 6 cm in the low back area and 4 cm on the forearm region. Due to different electrode designs in ExpBack and ExpArm stimulation, intensity of test stimulation and LFS within the two experiments varied. Test and conditioning stimulation were performed with fourfold IP in ExpBack and threefold IP in ExpArm. Preliminary testing aimed at obtaining ratings within a similar rating magnitude for both kinds of electrodes.
Stimulus intensities elicited a definite pinprick-like painful sensation in all subjects during both experiments. No gender differences were found. Statistics revealed no differences between volunteers who started with LFS IE or OE and LFS cRF, mRF, or oRF, respectively. Mean pain ratings of test stimuli for ExpBack under precondition were 23.4 ± 2.3 and 31.5 ± 3.3 for ExpArm, respectively.
ExpBack (n = 20)
Twenty subjects participated in LFS IE and OE in a randomized order. LFS was applied to either inner (IE) or outer (OE) electrode, whereas test stimulation series were always applied to master electrode (ME). Mean test stimulus intensity (IS) of ME for LFS IE was 3.2 ± 0.2 mA (mean ± sem). Conditioning LFS to IE was applied with a mean IS of 2.8 ± 0.2 mA. Mean IS for ME during LFS OE experiment was set to 2.8 ± 0.2 mA. LFS stimulation intensity for LFS OE was adjusted to 2.4 ± 0.1 mA. All intensities corresponded to fourfold IP. Statistical comparison demonstrated no differences between pain thresholds under precondition within and between LFS IE and OE experiment (Table 1).
Rating statistics revealed significant interaction between time and site of LFS application (F = 31.9, P < 0.001). Comparison of ME pre- and posttest stimuli rating indicated a significant pain reduction after LFS application to IE (DM = 6.9, P < 0.001 whereas LFS to OE did not induce any rating changes at ME (Fig. 5). Statistics revealed a stronger pain perception depression after LFS IE compared with LFS OE (DM = 5.4, P < 0.01). No difference in rating was found under precondition between LFS IE and LFS OE.
Comparison between LFS to inner electrode (IE) and outer electrode (OE) in 20 volunteers during ExpBack. Test stimulation was always applied to master electrode (ME). Box plot shows grand mean average of absolute pain rating data under test stimulation before and after LFS IE (preIE, postIE) and before and after LFS OE (preOE, postOE). Rating under LFS IE revealed a significantly stronger decrease than under LFS OE. Solid line indicates median value, dotted line shows mean value. Asterisks mark significant changes as analyzed by (time course and site of LFS application) two-way repeated measures ANOVA (**P < 0.01, ***P < 0.001)
ExpArm (n = 20)
Sixty randomized sessions were performed in 20 healthy volunteers in order to obtain information about the location of involved RF areas in LTD induction. LFS was either applied to central (cRF), marginal (mRF), or outlying area (oRF) of the RF, whereas test stimulation was always applied to centrally located electrodes (cRF). Mean IP of cRF electrodes for LFS cRF experiment was 0.8 ± 0.1 mA. LFS mRF and oRF both resulted in an IP of 1.0 ± 0.1 mA (Table 1). Statistical comparison demonstrated no differences between IP of cRF under precondition between the three different experiments. Mean test stimulation intensity was set to 2.8 mA corresponding to threefold IP (0.9 ± 0.1 mA). Conditioning LFS to cRF was applied with a mean IS of 2.5 ± 0.2 mA, corresponding to threefold IP (0.8 ± 0.1 mA), to mRF with 2.8 ± 0.2 mA (IP: 0.9 ± 0.1 mA), and to oRF with 2.4 ± 0.1 mA (IP: 0.8 ± 0.1 mA).
Rating statistics revealed significant interaction between time and site of LFS application (F = 7.6, P < 0.01). LFS significantly suppressed pain ratings in all three experiments (cRF: DM = 8.5, P < 0.001; mRF: DM = 5.4, P < 0.001; oRF: DM = 2.7, P < 0.05) (Fig. 6). However, comparison of postrating within the three sessions showed significant differences between LFS oRF and LFS cRF (DM = 5.8, P < 0.05) and mRF (DM = 4.9, P < 0.05). Reduction in pain perception was strongest after LFS cRF and mRF compared with LFS oRF. Effects after LFS application to cRF and mRF did not statistically differ. No difference in rating was found under precondition between LFS cRF, mRF, and oRF.
Comparison between LFS to central receptive field (cRF), marginal RF (mRF), and outlying RF (oRF) in 20 volunteers during ExpArm. Test stimulation was always applied to cRF. Box plot shows grand mean average of absolute pain rating data under test stimulation before and after LFS cRF (precRF, postcRF), mRF (premRF, postmRF), and oRF (preoRF, postoRF). Rating under LFS cRF and mRF revealed a significantly stronger decrease than under LFS oRF. Solid line indicates median value, dotted line shows mean value. Asterisks mark significant changes as analyzed by (time course and site of LFS application) two-way repeated measures ANOVA (*P < 0.05, **P < 0.01, ***P < 0.001)
Discussion
The present study demonstrates sustained pain reduction after LFS application to different RFs on the forearm and low back. The idea of focusing on the specific involvement of different RF areas in human LTD induction is the unique feature of this study. For the first time, it was shown that the most efficient LTD effect was obtained in the central and marginal area of the RF compared with the less affected outlying area on the forearm. A similar conclusion accounts for the low back experiment. A pain depression was exclusively found within the same central RF whereas the outlying area was not affected. As LFS was able to decrease a pain rating of test stimuli that were applied to a different electrode location, this phenomenon can be referred to as heterotopic pain reduction. Both experimental setups include electrical stimulation to different electrode locations and therefore indicate a heterotopic pain depression within the same central RF which corroborates the proposed hypothesis.
In ExpBack, pain, perception was exclusively inhibited after LFS application within the same RF but did not change after LFS application to a distinct dermatome. These results are congruent with data from in vitro studies that showed pure homotopic LTD of the electrically conditioned pathway in various brain regions including hippocampus (Dudek and Bear 1992; Mulkey and Malenka 1992; Kerr and Abraham 1995), visual cortex (Kirkwood et al. 1993), and amygdala (Wang and Gean 1999). Literature suggests that LTD is input specific and confined to the stimulated synapses. Electrical LFS of one afferent input to a postsynaptic neuron selectively evoked LTD of the same synapse (Dudek and Bear 1992; Mulkey and Malenka. 1992; Chen and Sandkuhler 2000).
However, differences in the experimental setup between the present study and the described in vitro literature need to be discussed. In animal studies, LFS with Aδ fiber intensity was applied to primary afferent fibers of the dorsal root. Intracellular recordings from rat dorsal horn neurons showed homosynaptic LTD at the synapse between Aδ fibers and second-order neurons in the superficial spinal dorsal root (Chen and Sandkuhler 2000). Even though the results from this study are consistent with those data, restrictions of experimental setups in human experiments should be taken into consideration. The described in vitro experiments focused on effects on the first nociceptive synapse whereas this study provides information about involved RFs of higher-order neurons. Many axons from primary neurons converge onto a single second-order sensory neuron in the dorsal horn. This information is conveyed via third-order neurons to the cerebral cortex where sensory perception occurs. Consequently, RFs of second- and higher-order sensory neurons are larger and more complex than those of receptor neurons as they receive convergent input from many hundreds of receptors, each with a slightly different but overlapping primary RF. Thus, present data give rather evidence about central RF involvement in LTD induction than information about possible homosynaptic mechanisms.
Results of ExpBack are in agreement with recently published studies in human volunteers investigating spatial organization of LTD. Application of LFS to right-hand dorsum solely depressed rating on right hand but had no effect on left-hand rating. Stimulation of radial side of right-hand dorsum exclusively decreased perception on radial but not on ulnar side of the same hand (Rottmann et al. 2008). In contrast to this homotopic organization in the spinal system, investigations in the trigeminal system revealed a heterosynaptic LTD (Yekta et al. 2006). The trigemino-facial blink reflex (BR) was used as a model to monitor spatial organization of nociceptive processing within the brainstem (Schorr and Ellrich 2002). LTD-inducing electrical stimuli were found to elicit bilateral, heterotopic LTD of the BR also on the unstimulated side. In contrast, pain perception solely decreased after ipsilateral LFS but was not affected after contralateral LFS (Yekta et al. 2006). Long-term depression of the BR by contralateral LFS may be due to bilateral projections of craniofacial nociceptive afferents onto sensory neurons of the spinal trigeminal nucleus that is known to be exceptionally convergent (Sessle et al. 1986; Ellrich et al. 1998; Sessle 2000). The divergent effect on pain perception might be explained by a preferential contralateral projection of nociceptive afferents onto reflex interneurons but not onto trigeminothalamic projection neurons. An electrophysiological study in rats that recorded secondary sensory neurons in the spinal trigeminal nucleus with afferent input from the ipsilateral supraorbital region (Ellrich and Messlinger 1999) supported a bilateral projection. Results showed that the majority of neurons received excitatory input from the contralateral face as well as the ipsilateral supraorbital nerve area. These described anatomical differences between the spinal and the trigeminal nociceptive pathways might explain the demonstrated differing results for spatial organization of LTD.
A subsequent study focused on the unilateral trigeminal neural integration of the forehead (Aymanns et al. 2009). The effect of noxious LFS that was applied to all three sensory branches of the trigeminal nerve on the BR at the forehead was investigated in healthy man. Trigeminal nociception and pain were inhibited by homotopic LFS at the forehead but not after heterotopic LFS of infraorbital and mental nerve skin afferents.
These studies indicate that spatial organization underlying this prolonged antinociception in man is still not well understood. Due to the small size of RFs on the investigated hand dorsum and face region, respectively, it was hardly possible to selectively stimulate skin area that is innervated by terminals of only one receptor neuron. Hence, previous human studies exclusively allowed conclusions about homotopic effects and could not provide information about involved central RFs.
According to the sensory homunculus, the forearm and low back were chosen for this study as central RFs in these areas are relatively large adding up to 40 mm (Penfield and Jasper 1954; Weinstein 1968). Thus, these large RFs allow a fixation of electrodes apart from each other within and outside the same RF. A concentric electrode design was applied that previously proved a preferential activation of nociceptive Aδ fibers (Kaube et al. 2000; Katsarava et al. 2006). In order to increase the stimulation area, the general design of this electrode was copied and modified resulting in a multiarray electrode. The size of RFs at forearm in combination with a multiarray electrode allowed a specific pin electrode positioning in different RF areas. Consequently, the involvement of these areas in LTD induction could be investigated very precisely.
2PD is a well-established property of the sensory systems in order to discriminate and separate mechanical and noxious inputs (Dellon et al. 1987). Information regarding mechanical sensitive RF distribution in humans is very broad (Weinstein 1968). However, literature focusing on the distribution of nociceptive RFs in human is still rare (Price et al. 1989). According to the study hypothesis, the 2PD was performed with a compass that featured two pricking spikes in order to define nociceptive RFs. However, the corresponding electrical RF shape might differ from the RF size determined by physical perturbation of the mechanoreceptor. Therefore, each RF obtained by compass testing was confirmed with electrical stimulation. Electrically and mechanically determined RFs strongly resembled.
As this experimental setup did not include a precise measurement of RF size before and after LFS, it is difficult to judge the impact of RF plasticity caused by LTD induction. Even though this phenomenon seems rather unlikely, it cannot be completely ruled out. Further investigation is needed to uncover the effects of LTD on different nerve fiber functions and RF size and shape.
In ExpBack, LFS application on OE had no effect on ME, whereas in ExpArm, application of LFS on oRF resulted in a pain reduction in cRF. One reason for these opposed results for the outlying RF area might be differences in the distance to the central electrodes. During ExpBack, OE was placed with a minimum distance of 12 cm to the ME. Additionally, electrodes were placed within two different dermatomes (T8 and T12). Consequently, the electrode that was receiving test stimuli (ME) was cardinally separated from the electrode where LFS was applied to (OE), ruling out a possible involvement of the same central RF. In contrast, the design of the multiarray electrode did not allow such a distant separation of oRF and cRF. Here, the distance between cRF and oRF was not more than 4 cm which might explain a possible influence of LFS applied to oRF on pain suppression at cRF.
Due to the multiarray electrode design, the largest distance between electrodes that receive test stimuli and the electrodes that received conditioning LFS was 4 cm. This fact and the corresponding results might indicate that both electrode positions might still be under the influence of the same expansive RF. In contrast, electrodes during low back experiments were located in two different dermatomes and the separation distance between these electrodes was approx. 12 cm, therefore ruling out any influences of the same receptive field.
Descending nociceptive pathways were recently shown to play a role in LTD induction (Liu et al. 1998; Rottmann et al. 2010b). In rats under general anesthesia, prolonged burst Aδ fiber stimulation evoked decreased field potentials with intact descending pathways. In contrast, the same conditioning stimulation induced long-term potentiation in spinalized animals (Liu et al. 1998). This finding emphasizes the necessity of intact descending nociceptive pathways as they seem to be a prerequisite for the onset of an analgesic effect after LFS application. The significance of top down modulation via the descending analgesic pathways in LTD induction was further confirmed by a recently performed imaging study. This fMRI study in humans indicated an involvement of peripheral and central processes in LTD (Rottmann et al. 2010b). On one hand, LFS reduced synaptic transmission at nociceptive synapse. On the other hand, a positive correlation between pain relief and increased cerebral activation of brain areas that are involved in the endogenous pain control mechanisms after LFS was shown. Consequently, it seems inevitable to select an experimental setup that is able to take pre- and postsynaptic influences of LTD on pain perception into consideration.
The presented observations support the hypothesis that LTD is restricted to the RF where LFS is applied. Data indicate that noxious LFS is able to induce LTD not only at the exact stimulation site but also within a certain area of the same central RF. The applied optimized electrode design showed the strongest LTD effect in the marginal and central RF area compared with the outlying region. These findings play an important role for future introduction of this electrostimulation in clinical use. By developing an electrode array that is able to stimulate a wide skin area, this non-pharmacological treatment can induce a more widespread analgesic effect corresponding to the dimension of the involved central RFs. Consequently, profound knowledge about RF involvement on LTD in humans is crucial in order to judge the quality of LFS as a possible neuromodulatory treatment of pain.
Abbreviations
- 2PD:
-
Two-point discrimination test
- cRF:
-
Central receptive field
- ExpArm:
-
Experiments performed on the forearm
- ExpBack:
-
Experiments performed on the low back region
- IE:
-
Inner electrode
- IP :
-
Pain threshold
- IS :
-
Stimulus intensity
- LFS:
-
Low-frequency stimulation
- LTD:
-
Long-term depression
- ME:
-
Master electrode
- mRF:
-
Marginal receptive field
- OE:
-
Outer electrode
- oRF:
-
Outlying receptive field
- RF:
-
Receptive field
- ROI:
-
Region of interest
References
ASTH, American Society of Hand Therapists (1992) Clinical assessment recommendations. The Society, Chicago
Aymanns M, Yekta SS, Ellrich J (2009) Homotopic long-term depression of trigeminal pain and blink reflex within one side of the human face. Clin Neurophysiol 120:2093–2099
Bliss TV, Lomo T (1973) Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol 232:331–356
Bromm B, Meier W (1984) The intracutaneous stimulus: a new pain model for algesimetric studies. Methods Find Exp Clin Pharmacol 6:405–410
Bromm B, Meier W, Scharein E (1983) Antagonism between tilidine and naloxone on cerebral potentials and pain ratings in man. Eur J Pharmacol 87:431–439
Chen J, Sandkuhler J (2000) Induction of homosynaptic long-term depression at spinal synapses of sensory a delta-fibers requires activation of metabotropic glutamate receptors. Neuroscience 98:141–148
Dellon AL, Mackinnon SE, Crosby PM (1987) Reliability of two-point discrimination measurements. J Hand Sur Am 12:693–696
Dudek SM, Bear MF (1992) Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA 89:4363–4367
Ellrich J (2006) Long-term depression of orofacial somatosensory processing. Suppl Clin Neurophysiol 58:195–208
Ellrich J, Messlinger K (1999) Afferent input to the medullary dorsal horn from the contralateral face in rat. Brain Res 826:321–324
Ellrich J, Schorr A (2002) Long-term depression of the human masseter inhibitory reflex. Neurosci Lett 329:265–268
Ellrich J, Andersen OK, Treede RD, Arendt-Nielsen L (1998) Convergence of nociceptive and non-nociceptive input onto the medullary dorsal horn in man. Neuroreport 9:3213–3217
Jung K, Rottmann S, Ellrich J (2009) Long-term depression of spinal nociception and pain in man: influence of varying stimulation parameters. Eur J Pain 13:161–170
Katsarava Z, Ayzenberg I, Sack F, Limmroth V, Diener HC, Kaube H (2006) A novel method of eliciting pain-related potentials by transcutaneous electrical stimulation. Headache 46:1511–1517
Kaube H, Katsarava Z, Kaufer T, Diener H, Ellrich J (2000) A new method to increase nociception specificity of the human blink reflex. Clin Neurophysiol 111:413–416
Kerr DS, Abraham WC (1995) Cooperative interactions among afferents govern the induction of homosynaptic long-term depression in the hippocampus. Proc Natl Acad Sci USA 92:11637–11641
Kirkwood A, Dudek SM, Gold JT, Aizenman CD, Bear MF (1993) Common forms of synaptic plasticity in the hippocampus and neocortex invitro. Science 260:1518–1521
Larsen LE, Jung K, Ellrich J (2010) Heterotopic low-frequency stimulation induces nociceptive LTD within the same central receptive field in man. Act Physiol 198(Suppl.677):117
Liu XG, Morton CR, Azkue JJ, Zimmermann M, Sandkuhler J (1998) Long-term depression of C-fibre-evoked spinal field potentials by stimulation of primary afferent A delta-fibres in the adult rat. Eur J Neurosci 10:3069–3075
Lundborg G, Rosen B (2004) The two-point discrimination test—time for a re-appraisal? J Hand Sur Br 29B:418–422
Malenka RC, Kauer JA, Perkel DJ, Mauk MD, Kelly PT, Nicoll RA, Waxham MN (1989) An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature 340:554–557
Malenka RC, Lancaster B, Zucker RS (1992) Temporal limits on the rise in postsynaptic calcium required for the induction of long-term potentiation. Neuron 9:121–128
Moberg E (1978) The upper limb in tetraplegia: a new approach to surgical rehabilitation. George Thieme, Stuttgart
Moberg E (1990) Two-point discrimination test a valuable part of hand surgical rehabilitation in tetreplegia. Scand J Rehabil Med 22:127–134
Mulkey RM, Malenka RC (1992) Mechanisms underlying induction of homosynaptic long-term depression in area CA1 of the hippocampus. Neuron 9:967–975
Mulkey RM, Herron CE, Malenka RC (1993) An essential role for protein phosphatases in hippocampal long-term depression. Science 261:1051–1058
Novak CB, MacKinnon SE, WilliamsJI Kelly L (1993) Establishment of reliability in the evaluation of hand sensibility. Plast Reconstr Surg 92:311–322
Penfield W, Jasper H (1954) Epilepsy and the functional anatomy of the human brain. J Am Med Assoc 155:86
Price DD, Mchaffie JG, Larson MA (1989) Spatial summation of heat-induced pain—influence of stimulus area and spatial separation of stimuli on perceived pain sensation intensity and unpleasantness. J Neurophysiol 62:1270–1279
Randic M, Jiang MC, Cerne R (1993) Long-term potentiation and long-term depression of primary afferent neurotransmission in the rat spinal cord. J Neurosci 13:5228–5241
Rottmann S, Jung K, Ellrich J (2008) Electrical low-frequency stimulation induces homotopic long-term depression of nociception and pain from hand in man. Clin Neurophysiol 119:1895–1904
Rottmann S, Jung K, Ellrich J (2010a) Electrical low-frequency stimulation induces long-term depression of sensory and affective components of pain in healthy man. Eur J Pain 14:359–365
Rottmann S, Jung K, Vohn R, Ellrich J (2010b) Long-term depression of pain-related cerebral activation in healthy man: an fMRI study. Eur J Pain 14:615–624
Sandkuhler J, Chen JG, Cheng G, Randic M (1997) Low-frequency stimulation of afferent Adelta-fibers induces long-term depression at primary afferent synapses with substantia gelatinosa neurons in the rat. J Neurosci 17:6483–6491
Schorr A, Ellrich J (2002) Long-term depression of the human blink reflex. Exp Brain Res 147:549–553
Sessle BJ (2000) Acute and chronic craniofacial pain: brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med 11:57–91
Sessle BJ, Hu JW, Amano N, Zhong G (1986) Convergence of cutaneous, tooth pulp, visceral, neck and muscle afferents onto nociceptive and nonnociceptive neurones in trigeminal subnucleus caudalis (medullary dorsal horn) and its implications for referred pain. Pain 27:219–235
Silverthorn DU (2007) Sensory physiology. In: Silverthorn DU (ed) Human physiology: an integrated approach. Benjamin Cummings, San Francisco, p 332
Wang SJ, Gean PW (1999) Long-term depression of excitatory synaptic transmission in the rat amygdala. J Neurosci 19:10656–10663
Weinstein S (1968) Intensive and extensive aspects of tactile sensitivity as a function of body part, sex, and laterality. In: Kenshalo DR (ed) The Skin Senses. Thomas CC, Springfield, pp 195–222
Yekta SS, Lamp S, Ellrich J (2006) Heterosynaptic long-term depression of craniofacial nociception: divergent effects on pain perception and blink reflex in man. Exp Brain Res 170:414–422
Ziegler EA, Magerl W, Meyer RA, Treede RD (1999) Secondary hyperalgesia to punctate mechanical stimuli. Central sensitization to A-fibre nociceptor input. Brain 122:2245–2257
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, K., Larsen, L.E., Rottmann, S. et al. Heterotopic low-frequency stimulation induces nociceptive LTD within the same central receptive field in man. Exp Brain Res 212, 189–198 (2011). https://doi.org/10.1007/s00221-011-2718-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2718-8