Abstract
In two experiments we studied the role of active movements for adaptation to a visuo-motor rotation by way of adding external forces. In the first experiment we compared practice with target guidance and path guidance with a no-guidance control condition. With target guidance the arm was driven to the target on a straight path, whereas with path guidance active movements were required which were driven to the correct straight path of the hand whenever there was a deviation. During practice target guidance resulted in faster movements with smaller initial direction errors than in the other two conditions. However, in subsequent visual open-loop tests the adaptive shifts turned out to be smaller after both target-guidance and path-guidance practice than after no-guidance practice. In the second experiment resistive path guidance during practice was compared with a no-guidance control condition. With resistive path guidance the hand was driven away from the correct straight path whenever there was a deviation. Thus, corrections required active movements and could not be passive as with assistive path guidance in the first experiment. During practice resistive path guidance resulted in longer movement time than in the no-guidance group. Adaptive shifts, as assessed in subsequent open-loop tests, were not different between the two groups. According to these findings adaptation to a visuo-motor rotation is driven by active error corrections.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Planning and control of human movement invokes multimodal representations of space which embrace various sensory signals as well as motor commands, that is, efference copies (Andersen et al. 1997). The relations between the different sensory and motor maps must be flexible to enable adaptation to changing characteristics of the body and its environment. A well-established experimental paradigm for the study of such flexibility is adaptation to visuo-motor rotations (e.g., Abeele and Bock 2001a, b, 2003; Cunningham 1989; Krakauer et al. 2000). In the present experiments we address the role of active movements for the adjustment to a visuo-motor rotation by way of adding external forces. Specifically we compare adjustment to a visuo-motor rotation after unguided practice with the adjustment after practice with different types of robot guidance. In the first experiment these are target guidance and assistive path guidance, in the second experiment resistive path guidance (cf. Table 1).
Visuo-motor rotations are similar to prismatic displacements. However, in prismatic displacement the rotation is egocentric, but in visuo-motor rotation it is allocentric. In addition, with prismatic displacement motor commands, proprioception, and vision all refer to the hand, whereas with visuo-motor rotations vision refers to a different object, the cursor. Nevertheless, findings from prism-adaptation studies are at least suggestive as far as adjustment to visuo-motor rotations is concerned, even though the adaptive mechanisms involved in these paradigms may be different (cf. Bedford 1993, 1995; Clower and Boussaoud 2000; Welch 1972).
A classic finding of prism-adaptation studies is that of Held and Hein (1958), who observed an after-effect only when active movements were performed during prismatic exposure. However, later studies revealed that active movements are not a critical requirement for prism adaptation to occur, but that active movements may facilitate the adaptive process, nevertheless (cf. Welch 1974). As far as visuo-motor rotations are concerned, we are not aware of a comparison of the effects of active and passive movements during exposure. But there are some data on the role of proprioception. Note that with a visuo-motor rotation a novel relation between the visual information on cursor direction and the proprioceptive and/or efferent information on the direction of hand movement must be learned. Thus, the observation that a degradation of proprioception by means of concurrent agonist and antagonist vibration has essentially no effect on adaptation to a visuo-motor rotation (Pipereit et al. 2006) strongly suggests a critical role of motor commands. However, observations on deafferented patients are not fully consistent regarding the role of proprioception (and implicitly of motor commands) for adaptation to visuo-motor transformations (e.g., Guedon et al. 1998; Ingram et al. 2000).
In the present experiments we vary the efferent commands during practice by way of different types of robot guidance. Target guidance is implemented by a force field that drives the hand to the correct target position. In principle the hand can be driven passively to the target, but the movements are not fully passive and are not instructed to be so. Assistive path guidance is implemented by a force field that drives the hand to the correct straight path from start to target. Thus, an active movement to the target is required, but deviations from the straight path are corrected passively. As listed in Table 1, both types of guidance used in the first experiment serve to demonstrate the correct straight movement of the hand, but only target guidance allows passive movements to the target. In addition, with both kinds of guidance the motor commands are distorted as compared with those in unguided practice.
If active movements indeed facilitate the adjustment to visuo-motor rotation, guided practice should result in a deterioration. Of course, the deterioration would not be expected for performance during guided practice, but for subsequent tests without guidance. Such an expectation is consistent with the majority of findings on the effects of physical guidance on the acquisition of various types of motor skill (cf. Schmidt and Lee 1999, pp. 316–317) as well as with the general effects of robotic guidance (cf. Reinkensmeyer and Patton 2009). However, certain types of robotic guidance can be beneficial for certain types of task (e.g., Feygin et al. 2002). With respect to the present study, path guidance requires active movements to the target. Thus, with path guidance during practice the deterioration of adjustment to a novel visuo-motor rotation might be smaller than with target guidance or even totally absent.
Both target guidance and path guidance distort the motor commands as compared with a no-guidance control condition. For target guidance efferent commands could, in principle, even be completely absent. On the other hand, both types of guidance demonstrate the correct hand paths, which can be sensed proprioceptively. This is the case from the very start of practice. Thus, if adjustment to a visuo-motor rotation involves proprioceptive information, the experience of the correct proprioceptive information provided by physically guided correct movements could enhance the adjustment. Specifically it could enhance the acquisition of a perceptual trace (Adams 1971) of the movement in the correct direction. The beneficial effect of proprioceptively perceiving the correct movement seems to be more likely in the case of slower movements, compared to rapid ones. Indeed, studies with prismatic displacement have shown that adaptation of slower movements is mostly dependent on proprioception, whereas rapid ones are more likely to adapt through the processing of efferent commands (Baily 1972; Kitazawa et al. 1997). To enhance the role of proprioception, in this study, participants performed rather slow movements during practice with the requirement to reach the target accurately.
In the second experiment we compare the effects of resistive path guidance during practice with the effects of unguided practice. This experiment was motivated by the results of the first experiment, which revealed a decline of adjustment to a visuo-motor rotation with both types of assistive guidance. Resistive path guidance is implemented by a force field which drives the hand away from the correct straight path except when the position of the hand is within a narrow tolerance. With resistive path guidance motor commands are distorted as compared with unguided practice. In this respect resistive path guidance is comparable with assistive path guidance, even though the nature of the distortion will be different. However, resistive path guidance differs from assistive guidance in that errors are not corrected passively, but have to be corrected actively (cf. Table 1). There is some reason to expect that the decline of adjustment to a visuo-motor rotation, that is present with assistive path guidance, should be absent with resistive path guidance because active error corrections are required as they are present in unguided practice.
An important role of error corrections for adaptation is suggested by a couple of findings. In a review paper Shadmehr et al. (2010) claim that visuo-motor adaptation is driven by prediction errors. These are deviations of the visual direction from the one predicted on the basis of an internal model of the visuo-motor transformation (cf. Wolpert and Kawato 1998). This model uses motor commands and/or proprioceptive information as its input. Consistent with the claim of an important role of error corrections, in prism-adaptation studies a role of error information for adaptation has been found (e.g., Coren 1966; Welch and Rhoades 1969). Note that the presence of targets may stimulate predictions and thus the occurrence of prediction errors.
Divergent force fields, as used for the implementation of resistive guidance, have received a good deal of attention during the last couple of years. Benefits have been shown for motor learning in healthy adults (e.g., Burdet et al. 2001) as well as for neurorehabilitation (Cesqui et al. 2009). In part the benefits could be due to the increase of impedance that is induced by diverging force fields. This adjustment is directionally specific (cf. Franklin et al. 2007). Thus, with the resistive guidance of the second experiment, impedance should be high roughly orthogonal to the movement paths. The increased impedance should serve to reduce errors during practice. However, these are only those components of errors which result from the external forces, whereas those components which result from incorrectly planned directions of hand movements should not be affected by increased impedance. Thus, active error corrections are required with resistive guidance as in unguided practice, but excess errors induced by the divergent forces could be more or less abolished by increased impedance.
Adjustment to a visuo-motor rotation embraces different processes, and their relative importance depends on practice conditions (e.g., Saijo and Gomi 2010). Thus, the different types of guidance of the present experiments may affect different components of adjustment. A particularly important distinction is that between explicit (or strategic) and implicit components (cf. Heuer et al. in press), which in general develop in parallel and are functionally independent (Mazzoni and Krakauer 2006; Sülzenbrück and Heuer 2009; Taylor and Ivry 2011). An abrupt introduction of a (sufficiently large) visuo-motor rotation is likely to give rise to both explicit and implicit adjustments, whereas a gradual introduction, which is known as prismatic shaping for prismatic displacement, is likely to give rise to implicit adjustments only (cf. Buch et al. 2003; Saijo and Gomi 2010).
In the present experiments we studied adjustment to a visuo-motor rotation of −75° clockwise that was introduced abruptly. Explicit and implicit components of adjustment were assessed by different tests presented after the practice period. Visual open-loop tests in the presence of the transformation assess total adjustment, that is, a combination of both implicit changes of an internal model and explicit or strategic corrections such as side-pointing. Visual open-loop tests without the transformation serve to assess after-effects. Provided that participants are informed about the absence of the transformation, strategic corrections should no longer be made. Thus, after-effects serve as a measure of implicit adjustments. Whereas after-effects are frequently used to assess visuo-motor adaptation, separate tests of explicit knowledge of the visuo-motor transformation are rare. In the present study we used such tests.
Method
Participants
In the first experiment there were sixty participants, 41 female and 19 male, aged 15–35 years (mean: 23.2 years, SD: 3.3 years). They were randomly assigned to the no-guidance group (mean age: 23.5 years, SD: 2.1 years; 12 female, 8 male), the target-guidance group (mean age: 22.8 years, SD: 2.9 years; 15 female, 5 male), or the path-guidance group (mean age: 23.3 years, SD: 4.5; 14 female, 6 male). In the second experiment there were forty participants, 30 female and 10 male, aged 19–34 years (mean: 23.4 years; SD 3.3 years), who were randomly assigned to the no-guidance group (mean age: 22.8 years, SD: 2.8 years; 16 female, 4 male) or to the resistive path-guidance group (mean age: 24.1 years, SD: 3.5 years; 14 female, 6 male). Participants received course credit or a compensation of 15€. All of them were self-declared right-handers and were able to discriminate the red and green colour of the cues used in the experiments. They had given written informed consent prior to the start of the experiment that was done in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Apparatus
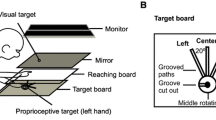
A picture of the apparatus is shown in Fig. 1. Participants faced a 22 inch CRT Monitor (iiyama Vision Master™ Pro 513) in about 55 cm distance from their eyes. They grasped a vertical handle of 9.5 cm length placed in the centre of a circular slide of 10 cm diameter that could be slid on the table top with only little friction. The vertical handle was the customized peripheral segment of the arm of a Phantom Premium 3.0 (SenseAble Technologies). A black curtain prevented direct sight of the right hand of the participant and of the robot. The horizontal position of the handle was recorded with a rate of 100 samples/s. With the same rate the forces exerted by the robot arm were updated.
Task
Participants performed aimed movements from a central start location to eight equally spaced targets arranged on a circle of 10 cm radius around the start location. Movements were instructed to be as swift and accurate as possible. The central start location on the monitor corresponded to a start location of the hand on the table such that all target positions could be reached without fully stretching the arm and without angles of less than 90° between upper and lower arm. Without visuo-motor rotation hand movements to the right corresponded to motions of a cursor on the monitor to the right as well. Absence of the visuo-motor rotation was cued by the green colour of the octagon which marked the start location. When the visuo-motor rotation was in effect, this was cued by the red colour of the start octagon. In this case hand movements to the right (0°) resulted in almost downward cursor motions (−75°). Whereas the cursor was visible during practice trials (visual closed-loop trials), it was invisible during test trials (visual open-loop trials).
The experimental groups differed with respect to their practice conditions. For all of them there was continuous visual feedback, and the cursor had to reach the visual target accurately before the trial was ended. For the no-guidance groups of both experiments there was no physical guidance at all. For the target-guidance, path-guidance and resistive path-guidance groups of both experiments potentials as shown in Fig. 2a, b, c were defined. The corresponding force fields are illustrated in Fig. 2d, e, f. In the experiment the forces were cut off at 6 N. With target guidance the hand was driven to its target so that the cursor reached the visual target accurately. This was the case even when there was no active movement at all. With path-guidance the hand was driven back to the correct straight path whenever it deviated from it, but the movement along the straight path had to be active for the cursor to reach the visual target. Note that during practice deviations from straight paths generally become less, so that assistance is faded without an explicit fading algorithm. With resistive path guidance the hand was driven away from the correct straight path whenever it deviated from it, but there was a tolerance range around the correct path within which there were no divergent forces. Again, to the extent that correct movements are performed, resistive guidance is faded.
The potential for target guidance was defined as
with A = 1 and n = 1.75. For each point (x, y) the force was given as
For path guidance, with a path from \( (x_{s} ,0) \) to \( (x_{t} ,0) \), \( x_{s} <\, x_{t} \), the potential was defined as
with the same parameters as for target guidance. For resistive path guidance, again with the correct path along the x axis, the potential was not bounded at the start and target position. It was defined as
with the same parameters A and n and the additional parameter d = 5.
Design and procedure
The experiments consisted of six phases each, baseline practice, pre-tests, first practice period, intermediate post-tests, second practice period, final post-tests. Baseline practice consisted of 48 visual closed-loop trials without the visuo-motor rotation as cued by the green colour of the start octagon. Each target was presented six times in a pseudo-random order, that is, there were six permutations of the eight targets which where presented one after the other. In the first and second practice periods the visuo-motor rotation was present, cued by the red colour of the start octagon. Targets were presented pseudo-randomly as in the baseline block. The first practice period consisted of two blocks, each with 48 visual closed-loop trials, the second practice period consisted of six blocks of trials.
The pre-tests were a visual open-loop test without rotation followed by an explicit test, again without rotation. The intermediate post-test was a visual open-loop test with rotation. The final post-tests were, first, a visual open-loop test with rotation, second, a visual open-loop test without rotation, third, an explicit test with rotation. In all test trials without rotation the colour of the start octagon was green, in all trials with rotation it was red. The colour of the start octagon and thus the knowledge of the participants about the presence or absence of the visuo-motor rotation was the only difference between the post-tests with and without rotation. Each of the visual open-loop tests consisted of three blocks, each of eight trials, each target being presented once in a pseudo-random order. Each of these three test blocks was preceded by a maintenance block of eight trials, which were identical to trials of the preceding practice phase or baseline phase. Maintenance blocks were included to prevent decay of adaptation in the course of the series of test blocks. Explicit tests consisted of only two blocks of 8 trials each. Again each block was preceded by a maintenance block. The whole experiment took about one and a half hour.
A movement trial began with guiding the participant to the start location. Arrows at the edges of the monitor indicated the direction of the appropriate hand movement. For example, an arrow presented at the left edge and pointing to the right indicated a required hand movement to the right; two arrows at the top and at the right edge, pointing downward and to the left, indicated a required hand movement toward the participant and toward the left; etc. The start location was indicated by a grey outline octagon of 9 mm diameter. The cursor, a grey filled octagon of 7 mm diameter, was displayed when its centre was less than 15 mm away from the start location to assist in reaching it accurately. When its centre was less than 2.5 mm away from the start location, the colour of both the start octagon and the cursor turned green or red, depending on the absence or presence of the visuo-motor rotation in the forthcoming trial. After a random waiting period between 0.5 and 1.3 s, which was re-started when the cursor left the start position, the target was presented, a blue octagon of 7 mm diameter, and the start octagon disappeared. In visual open-loop trials the cursor disappeared as well, and in closed-loop trials it turned blue.
The movement trial ended when the distance between positions of the hand in successive samples had been less than 0.25 mm for 400 ms, provided the cursor was located within 2.5 mm of the target. In visual open-loop trials there was no accuracy criterion, but besides the velocity criterion the distance between cursor and start location had to exceed 15 mm. Only in the first experiment a trial was aborted when the criterion for its end had not been reached within 10 s after appearance of the target. In this case a message was presented on the monitor, indicating that the time had expired. Participants were instructed that such trials had to be repeated. The repetition took place at the end of the block. In the second experiment there was no time limit.
In explicit-judgment trials a target was presented together with a filled start octagon and a line of 10 mm length and 1.6 mm width, which could be rotated around the start position. Initially its orientation was set randomly. Participants were told to instruct the experimenter to rotate the line until its orientation corresponded to the direction of hand movement the participant judged to be appropriate for the cursor to reach the target.
Data analysis
The position-time curves of the hand movements were low-pass filtered (fourth-order Butterworth, cut-off frequency 10 Hz, dual-pass) and differentiated (central-difference algorithm). Start and end of each movement were defined based on tangential velocity. In a forward search, the start of the movement was defined as that time when velocity exceeded 15 mm/s and remained above this threshold for at least 150 ms; the end of the movement was defined as the time at which velocity became less than 15 mm/s and remained below the criterion for at least 150 ms. Only in practice trials there was an additional accuracy criterion of less than 5 mm deviation from the target position.
For each trial the total length of the trajectory was determined. Trials were excluded when it was less than 15 mm or longer than three times (“Experiment 1”) or five times (“Experiment 2”) the target amplitude. In the first case there was typically more than one movement in the trial, the second case mostly resulted from “circling” around the target. Six participants of the first experiment, three in the no-guidance group, one in the target-guidance group, and two in the path-guidance group, had produced more than 50 invalid movements, and their data were neglected. The remaining 54 participants had an average of 5.0 (SD: 4.6) invalid movements. In the second experiment no participants were excluded from further analysis. The average of invalid trials per participant was 6.9 (SD: 8.4).
For each movement final and initial direction errors and movement time were determined. Final direction error was the angle between the vectors from start to target location and from start to end position of the movement. Initial direction error differed from the final direction error in that the end position of the movement was replaced by the position after 200 ms. The initial direction error is of particular interest for visual closed-loop practice trials in which curved movements are produced that reach the target accurately, so that final direction errors become zero. For each practice block or test phase individual medians were computed for each of the eight target directions. Similarly median errors of the judgments in the explicit tests were computed for each target direction. For post-tests the individual medians of the pre-tests were subtracted to obtain pre-test-to-post-test changes. Thereby directional biases related to the start location in the right workspace (cf. Fig. 1; Ghilardi et al. 1995) and biases of other origins should not affect the measures. Before the individual medians were entered in the statistical analyses they were averaged across target directions. The results will be reported for practice and tests in sequence.
Results
Example trajectories
Figure 3 shows example trajectories of four different participants, one representative for each of the four conditions of the two experiments. For each participant trajectories in the first block of practice trials, in the last block of practice trials, and in a test block (open-loop test with transformation) are shown. The open circle marks the correct end position of the hand movement for a visual target to the right of the start location. During practice trajectories became straighter, and in the post-test they were essentially straight or slightly curved in the direction opposite to that during practice. During visual closed-loop practice movements end at their targets, but not during open-loop post-tests.
Example trajectories taken from one participant of each experimental group from the first and last practice block and the middle visual open-loop post-test block with visuo-motor rotation. Circles mark the targets for correct hand movements. The open circle marks the target position for the hand movement when the visual target for the cursor motion is to the right of the start location; the dotted curve is the associated trajectory
Comparing the trajectories of the four participants from different groups, during early practice curvature appears reduced with target guidance as compared with the no-guidance control condition. Note that curvature is reflected by the initial direction errors. With resistive path guidance the trajectories appear somewhat more erratic, and curvature appears stronger than in the control condition. This difference, however, was specific to the example trajectories and not visible in the group means of the initial direction errors. At the end of practice movements were straighter, but the group differences seemed to persist. In the test the final direction errors were no longer zero, but positive. As an example, consider movements to the right visual target. For the cursor to reach this target, the hand should stop at a location marked by the open circle in Fig. 3. In the control condition and the resistive path-guidance condition the hand movements approached this target, but in the target-guidance and path-guidance conditions the final direction error was larger and the hand movement was more in the direction of the visual target than in its appropriately rotated direction.
Practice
During visual closed-loop practice, movements were accurate. Progressive adjustment to the visuo-motor rotation can be evidenced from the changes of the initial direction errors, which become smaller in the course of practice (e.g., Heuer and Hegele 2008). In addition we report the changes of movement time. For the ANOVA tests Greenhouse-Geisser corrections were applied when appropriate, but uncorrected degrees of freedom are reported together with the Greenhouse-Geisser epsilon. As measures of effect size partial eta-squared are given.
The mean initial direction errors and movement times of both experiments are shown in Fig. 4. In the course of practice (negative) initial direction errors declined in absolute terms, and movement time became faster in both experiments. In the first experiment (Fig. 4a) there was a consistent ranking of the three practice groups. Without guidance (negative) initial direction errors were largest, and movement time was longest. With target guidance initial direction errors were smallest, and movement time was shortest. Performance characteristics of the path-guidance group were in-between the other two groups, but closer to the no-guidance group than to the target-guidance group. In the second experiment (Fig. 4b) the initial direction error was essentially the same in both groups, and movement time was consistently longer with resistive path guidance than in the no-guidance control group.
For the first experiment the ANOVAs with the between-participant factor practice group and the within-participant factor block revealed significant main effects of block, which reflect the improvement in the course of practice, F(7,357) = 16.0, P < 0.01, ε = 0.44, \( \eta_{p}^{2} = 0.24 \), for initial direction error and F(7,357) = 86.2, P < 0.01, ε = 0.30, \( \eta_{p}^{2} = 0.63 \), for movement time. For both variables also the main effect of practice group was significant, F(2,51) = 14.9, P < 0.01, \( \eta_{p}^{2} = 0.37 \), for initial direction error and F(2,51) = 7.7, P < 0.01, \( \eta_{p}^{2} = 0.23 \), for movement time. The interactions of practice group and block failed to reach statistical significance, F(14,357) = 1.4, P > 0.20, ε = 0.44, and F(14,357) = 2.1, P > 0.05, ε = 0.30. With respect to the difference between the three practice groups, Tukey’s HSD post-hoc tests indicated significant differences between the target-guidance group on the one hand and the no-guidance group and path-guidance group on the other hand for both initial direction error and movement time (P < 0.05), but no significant differences between the no-guidance group and the path-guidance group (P > 0.20).
For the second experiment the ANOVAs with the between-participant factor practice group and the within-participant factor block again revealed significant main effects of block, F(2,266) = 24.4, P < 0.01, ε = 0.29, \( \eta_{p}^{2} = 0.39 \), for initial direction error and F(2,266) = 130.0, P < 0.01, ε = 0.46, \( \eta_{p}^{2} = 0.77 \), for movement time. However, the main effect of practice group was significant only for movement time, F(1,38) = 11.0, P < 0.01, ε = 0.22, but not for initial direction error, F < 1. The interactions of practice group and block were not significant, both F < 1.
After the second block of practice trials and after the last block there were series of blocks of test trials. To prevent decay of adjustment to the visuo-motor rotation during these visual open-loop trials, each test block was preceded by a maintenance block in which trials were of the same type as during practice. Initial direction errors, which serve to assess adjustment during practice trials, should remain constant across the maintenance blocks and thus throughout the test phases. This was, in fact, the case. In the first experiment the mean initial direction errors were −25.4° in the second practice block and −26.0°, −26.6°, and −22.1° in the three subsequent maintenance blocks. An ANOVA with the between-participant factor practice group and the within-participant factor block (practice block 2 and 3 subsequent maintenance blocks) revealed neither a significant effect of block nor a significant interaction with practice group. The same finding was obtained with the last practice block and the eight subsequent maintenance blocks, with initial direction errors ranging between −22.6 and −18.4°. For the second experiment the respective ANOVAs revealed no reliable variations at all. The means were −45.7°, −45.1°, −45.0°, and −43.8° for the second practice block and the three subsequent maintenance blocks, and for the last practice block and the eight subsequent maintenance blocks the initial direction errors ranged between −40.0° and −35.8°.
Tests: experiment 1
With respect to the test phases, the main interest is in the changes of the directions of hand movements relative to the pre-test directions. For the post-tests with cued presence of the visuo-motor rotation these changes are called adaptive shifts, for the tests with cued absence of the visuo-motor rotation they are after-effects, and for the tests in which explicit judgements were required they are called explicit shifts. Adaptive shifts were assessed after two and eight blocks of practice, after-effects and explicit shifts only after eight blocks. The means of the first experiment are shown in Fig. 5a.
Adaptive shifts were strongest in the no-guidance group. They were smaller and hardly different from each other in the target-guidance and the path-guidance groups. From the first to the second test phase adaptive shifts became stronger, in particular for the no-guidance group. A two-way ANOVA with the between-participant factor practice group and the within-participant factor test (intermediate vs. final) revealed significant main effects of practice group, F(2,51) = 4.0, P < 0.05, \( \eta_{p}^{2} = 0.13 \), and test, F(1,51) = 14.1, P < 0.01, \( \eta_{p}^{2} = 0.22 \), and an almost significant interaction of these two factors, F(2,51) = 3.1, P = 0.053, \( \eta_{p}^{2} = 0.11 \). Post-hoc comparisons of the three practice groups by means of Tukey’s HSD test revealed a significant difference of the no-guidance group from the target-guidance group (P < 0.05) and an almost significant difference from the path-guidance group (P < 0.10), whereas the difference between the target-guidance group and the path-guidance group did not approach statistical significance (P > 0.20).
After-effects were only slightly larger in the no-guidance group than in the two guidance groups. A one-way ANOVA revealed that the differences between the three groups were not significant, F < 1. However, overall the after-effects were significantly different from zero, F(1,51) = 37.4, P < 0.01, \( \eta_{p}^{2} = 0.42 \), and this was also the case for each single group.
Explicit shifts were largest in the no-guidance group and smallest in the target-guidance group. However, the difference between the three practice groups failed to reach statistical significance, F(2,51) = 1.9, P < 0.20. Overall the explicit shifts were significantly different from zero, F(1,51) = 17.8, P < 0.01, \( \eta_{p}^{2} = 0.26 \), but when tested for each single group, the explicit shift in the target-guidance group turned out not to be significant.
Tests: experiment 2
The mean adaptive shifts in the intermediate post-test after two blocks of practice and in the final post-test after eight blocks of practice are shown in Fig. 5b together with the after-effects and explicit shifts assessed after the end of practice. Adaptive shifts became stronger from the intermediate to the final test, and they were numerically slightly stronger in the no-guidance group than in the resistive path-guidance group. A two-way ANOVA with the between-participant factor practice group and the within-participant factor test (intermediate vs. final) revealed only a significant main effect of test, F(1,38) = 9.3, P < 0.01, \( \eta_{p}^{2} = 0.20 \), whereas the main effect of practice group failed to approach statistical significance, F(1,38) = 1.7, P > 0.20. The interaction was not significant, F < 1.
After-effects were almost identical in both groups and not significantly different from each other, as indicated by a t test, t(38) = 0.19, P > 0.20. Overall they were significantly different from zero, t(39) = 7.8, P < 0.01, and this was the case for each of the two groups.
Explicit shifts were slightly larger in the no-guidance group than in the resistive path-guidance group, but the difference did not approach statistical significance, t(38) = 0.7, P > 0.20. Overall the explicit shifts were significantly different from zero, t(39) = 3.0, P < 0.01, but when tested for each group separately, this was the case only for the no-guidance group.
Discussion
The present experiments were designed to explore the role of active movements and thus of efferent commands for adaptation to visuo-motor rotations. For adaptation to prismatic displacement a facilitative role of active movements is known (cf. Welch 1974), even though the original claim of Held and Hein (1958), that adaptation to prismatic displacement is absent without active movements, could not be upheld. All in all the present findings suggest a facilitative role of active movements also for adaptation to visuo-motor rotation. However, it is not the absence of active movements per se which impedes adaptation to a visuo-motor adaptation, but the absence of certain characteristics of active movements.
The first experiment revealed an impediment of the adjustment to a visuo-motor rotation when movements were physically guided during practice. This impediment was observed in spite of the immediate benefits of target-guidance both in terms of initial direction error and movement time. The impediment was also observed with path guidance, even though during practice initial direction errors and movement time were basically the same as in the no-guidance group. Thus, whenever motor commands were distorted by complete or partial physical guidance, the acquisition of an internal representation of the visuo-motor rotation suffered.
The poorer adjustment to a novel visuo-motor transformation after physically assisted practice is consistent with the notion that motor commands play an important role for visuo-motor adaptation. Adjustment to a visuo-motor rotation requires the learning of novel relations between proprioceptive and efferent information on the one hand and visual information on the other. The present findings therefore complement the findings of Pipereit et al. (2006) that a degradation of proprioception has essentially no effect on adaptation by showing that a degradation of efferent commands has such an effect. However, physical guidance does not completely abolish the adjustment, but only partially. This finding is consistent with observations from prism-adaptation studies (cf. Welch 1974). Thus, even though the relation between efferent and visual information seems to be more important than the relation between proprioceptive and visual information, the latter relation plays some role as well.
With respect to the components of adjustment that suffer from physical guidance the present results are somewhat inconclusive. Adaptive shifts embrace both implicit and explicit adjustments (cf. Sülzenbrück and Heuer 2009; Heuer et al. in press; Taylor and Ivry 2011), and they were clearly stronger for the no-guidance group than for the two guidance groups of the first experiment. After-effects are supposed to reflect implicit adjustments only; they were somewhat stronger for the no-guidance group than for the two guidance groups, but the variation was statistically unreliable. Similarly explicit shifts were strongest for the no-guidance group, but the differences between the three experimental groups only approached statistical significance. Thus, physical guidance impedes perhaps both implicit and explicit adjustments, but only when both impediments were combined the effect was large enough to reach statistical significance.
The second experiment revealed no impediment of the adjustment to a visuo-motor rotation with resistive path guidance during practice. With respect to the distorted motor commands, resistive path guidance is similar to assistive path guidance and target guidance of the first experiment, but with respect to active error corrections it is similar to the no-guidance condition (cf. Table 1). Thus, if the distortion of motor commands were the critical factor for the detrimental effect of physical guidance on visuo-motor adaptation, the impediment should also be found with resistive path-guidance. In contrast, if active error corrections were the critical factor, visuo-motor adaptation should not be impeded by resistive path guidance. The absence of a reliable difference between the no-guidance group and the resistive path-guidance group suggests a critical role for active error corrections in visuo-motor adaptation.
The initial direction errors that participants produced during practice with assistive and resistive path guidance were not reliably different from those of the respective no-guidance control groups in the two experiments. In all three conditions participants had to initiate a movement to the target, and movements started in the wrong direction. Correspondingly the motion of the cursor was not in the expected direction, which was toward the target. The prediction errors, that is, the deviations of the cursor direction from the one expected for a certain direction of hand movement, should have been roughly comparable. Thus, it may not just be prediction errors that facilitate adjustment to novel visuo-motor transformations as claimed by Shadmehr et al. (2010), but the active correction of such errors.
Adaptation to a visuo-motor rotation can be conceived as an instance of learning novel intermodal relations. This seems to require active corrections of errors which result from no longer correct internal representations of the intermodal relations. Similarly, implicit learning of intermodal visual-auditory sequences was found only when responses to both types of stimuli were required, that is, when stimuli of both modalities were behaviourally relevant (Schmidtke and Heuer 1997, Exp. 3). Perhaps it is not responding per se that is critical, but the prerequisite that stimuli of both modalities are attended (cf. Keele et al. 2003). Attention here is claimed to provide the gating mechanism which selects stimuli to be related to each other by way of basic learning mechanisms. The role of active error corrections may be similar for adaptation to visuo-motor rotations.
When participants move their hand into an incorrect direction, the motion of the cursor signals two different errors. The first one is the discrepancy between the direction of cursor motion and the target direction. This is the error measured in the experiment. The second error is the discrepancy between the direction of cursor motion and the predicted direction, with the prediction being based on the efferent and proprioceptive information. Under the assumption that the predicted direction of cursor motion matches the target direction, both errors are identical. The second error is assumed to be functionally relevant for adaptation (Shadmehr et al. 2010). However, the first error could be critical for explicit strategic adjustments (Taylor and Ivry 2011). From the findings of the present study the effects of the errors are bound to situations in which they are behaviourally relevant and thus require attention. This is the case when active error corrections are required, but not—or to a lesser extent—when error corrections are passive, driven by external forces.
References
Abeele S, Bock O (2001a) Mechanisms for sensorimotor adaptation to rotated visual input. Exp Brain Res 139:248–253
Abeele S, Bock O (2001b) Sensorimotor adaptation to rotated visual input: different mechanisms for small versus large rotations. Exp Brain Res 140:407–410
Abeele S, Bock O (2003) Transfer of sensorimotor adaptation between different movement categories. Exp Brain Res 148:128–132
Adams JA (1971) A closed-loop theory of motor learning. J Mot Behav 3:111–155
Andersen RA, Snyder LH, Bradley DC, Xing J (1997) Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Ann Rev Neurosci 20:303–330
Baily JS (1972) Adaptation to prisms: do proprioceptive changes mediate adapted behavior without ballistic arm movements? Quart J Exp Psychol 24:8–20
Bedford FL (1993) Perceptual and cognitive spatial learning. J Exp Psychol Hum Percept Perform 19:517–530
Bedford FL (1995) Constraints on perceptual learning: objects and dimensions. Cognition 54:253–297
Buch ER, Young S, Contreras-Vidal JL (2003) Visuomotor adaptation in normal aging. Learn Mem 10:55–63
Burdet E, Osu R, Franklin DW, Milner TE, Kawato M (2001) The central nervous system stabilizes unstable dynamics by learning optimal impedance. Nature 414:446–449
Cesqui B, Aliboni S, Mazzoleni S, Carrozza MC, Posteraro F, Micera S (2009) On the use of divergent force fields in robot-mediated neurorehabilitation. In: Biomedical robotics and biomechatronics, BioRob 2008. IEEE, Scottsdale, AZ, pp 854–861. doi:10.1109/BIOROB.2008.4762927
Clower DM, Boussaoud D (2000) Selective use of perceptual recalibration versus visuomotor skill acquisition. J Neurophysiol 84:2703–2708
Coren S (1966) Adaptation to prismatic displacement as a function of the amount of available information. Psychon Sci 4:407–408
Cunningham HA (1989) Aiming error under transformed spatial mappings suggests a structure for visual-motor maps. J Exp Psychol Hum Percept Perform 15:493–506
Feygin D, Keehner M, Tendick F (2002) Haptic guidance: experimental evaluation of a haptic training method for a perceptual motor skill. In: Proceedings of the 10th international symposium on haptic interfaces for virtual environment and teleoperator system (Haptics 2002). IEEE computer society, Orlando, pp 40–47
Franklin DW, Liaw G, Milner TE, Osu R, Burdet E, Kawato M (2007) Endpoint stiffness of the arm is directionally tuned to instability in the environment. J Neurosci 27:7705–7716
Ghilardi MF, Gordon J, Ghez C (1995) Learning a visuomotor transformation in a local area of work space produces directional biases in other areas. J Neurophysiol 73:2535–2539
Guedon O, Gauthier G, Cole J, Vercher J-L, Blouin J (1998) Adaptation in visuomotor tracking depends on intact proprioception. J Mot Behav 30:234–248
Held R, Hein A (1958) Adaptation to disarranged hand-eye coordination contigent upon reafferent stimulation. Percept Mot Skills 8:87–90
Heuer H, Hegele M (2008) Adaptation to direction-dependent visuo-motor rotations and its decay in younger and older adults. Acta Psychol 127:369–381
Heuer H, Hegele M, Sülzenbrück S (in press) Implicit and explicit adjustments to extrinsic visuo-motor transformations and their age-related changes. Hum Mov Sci. doi:10.1016/j.humov.2010.07.004
Ingram HA, van Donkelaar P, Cole J, Vercher J-L, Gauthier GM, Miall RC (2000) The role of proprioception and attention in a visuomotor adaptation task. Exp Brain Res 132:114–126
Keele SW, Ivry RB, Mayr U, Hazeltine E, Heuer H (2003) The cognitive and neural architecture of sequence representation. Psychol Rev 110:316–339
Kitazawa S, Kimura T, Uka T (1997) Prism adaptation of reaching movements: specificity for the velocity of reaching. J Neurosci 17:1481–1492
Krakauer JW, Pine ZM, Ghilardi MF, Ghez C (2000) Learning of visuomotor transformations for vectorial planning of reaching trajectories. J Neurosci 20:8916–8924
Mazzoni P, Krakauer JW (2006) An implicit plan overrides an explicit strategy during visuomotor adaptation. J Neurosci 26:3642–3645
Pipereit K, Bock O, Vercher J-L (2006) The contribution of proprioceptive feedback to sensorimotor adaptation. Exp Brain Res 174:45–52
Reinkensmeyer DJ, Patton JL (2009) Can robots help the learning of skilled actions? Exerc Sport Sci Rev 37:43–51
Saijo N, Gomi H (2010) Multiple motor learning strategies in visuomotor rotation. PLoS ONE 5(2):e9399
Schmidt RA, Lee T (1999) Motor control and learning: a behavioral emphasis, 3rd edn. Human Kinetics Publishers, Champaign
Schmidtke V, Heuer H (1997) Task integration as a factor in secondary-task effects on sequence learning. Psychol Res 60:53–71
Shadmehr R, Smith MA, Krakauer JW (2010) Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci 33:89–108
Sülzenbrück S, Heuer H (2009) Functional independence of explicit and implicit motor adjustments. Conscious Cogn 18:145–159
Taylor JA, Ivry RB (2011) Flexible cognitive strategies during motor learning. PloS Comput Biol 7(3):e1001096
Welch RB (1972) The effect of experienced limb identity upon adaptation to simulated displacement of the visual field. Percept Psychophys 12:453–456
Welch RB (1974) Research on adaptation to rearranged vision : 1966–1974. Perception 3:367–392
Welch RB, Rhoades RW (1969) The manipulation of informational feedback and its effects upon prism adaptation. Can J Psychol 23:415–428
Wolpert DM, Kawato M (1998) Multiple paired forward and inverse models for motor control. Neural Netw 11:1317–1329
Acknowledgments
The research reported in this paper was supported by the European Community’s Seventh Framework Programme, Grant Agreement Number 231724 (HUMOUR). We thank Johanna Maag, Tuğba Özcan, and Philipp Quiring for their support in running the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heuer, H., Rapp, K. Active error corrections enhance adaptation to a visuo-motor rotation. Exp Brain Res 211, 97–108 (2011). https://doi.org/10.1007/s00221-011-2656-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2656-5