Abstract
This paper reviews results that support a model in which memory for VOR gain is initially encoded in the flocculus, and in which cerebellar LTD and LTP are responsible for gain increases and gain decreases, respectively. We also review data suggesting that after it is encoded, motor memory can either be disrupted, possibly by a local mechanism, or else consolidated. We show that consolidation can be rapid, in which case the frequency dependence of learning is unchanged and we will argue that this is consistent with a local mechanism of consolidation. In the longer term, however, the available evidence supports the transfer of memory out of the flocculus. In new experiments reported here, we address the mechanism of memory encoding. Pharmacological evidence shows that both mGluR1 and GABAB receptors in the flocculus are necessary for gain-up, but not for gain-down learning. Immunohistochemical experiments show that the two receptors are largely segregated on different dendritic spines on Purkinje cells. Together with what is already known of the mechanisms of cerebellar LTD and LTP, our data suggest that the direction of learning may be determined by interactions among groups of spines. Our results also provide new evidence for the existence of frequency channels for vestibular signals within the cerebellar cortex.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The vestibulo-ocular reflex (VOR) pathway is perhaps the most thoroughly studied of all vestibular pathways, and its response to horizontal rotatory head movements has been the focus of the most attention. The VOR stabilizes gaze during head movements, making it possible to move and see at the same time. The sensory input driving the VOR is exclusively vestibular. This means that the reflex can respond rapidly to head velocity and acceleration signals. However, the vestibular system does not provide feedback about accurate gaze stabilization; therefore, it is necessary to recalibrate the gain and phase of the VOR’s response to movement whenever conditions change in either the sensory or motor periphery. The gain of the VOR can be increased or decreased; phase leads or lags can also be introduced. This recalibration is a simple form of motor learning.
Two conditions are sufficient to cause learning in the VOR: head movement and a visual error signal. Unlike the immediate modulation of VOR gain by VOR cancellation or enhancement, learning causes changes that are expressed when the VOR gain is measured in darkness. Gain increases (gain-up learning) can be induced by having cats wear 2× magnifying lenses during passive rotation, and gain decreases (gain-down learning) are induced using 0.25× miniaturizing lenses. Figure 1 illustrates the rapid learning in the VOR of alert cats. Details of the methods for Fig. 1 have recently been published (Titley et al. 2007, 2010). In 1 h, during which cats were rotated using a sum-of-sines waveform that included frequencies from 0.2 to 8 Hz, the gain decreased reliably at 0.5, 2, and 8 Hz. The learned gain decrease or increase is significant after 30 min and in fact is most rapid during the first 30 min of learning.
The VOR gain changes rapidly in both gain-up and gain-down learning protocols. Cats are rotated using a sum-of-sines velocity profile for 60 min. For gain-up, magnifying lenses are worn, and for gain-down, the lenses are miniaturizing. Otherwise, the two protocols are identical. VOR gain is measured in darkness at 0.5, 2 and 8 Hz. For each frequency, the traces to the right show examples of the averaged eye and head velocity traces, before and after the learning period. Details of the experimental methods, sample sizes, and subjects are described in Titley et al. (2010). These data were obtained in the vehicle control trials
The frequency content of the vestibular signal is an important parameter that depends on the source of movement (for example, running, walking, or postural changes). Learning is more effective at frequencies below about 4 Hz than it is at higher frequencies, both for gain decreases (Raymond and Lisberger 1996; Broussard et al. 1999) and for gain increases (Broussard et al. 1999; Fig. 1). Furthermore, when passive rotation is used to control the frequency of rotation and induce changes in VOR gain, the learned change in gain (whether an increase or a decrease) is greatest at the frequency of rotation (Robinson 1976; Lisberger et al. 1983; Raymond and Lisberger 1996; de Zeeuw et al. 1998; Kimpo et al. 2005; Titley et al. 2009). This selectivity is more pronounced for gain increases than it is for decreases (Kimpo et al. 2005). The selectivity of learning has led to the idea that VOR signals are segregated into frequency “channels” or adaptive filters (Lisberger et al. 1983), which may be due to properties of the cerebellar cortical circuitry (Dean et al. 2010).
Memory consolidation in the VOR
After only 1 h of learning, it is reasonable to ask whether the newly encoded change in gain is stable when faced by disruptive stimuli. For other motor systems, we can distinguish between short-term memory, which is labile, and long-term memory, which becomes progressively less labile over time (Scavio et al. 1992; Brashers-Krug et al. 1996). The process that converts short-term to long-term memory is known as consolidation.
Normally, rotation in darkness does not change the gain of the VOR (Fig. 2a). However, immediately after the VOR encodes a new memory, rotation in darkness can change the VOR gain toward its initial value, apparently disrupting the memory (Cohen et al. 2004; Kassardjian et al. 2005; Titley et al. 2007; Fig. 2a). For gain decreases, it is possible to prevent disruption by inserting a 60-min “neutral period” during which the subject is stationary with the eyes covered (Titley et al. 2007; Fig. 2b, dotted line). After the neutral period, disruption is ineffective (Fig. 2b), indicating that VOR motor memory consolidates rapidly, at least for gain decreases. After 60 min, gain increases are only partially consolidated (Titley et al. 2007; Fig. 2b). It is possible that, as in other memory systems (Dudai 2004), such “rapid consolidation” is a process distinct from longer-term consolidation. Furthermore, rapid consolidation is independent of the test frequency; in other words, it does not share the frequency selectivity of the original learning (Fig. 2c; Titley et al. 2009). One possibility is that rapid consolidation may act at the same set of synapses that were modified when the memory was encoded. In other words, as has been proposed for the eyeblink reflex (Cooke et al. 2004), the initial consolidation of VOR motor memory may take place within the cerebellar cortex.
Newly formed memory can be disrupted, but after 1 h of consolidation, disruption is much less effective. a The VOR gain at 2 Hz increases or decreases rapidly during the learning period (gray line) and returns to normal during the disruption period (black dashed line). The disruption stimulus presented alone causes no change (open symbols). b If a 1-h consolidation period (dotted line) intervenes between learning and disruption, disruption has a much smaller effect. Data for a and b from Titley et al. (2007). c When the training stimulus is a sinusoid, learning is greatest at the training frequency (filled symbols). After the consolidation period, this is still the case (open symbols), indicating that consolidation is not frequency dependent. d Disruption is greatest at frequencies that are different from the training frequency. e After consolidation, disruption is no longer effective. Data for c–e from Titley et al. (2009)
Extinction of a newly formed memory can be thought of as new learning that masks the original memory (Robleto et al. 2004). If this is the case for the VOR, then the frequency dependence that is characteristic of VOR motor learning should also be characteristic of disruption. However, we have found that disruption is frequency independent and as a result, the frequency tuning that appears during learning is not lost during disruption (Fig. 2d). This is the case even when single-frequency rotation is used as the disruption stimulus. In other words, disruption generalizes across stimulus frequencies. However, generalization does not fully explain the observation that memory is more easily disrupted at test frequencies that are very different from the frequency that was used for training (Fig. 2d). Surprisingly, this is the case even when the same frequency is used for disruption as for training. For example, if learning occurred at 1 Hz, disruption using 1-Hz rotation in darkness is more successful at 8 Hz than at 1 Hz (Titley et al. 2009).
The concept of frequency channels (Lisberger et al. 1983) is useful in interpreting these results. Frequency channels in the VOR, if they exist, may be broadly tuned and overlapping so that neighboring frequencies are affected by learning at a given frequency. This scenario is supported by “phase crossover”: During gain-down learning, at frequencies above the training frequency, the phase lag between head and eye increases but at frequencies below the training frequency, phase leads may appear (Lisberger et al. 1983; Raymond and Lisberger 1996; Kramer et al. 1998; Titley et al. 2009). Phase crossover is thought to be due to changes in overlapping channels.
Broadly tuned, overlapping frequency channels could be responsible for the unusual pattern of disruption shown in Fig. 2d. If memory is encoded at the parallel fiber-Purkinje cell synapse, as previous data suggest (de Zeeuw et al. 1998; Raymond and Lisberger 1998; Hansel et al. 2006; Titley et al. 2010), and if the channels have a physical basis in the distribution of activity within the cerebellar cortex, then Purkinje cells (PCs) that are located at the center of the channel may have a large proportion of their parallel fiber inputs active during learning at the center frequency of the channel. We found that the proportion of the memory that was lost during disruption increased slightly as the training and test frequencies diverged (Fig. 2d). The selective loss would be explained if the speed of stabilization depends on the preponderance of nearby synapses that have also been modified. Mechanisms for such selectivity, based upon synaptic tagging and capture, have been described in other brain regions (see for example (Frey and Morris 1997)).
Mechanisms of memory encoding
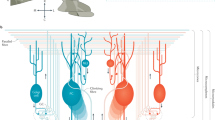
A great deal of effort has been directed toward understanding the mechanisms that encode memory in the VOR (for a recent review, see (Boyden et al. 2004)). Relevant details of the VOR circuitry are illustrated by the diagram in Fig. 3c. Ito originally proposed that cerebellar long-term depression at the parallel fiber-PC synapses, or PF-LTD, was a mechanism for VOR motor learning (Ito 1972). More recently, LTP at the same synapses was proposed to contribute (Boyden and Raymond 2003). PF-LTP might be responsible for gain-down learning while PF-LTD is responsible for gain-up learning (Boyden and Raymond 2003). Because PCs inhibit neurons in the direct VOR pathway, a learned decrease in the efficacy of the sensory input to Purkinje cells would have the downstream effect increasing the amplitude of the signal transmitted from the flocculus target neuron (FTN) to the motoneuron (Fig. 3c).
Injection of the glutamate antagonist CNQX bilaterally into the flocculus prevents the expression of a short-term, recently encoded motor memory, but not of a long-term memory. Panel C illustrates the experimental design. All synapses indicated in the diagram are possible sites of plasticity. Those considered most likely are the granule-cell inputs to Purkinje cells and the canal-afferent inputs to flocculus target neurons. The CNQX injection included inputs to Purkinje cells but did not include synapses on flocculus target neurons. a Following the learning period in a gain-down protocol (gray bar), the VOR gain at 2 Hz was decreased. After CNQX was injected bilaterally (black bar, filled symbols), the VOR gain returned to normal. If the PBS vehicle was injected, the gain did not change significantly. b When lenses were worn continuously for 3 days, including passive rotation indicated by the gray arrows, the VOR gain showed a larger decrease. On the third day, there was no passive rotation; instead, CNQX was injected. The VOR gain did not return to normal. In d, results from individual cats are represented by different symbols. For the short-term experiments (circles and triangles), the effect of CNQX on memory expression was inversely correlated with the cat’s ability to cancel the VOR, an indicator of floccular function. In the long-term experiments, cancelation was affected by the injection, but memory was not. The dashed lines divide long-term from short-term and PBS (vehicle) from CNQX injections. Data from Kassardjian et al. (2005)
FTNs receive vestibular sensory signals, directly and via brainstem pathways, which are modified in association with long-term changes in VOR gain (Lisberger et al. 1994). Whether memory is initially encoded in the floccular cortex or in the synaptic inputs to FTNs has been a topic of debate. Several authors have proposed that memory is first encoded in the flocculus and then transferred to the vestibular nuclei (the “transfer hypothesis”; Galiana 1986; Peterson et al. 1991; Raymond et al. 1996; Broussard and Kassardjian 2004). We tested this hypothesis by injecting CNQX, which blocks glutamatergic transmission, bilaterally into the flocculus (Kassardjian et al. 2005). When injected immediately after a new memory was encoded, CNQX prevents the expression of the memory (Fig. 3a). However, blocking excitatory floccular synapses does not reverse a gain change that has stabilized over a period of days (Fig. 3b). Importantly, in the short term, the degree of memory blockade is correlated with the interference with VOR cancelation, also a function of the flocculus, but the correlation is lost in the long term (Fig. 3d). Together, these results indicate that the flocculus is the site of memory encoding, but not of long-term memory storage, for the VOR (Kassardjian et al. 2005). These conclusions have now been confirmed in monkeys (Anzai et al. 2010).
So far, it is not clear whether the transfer of memory out of the flocculus is a consolidation mechanism in the sense of stabilizing memory, or whether transfer simply takes time, and optimizes some other features of VOR performance (such as speed). There is evidence that the initial response of the VOR pathway, which has a latency of only a few msec, participates in long-term memory storage (Broussard et al. 1992), suggesting that speed is optimized. A recent synthesis of modeling and slice electrophysiology suggests that memory transfer may begin immediately after memory encoding (Menzies et al. 2010). This would coincide with the onset of consolidation, which is detectable within minutes after learning has occurred. Further data are needed to clarify the relationship between consolidation and transfer.
Bidirectional learning
There is a body of evidence supporting the idea that LTD at the parallel fiber-PC synapse, or PF-LTD, is responsible for encoding gain increases (de Zeeuw et al. 1998; Hansel et al. 2006). If VOR motor learning is to be truly reversible, as it appears to be, then the underlying changes must be reversed by LTP at the same set of synapses. A complicating factor is the asymmetry of LTD and LTP. While LTP can operate by both pre-and postsynaptic mechanisms (Salin et al. 1996; Lev-Ram et al. 2002), LTD is primarily postsynaptic (Sakurai 1987; but see (Qiu and Knopfel 2009)). This asymmetry led to the prediction that motor learning should also be asymmetric, and gain-up learning might not be capable of truly reversing gain-down learning. In fact, Raymond and coworkers found that in the mouse, gain-up learning saturates at a lower value if it is preceded by gain-down learning than if the animal is naïve (Boyden and Raymond 2003). It should be noted that in the long term, VOR gain increases and decreases are reversible in the cat (Titley et al. 2010) as they are in the monkey (Miles and Eighmy 1980). However, in the short term, they may be asymmetric. We replicated Boyden and Raymond’s result in the cat model using sum-of-sines rotation in naïve cats (Titley and Broussard, unpublished data). The results are shown in Fig. 4. During the experiments shown here, we switched the cat’s visual field from magnified to miniaturized, or vice versa, during the experiment. As was previously reported in the mouse (Boyden and Raymond 2003), we found a residual effect of gain-down learning that caused gain-up learning to saturate at a lower value. In the inverse situation, there was no residual effect of gain-up learning. These results require verification in more subjects, but they support the conclusion of Raymond and coworkers that gain-up learning does not completely reverse gain-down learning, at least in the short term.
In naïve cats, gain-down learning limited subsequent gain-up learning. a–c show the time course of VOR gain over 2–3 h of learning in cats that had not previously worn lenses. In “up, up” and “down, down,” magnifying or miniaturizing lenses were worn for the entire 2-h protocol. In “down, up, up” and “up, down, down,” the lenses were changed after the first hour. d and e show summaries of the changes in gain and phase at each frequency in each protocol. In “down, up, up,” the 2 h of gain-up learning failed to increase the VOR gain above the pre-learning value. However, after “up, down, down,” the VOR gain was as low as it was after “down, down.” These results suggest that gain-down learning is not easily reversible
mGluR1 receptors and the two-threshold model
The data we have described so far are consistent with a scheme in which PF-LTD encodes memory for gain increases and PF-LTP encodes gain decreases. If this scheme is correct, disrupting known mechanisms that contribute to PF-LTD might disrupt gain-up learning selectively. PF-LTD in slices is known to require mGluR1 receptors (Kano et al. 2008). We were able to disrupt gain-up learning selectively by blocking mGluR1 receptors bilaterally in the flocculus (Titley et al. 2010) (Fig. 5). Strikingly, mGluR1 blockade inverts gain-up learning, resulting in a gain decrease when magnifying lenses are worn. This observation will be discussed below.
mGluR1 receptor activity in the flocculus is required for gain-up, but not for gain-down learning. In the gain-up protocol (left panels), the VOR gain decreased if these receptors were blocked by the mGluR1 antagonist YM 298198 (open symbols) by local injections into the flocculus before the learning period. However, in the gain-down protocol (right panels), there was little effect. In both protocols, PBS vehicle alone (filled symbols) was injected in control trials before starting the learning protocol. Data from Titley et al. (2010)
On the cellular level, activation of second-messenger pathways via mGluR1 and release of calcium from intracellular stores is thought to be important for the detection of coincident climbing-fiber and PF inputs to PC spines (Wang et al. 2000; Kano et al. 2008). Also, mGluR1 activation causes calcium influx from the extracellular space (Tempia et al. 2001). Some authors have suggested that mGluR1 receptors are activated as a result of action potentials in climbing fibers, which release large amounts of glutamate (Coesmans et al. 2004). The excess glutamate could reach mGluR1 receptors that are near the edge of the postsynaptic density (Nusser et al. 1994).
We found that gain-up learning was replaced by gain-down learning when mGluR1 was blocked. This outcome is consistent with a two-threshold model for motor learning in which the cytosolic calcium concentration in PCs is the key determinant of the direction of learning. In this model, a moderate calcium increase is sufficient to trigger PF-LTP (lower threshold; Kano et al. 2008), while a greater increase is required for PF-LTD (higher threshold; Coesmans et al. 2004; Jorntell and Hansel 2006). If the lower threshold is not reached, no long-term plasticity occurs. Together, the two thresholds determine the direction of synaptic plasticity. We think that blocking mGluR1 may restrict calcium levels in PC dendritic spines so that the threshold for PF-LTP can be reached within at least some spines, but the threshold for PF-LTD cannot. Then, in the presence of sensory stimuli that normally evoke LTD, LTP is evoked instead. In this scenario, gain-down learning should still be possible, as we found (Titley et al. 2010; Fig. 5), because the threshold for PF-LTP is below the limit imposed by blocking mGluR1. Our results indicate that mGluR1 receptors are necessary for bidirectional motor learning in the VOR.
GABAB receptors have a role in learning
Another receptor type that might participate in VOR motor learning is the GABAB receptor, which like mGluR1 is a G-protein-coupled receptor. GABAB receptors are present at the PF-PC synapse, both pre- and postsynaptically (Kulik et al. 2002), and have been proposed to play a facilitatory role in cerebellar LTD (Hirono et al. 2001; Kamikubo et al. 2007). The presumed source of GABA is the interneurons in the molecular layer. When we blocked GABAB receptors using the potent, selective antagonist CGP 52432, we found that once again, gain-up learning was inverted (Fig. 6). Increasing the activation of either mGluR1 or GABAB receptors using agonists slightly improved gain-up learning (Fig. 6). We therefore asked whether the GABAB receptor made its contribution to LTD, and hence to gain-up learning, via convergent second-messenger pathways that are known to be activated by the two receptor types (Kamikubo et al. 2007; Rives et al. 2009) or possibly even a direct interaction with the mGluR1 receptor (Tabata et al. 2004).
GABAB receptor activity in the flocculus is required for gain-up learning. a Injection of a GABAB antagonist, CGP 52432, resulted in a gain decrease in the gain-up protocol. b When the GABAB and mGluR1 antagonists were injected together, learning was also reversed but was not completely eliminated (see also E, YM298198 + CGP 52432). c Injection of the GABAB agonist baclofen enhanced gain-up learning. d The effect of baclofen was partially blocked by the mGluR1 antagonist (see also e, YM298198 + baclofen). e The change in VOR gain during the gain-up protocol could be an increase or a decrease, depending on the type of drug that was injected. The different test frequencies are represented by the fills in e. Learning was inverted by YM 298198, CGP 52432, both together, and YM298198 combined with baclofen. In each situation where learning was inverted, the frequency dependence of learning was eliminated
To understand how the interaction between the mGluR1 and the GABAB receptors pertains to learning, we attempted to increase the disruption of learning, preventing gain-down as well as gain-up learning, by blocking both receptors simultaneously. If the two receptors are acting by way of completely separate pathways, the effects of the antagonists should add, possibly driving the calcium concentration below the threshold even for gain-down learning. However, as Fig. 6a, b, e shows, when CGP 52432 and YM 298198 were administered together, the effect was qualitatively the same as the effect of either drug individually. This result was consistent with a downstream interaction between the two receptors, and indeed the existence of such interactions via diffusible second messengers, including the phospho-inositol (PI) pathway, has been demonstrated previously (Kamikubo et al. 2007; Rives et al. 2009).
If the interaction occurs within individual spines, blocking the contribution of mGluR1 to the local calcium concentration might prevent any effect of GABAB activation by ensuring that calcium remains below the threshold for LTD. In fact, we found that blocking mGluR1 receptors inverted gain-up learning even in the presence of the GABAB agonist, baclofen (Fig. 6c, d, e). However, the inverted learning was slightly increased by baclofen in the presence of YM 298198, as would be expected with an additive interaction (Fig. 6, bottom panel). In fact, this result is consistent with an additive interaction among spines, in which the total effect of YM 298198 is greater than the effect of baclofen, but baclofen still increases the calcium level in some spines, increasing the total amount of gain-down learning. The possible nature of this interaction is discussed further in a later section.
The frequency dependence of learning was affected when the direction of learning was inverted (Fig. 6). In all cases in Fig. 6e where learning was in the correct direction (a gain increase), the change was greatest at 0.5 Hz, as expected. However, in all cases where learning was in the “wrong” direction (a gain decrease), no such pattern was seen. A possible mechanism for this effect will be discussed below.
mGluR1 and GABAB receptors are largely segregated
mGluR1 and GABAB receptors have been assumed to be co-localized within the same dendritic spines (Kamikubo et al. 2007), because both are clearly present in spines (Lujan et al. 1997; Fritschy et al. 1999; Kulik et al. 2002; Rives et al. 2009). Such co-localization would strengthen the argument for a downstream interaction between the receptors that determines the direction of learning. We tested this assumption using double labeling with fluorescent antibodies and confocal microscopy. Mice were used for this experiment, because a high degree of non-specific labeling was seen when GABAB antibodies were used in cat tissue. The immunocytochemical procedure of Fritschy and coworkers (1995) was followed with minor modifications (see the “Appendix” for details).
The results are shown in Fig. 7 for GABAB R2. The results for GABAB R1 were qualitatively similar (data not shown). At low magnification, mGluR1a and GABAB R2 were found to have a partially overlapping pattern of immunoreactivity localized to the molecular layer (Fig. 7c). Higher magnification of the molecular layer revealed a bushy immunoreactive pattern of GABAB R2 labeling (Fig. 7d) that is indicative of PC dendritic staining. In contrast, mGluR1a immunoreactivity displayed no distinct pattern at high magnification, with punctate immunoreactivity throughout the molecular layer (Fig. 7e). Some of these mGluR1a immunoreactive puncta appeared to co-localize with GABAB (Fig. 7f). We analyzed co-localization by random selection and higher magnification of regions of interest, whereby individual puncta could be analyzed as objects. Representative images are shown for mGluR1a and GABAB R2 (Fig. 7g–i). To analyze the extent of co-localization, a measurement based on the distance between the geometric centers of mGluR1a and GABAB R2 immunoreactive puncta was used (Fig. 7j). Figure 7j only illustrates puncta above the threshold that we set for co-localization. The results are summarized in Fig. 7k both for GABAB R2 and for GABAB R1. The proportion of co-localizing puncta was as follows: mGluR1a with GABAB R1 (0.085 ± 0.012); GABAB R1 with mGluR1 (0.101 ± 0.010); mGluR1a with GABA-BR2 (0.076 ± 0.012); and GABAB R2 with mGluR1 (0.117 ± 0.013).
Only a small proportion of GABAB and mGluR1 receptors are co-localized at the subcellular level in the mouse cerebellum. Photomicrographs of the molecular layer are shown. a, d, g Expression of GABAB R2. b, e, h Expression of mGluR1. c, e, i The merged composite images. j An image mask was applied to the image in i, and co-localized immunoreactive puncta were identified based on the distance between their centers. k Summary of the co-localization (n = 6). The red (green) bars in each case represent the number of mGluR1 (GABAB) immunoreactive puncta above threshold that are close enough to GABAB (mGluR1) puncta, divided by the total number of mGluR1 (GABAB) puncta. Scale bars in a–c represent 50 μm; d–e, 20 μm; g–i, 5 μm
The puncta in Fig. 7g–i are roughly the size of dendritic spines, which in Purkinje cells are approximately 0.5 μm in diameter, and the bright puncta do not appear to be located within the same spines. Although the mGluR1a and GABAB receptors clearly were co-localized at a few puncta, the majority of the sites were labeled by only one of the two antibodies, and quantitative analysis suggested that the overwhelming majority of the receptors are not co-localized at the level of individual puncta and therefore are not co-localized within the same spines. This finding was surprising given the suggestion by Fritschy et al. (1999) that at least in the rat, the overwhelming majority of PC spines contain GABAB receptors. In that study, a sensitive pre-embedding electron microscopy procedure was used. Our use of light microscopy may have led to a failure to detect very low levels of labeling in some spines. However, it is clear from Fig. 7 that the spines that label most intensely with the GABAB antibody were not labeled with the mGLuR1 antibody.
The calcium concentration within individual dendritic spines is believed to be the most important factor determining the direction of plasticity at each synapse (Coesmans et al. 2004; Jorntell and Hansel 2006). This is particularly evident in situations where mGluR1 activation controls calcium signaling (Wang et al. 2000). The crucial importance of calcium concentrations within spines means that we must consider interactions between the mGluR1 and GABAB receptors located in different spines as determining factors in the direction of motor learning. For example, we considered the possibility that when baclofen and YM 298198 were administered together in Fig. 6, LTD and LTP both occurred but at different synapses, and the result was an effect on VOR gain that is a combined effect of all of such synapses (similar to a weighted average). This interpretation is reasonable because in our study the drugs are injected focally and diffuse outward, resulting in concentration gradients and possibly different effects at different distances from the focal point. Such a consensus may also be the normal mode of operation and the mechanism by which the direction of learning is determined. We asked whether this idea could explain the changes in VOR gain that occur at different test frequencies. The inverted learning caused by the mGluR1 antagonist failed to show the usual pattern of frequency dependence (Fig. 6e). We can explain this if we assume three things. First, LTP and LTD both occur at the same time but at different synapses, and the overall balance of the two processes determines the direction of learning. Second, 0.5- and 8-Hz responses are distributed differently within the floccular cortex, as Dean and coworkers have suggested (Dean et al. 2010). Third, 0.5 Hz has a larger region of representation than 8 Hz. If these assumptions are correct, our results for inverted gain-up learning can be explained by the concentration gradients generated by diffusion. Concentration gradients may result in no learning at the injection site, a predominance of LTP at a short distance from the injection site and LTD farther from it. Then at 8 Hz, LTP could predominate completely (if our injection is optimally located), resulting in a large gain decrease; at 0.5 Hz, the two processes would be more nearly balanced and the gain decrease smaller as a result. In sum, the results are consistent with the determination of the direction of synaptic plasticity by a two-threshold determination of the direction of plasticity within spines, and a determination of the direction of learning that is a consensus among Purkinje cell dendritic spines in the cortex of the cerebellar flocculus. Our conclusions do not discount the fact that downstream interactions, via second messengers, clearly do occur between mGluR1 and GABAB receptors (Kamikubo et al. 2007; Rives et al. 2009). Since the receptors are, for the most part, not co-localized in the same spines, the dendritic branches are the most likely site for such interactions. These interactions may have a role in memory processes such as consolidation and disruption. This possibility is a possible topic of future research.
Other mechanisms for VOR motor learning
Perhaps the most striking observation among the results in Fig. 6 is that in many situations, gain-down learning is substituted for gain-up learning, but in no situation were we able to completely eliminate gain-down learning. When we used a gain-down protocol, none of the drugs that we tested prevented learning (Fig. 5; additional data not shown). This would be surprising in a simple two-threshold model in which postsynaptic calcium completely determines both the direction and the amount of learning. Our observations are consistent with other mechanisms, in addition to cerebellar LTP and LTD, that contribute to gain-down learning and are unaffected by postsynaptic calcium. Two well-established examples are presynaptic LTP (Salin et al. 1996) and bidirectional plasticity at excitatory synapses on inhibitory interneurons in the cerebellar cortex (Jorntell and Ekerto 2002). Such alternate mechanisms can be important, as most recently indicated by a study in which abolishing PF-PC LTP selectively affected gain-up learning (Schonewille et al. 2010). The study employed a PC-specific calcineurin knockout mouse. Interestingly, the knockout also showed changes in excitability of Purkinje cells. Further study of this knockout may yield significant insights into VOR motor learning.
Summary
In this review, we summarize results supporting the initial encoding of motor memory in the flocculus, by cerebellar LTD and LTP. We also mention evidence that after it is encoded, motor memory can either be disrupted or else consolidated. Previously published results indicate that consolidation can be rapid, in which case the frequency dependence of learning is unchanged, consistent with a local mechanism of consolidation. In the longer term, however, the available evidence supports the transfer of memory out of the flocculus. We also describe some new results suggesting that both mGluR1 and GABAB receptors in the flocculus are necessary for gain-up, but not for gain-down learning, and that the two receptors are largely segregated on different dendritic spines on Purkinje cells. Together with what is already known of the mechanisms of cerebellar LTD and LTP, our data suggest that the direction of learning may be determined by interactions among groups of spines.
References
Anzai M, Kitazawa H, Nagao S (2010) Effects of reversible pharmacological shutdown of cerebellar flocculus on the memory of long-term horizontal vestibulo-ocular reflex adaptation in monkeys. Neurosci Res 68:191–198
Bolte S, Cordelieres FP (2006) A guided tour into subcellular colocalization analysis in light microscopy. J Microsc 224:213–232
Boyden ES, Raymond JL (2003) Active reversal of motor memories reveals rules governing memory encoding. Neuron 39:1031–1042
Boyden ES, Katoh A, Raymond JL (2004) Cerebellum-dependent learning: the role of multiple plasticity mechanisms. Ann Rev Neurosci 27:581–609
Brashers-Krug T, Shadmehr R, Bizzi E (1996) Consolidation in human motor memory. Nature 382:252–255
Broussard DM, Kassardjian CD (2004) Learning in a simple motor system. Learn Memory 11:127–136
Broussard DM, Bronte-Stewart HM, Lisberger SG (1992) Expression of motor learning in the response of the primate vestibuloocular reflex pathway to electrical stimulation. J Neurophysiol 67:1493–1507
Broussard DM, Bhatia JK, Hong JA (1999) The dynamics of the vestibulo-ocular reflex after peripheral vestibular damage. II. Comparison with dynamics after optically-induced learning. Exp Brain Res 125:365–374
Coesmans M, Weber JT, de Zeeuw C, Hansel C (2004) Bidirectional parallel fiber plasticity in the cerebellum under climbing fiber control. Neuron 44:691–700
Cohen MR, Meissner GW, Schafer RJ, Raymond JL (2004) Reversal of motor learning in the vestibulo-ocular reflex in the absence of visual input. Learn Mem 11:559–565
Cooke SF, Attwell PJE, Yeo CH (2004) Temporal properties of cerebellar-dependent memory consolidation. J Neurosci 24:2934–2941
de Zeeuw CI, Hansel C, Bian F, Koekkoek SKE, van Alphen AM, Linden DJ, Oberdick J (1998) Expression of a protein kinase C inhibitor in Purkinje cells blocks cerebellar LTD and adaptation of the vestibulo-ocular reflex. Neuron 20:495–508
Dean P, Porrill J, Ekerot C-F, Jorntell H (2010) The cerebellar microcircuit as an adaptive filter: experimental and computational evidence. Nat Rev Neurosci 11:30–43
Dudai Y (2004) The neurobiology of consolidations, or, how stable is the engram? Ann Rev Psychol 55:51–86
Frey U, Morris RGM (1997) Synaptic tagging and long-term potentiation. Nature 385:533–536
Fritschy JM, Mohler H (1995) GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol 359:154–194
Fritschy JM, Meskenaite V, Weinmann O, Honer M, Benke D, Mohler H (1999) GABAB-receptor splice variants GB1a and GB1b in rat brain: developmental regulation, cellular distribution and extrasynaptic localization. Eur J Neurosci 11:761–768
Galiana HL (1986) A new approach to understanding adaptive visual-vestibular interactions in the central nervous system. J Neurophysiol 55:349–374
Hampson DR, Theriault E, Huang X-P, Kristensen P, Pickering DS, Franck JE, Mulvihill ER (1994) Characterization of two alternatively spliced forms of a metabotropic glutamate receptor in the central nervous system of the rat. Neuroscience 60:325–336
Hansel C, de Jeu M, Belmeguenai A, Houtman SH, Buitendijk GH, Andreev D, De Zeeuw C, Elgersma Y (2006) αCaMKII is essential for cerebellar LTD and motor learning. Neuron 51:835–843
Hirono M, Yoshioka T, Konishi S (2001) GABAB receptor activation enhances mGluR-mediated responses at cerebellar excitatory synapses. Nature Neurosci 4:1207–1216
Ito M (1972) Neural design of the cerebellar motor control system. Brain Res 40:81–84
Jorntell H, Ekerto C-F (2002) Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron 34:797–806
Jorntell H, Hansel C (2006) Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron 52:227–238
Kamikubo Y, Tabata T, Kakizawa S, Kawakami D, Watanabe M, Ogura A, Iino M, Kano M (2007) Postsynaptic GABAB receptor signalling enhances LTD in mouse cerebellar Purkinje cells. J Physiol 585:549–563
Kano M, Hashimoto K, Tabata T (2008) Type-1 metabotropic glutamate receptor in cerebellar Purkinje cells: a key molecule responsible for long-term depression, endocannabinoid signalling and synapse elimination. Phil Trans Royal Soc Lond Biol 363:2173–2186
Kassardjian CD, Tan Y-F, Chung J-YJ, Heskin R, Peterson MJ, Broussard DM (2005) The site of a motor memory shifts with consolidation. J Neurosci 25:7979–7985
Kimpo R, Boyden ES, Katoh A, Ke MC, Raymond JL (2005) Distinct patterns of stimulus generalization of increases and decreases in VOR gain. J Neurophysiol 94:3092–3100
Kramer PD, Shelhamer M, Peng GCY, Zee DS (1998) Context-specific short-term adaptation of the phase of the vestibulo-ocular reflex. Exp Brain Res 120:184–192
Kulik A, Nakadate K, Nyin G, Notomi T, Malitschek B, Bettler B, Shigemoto R (2002) Distinct localization of GABAB receptors relative to synaptic sites in the rat cerebellum and ventrobasal thalamus. Eur J Hum Genet 15:291–307
Lev-Ram V, Wong ST, Storm DR, Tsien RY (2002) A new form of cerebellar long-term potentiation is postsynaptic and depends on nitric oxide but not cAMP. PNAS 99:8389–8393
Lisberger SG, Miles FA, Optican LM (1983) Frequency-selective adaptation: evidence for channels in the vestibulo-ocular reflex? J Neurosci 3:1234–1244
Lisberger SG, Pavelko TA, Broussard DM (1994) Neural basis for motor learning in the vestibulo-ocular reflex of primates: I. Changes in the responses of brain stem neurons. J Neurophysiol 72:928–953
Lujan R, Roberts JD, Shigemoto R, Ohishi H, Somogyi P (1997) Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 alpha, mGLuR2 and mGLuR5, relative to neurotransmitter release sites. J Chem Neuroanat 13:219–241
Menzies JRW, Porrill J, Dutia MB, Dean P (2010) Synaptic plasticity in medial vestibular nucleus neurons: comparison with computational requirements of VOR adaptation. PLoS ONE 5:e13182
Miles FA, Eighmy BB (1980) Long-term adaptive changes in primate vestibuloocular reflex. I. Behavioral observations. J Neurophysiol 43:1406–1425
Nagy GG, Al-Ayyan M, Andrew D, Fukaya M, Watanabe M, Todd AJ (2004) Widespread expression of the AMPA receptor GluR2 subunit at glutamatergic synapses in the rat spinal cord and phosphorylation of GluR1 in response to noxious stimulation revealed with an antigen-unmasking method. J Neurosci 24:5766–5777
Nusser Z, Mulvihill E, Streit P, Somogyi P (1994) Subsynaptic segregation of metabotropic and ionotropic glutamate receptors as revealed by immunogold localization. Neuroscience 61:421–427
Peterson BW, Baker JF, Houk JC (1991) A model of adaptive control of vestibuloocular reflex based on properties of cross-axis adaptation. Ann NY Acad Sci 627:319–337
Qiu D, Knopfel T (2009) Presynaptically expressed long-term depression at cerebellar parallel fiber synapses. Pflugers Arch 457:865–875
Raymond JL, Lisberger SG (1996) Behavioral analysis of signals that guide learned changes in the amplitude and dynamics of the vestibulo-ocular reflex. J Neurosci 16:7791–7802
Raymond JL, Lisberger SG (1998) Neural learning rules for the vestibulo-ocular reflex. J Neurosci 18:9112–9129
Raymond JL, Lisberger SG, Mauk MD (1996) The cerebellum: a neuronal learning machine? Science 272:1126–1131
Rives M-L, Vol C, Fukazawa Y, Tinel N, Trinquet E, Ayoub MA, Shigemoto R, Pin J-P, Prezeau L (2009) Crosstalk between GABAB and mGLu1a receptors reveals new insight into GPCR signal integration. EMBO 28:2195–2208
Robinson DA (1976) Adaptive gain control of vestibuloocular reflex by the cerebellum. J Neurophysiol 39:954–969
Robleto K, Poulos AM, Thompson RF (2004) Brain mechanisms of extinction of the classically conditioned eyeblink response. Learn Mem 11:517–524
Sakurai M (1987) Synaptic modification of parallel fibre-Purkinje cell transmission in in vitro guinea-pig cerebellar slices. J Physiol 394:463–480
Salin PA, Malenka RC, Nicoll RA (1996) Cyclic AMP mediates a presynaptic form of LTP at cerebellar parallel fiber synapses. Neuron 16:797–803
Scavio MJ, Clift PS, Wills JC (1992) Posttraining effects of amphetamine, chlorpromazine, ketamine, and scopolamine on the acquisition and extinction of the rabbit’s conditioned nictitating membrane response. Behav Neurosci 106:900–908
Schonewille M, Belmeguenai A, Koekkoek SKE, Houtman SH, Boele HJ, van Beugen BJ, Gao Z, Badura A, Ohtsuki G, Amerika WE, Hosy E, Hoebeek FE, Elgersma Y, Hansel C, De Zeeuw CI (2010) Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron 67:618–628
Tabata T, Araishi K, Hashimoto K, Hashimotodani Y, van der Putten H, Bettler B, Kano M (2004) Ca2+ activity at GABAB receptors constitutively promotes metabotropic glutamate signaling in the absence of GABA. PNAS 101:16952–16957
Tempia F, Alojado ME, Strata P, Knopfel T (2001) Characterization of the mGluR1-mediated electrical and calcium signaling in Purkinje cells of mouse cerebellar slices. J Neurophysiol 86:1389–1397
Titley HK, Heskin-Sweezie R, Chung J-YJ, Kassardjian CD, Razik F, Broussard DM (2007) Rapid consolidation of motor memory in the vestibulo-ocular reflex. J Neurophysiol 98:3809–3812
Titley HK, Heskin-Sweezie R, Broussard DM (2009) Consolidation and disruption of motor memory generalize across stimulus conditions in the vestibulo-ocular reflex. Brain Res 1267:37–43
Titley HK, Heskin-Sweezie R, Broussard DM (2010) The bidirectionality of motor learning in the VOR is a function of cerebellar mGluR1 receptors. J Neurophysiol 104:3657–3666
Wang SS-H, Denk W, Hausser M (2000) Coincidence detection in single dendritic spines mediated by calcium release. Nat Neurosci 3:1266–1273
Acknowledgments
We thank R. Heskin-Sweezie and D. Adusei for technical assistance. This work was funded by the Natural Sciences and Engineering Research Council of Canada and by the Canadian Institutes of Health Research. H. K. T. was supported by a fellowship from the Vision Science Research Program.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Methods for immunohistochemistry
Two adult C57/Bl6 mice were deeply anesthetized with ketamine (75 mg/kg) and xylazine (5 mg/kg) before transcardial perfusion with 0.1 M PBS followed by fixative (4% para-formaldehyde containing 15% picric acid, pH 7.40). The brains were removed, bisected sagittally along the midline, and postfixed for an additional 2 h at room temperature. The brains were then transferred to a citrate buffer (pH 4.5) and stored overnight at 4°C. Next, brains were irradiated in a microwave oven, on low power, for 3 min in fresh citrate buffer before cryoprotection in 10% DMSO for 3 h at room temperature. Finally, the brains were flash frozen and embedded in O.C.T. compound (Sakura-Finetek, USA) before sagittal sectioning of 30-μm sections.
Unmasking of GABAB epitopes was accomplished by the treatment of the free-floating sections with porcine pepsin (Sigma; 0.5 mg/ml in 0.2 N HCl) for 3 min at 37°C (Nagy et al. 2004). The sections were incubated in a blocking solution containing 5% goat serum (Sigma) and 0.3% Triton X-100 for 1 h at room temperature before overnight incubation at 4°C in primary antibodies diluted in blocking solution. After washing in 0.1 M PBS, the sections were incubated for 2 h with secondary antibodies diluted in 5% goat serum at room temperature and then washed again in 0.1 M PBS before being mounted in ProLong Gold Antifade (Molecular Probes, Invitrogen). Visualization of immunostaining was done on a Zeiss LSM 510 confocal microscope. Co-localization was analyzed using object-based methods in the JACoP plugin (Bolte and Cordelieres 2006) for Image J, version 1.43u (NIH).
Anti-GABAB R1 and R2 (1:100) antibodies were purchased from Neuromab. The anti-mGluR1a-specific antibody was generated as previously described (Hampson et al. 1994), and the secondary goat anti-mouse DyLight 488 (1:1,000) and goat anti-rabbit DyLight 549 (1:1,000) antibodies were purchased from Jackson Immunoresearch.
Three 100× images were taken from each of the two mice and within each of those three images, another four were taken at 500× and averaged. Each 100× field of view was counted as an independent sample.
Rights and permissions
About this article
Cite this article
Broussard, D.M., Titley, H.K., Antflick, J. et al. Motor learning in the VOR: the cerebellar component. Exp Brain Res 210, 451–463 (2011). https://doi.org/10.1007/s00221-011-2589-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2589-z