Abstract
From observation of human behavior, we know that speed of movement initiation and execution can be influenced by motivational factors, for example we walk faster when in a hurry (sense of urgency) or write faster during an exam (potential reward of good results). However, there is scant experimental evidence for the motivational modulation of movement in man. Experiments in non-human primates have demonstrated shortening of reaction times in response to reward. However, it is not clear how reward might affect performance of reaction time (RT) tasks in humans, and specifically whether warned and unwarned simple and uncued and precued choice RTs are similarly or differentially affected by reward. The effect of monetary incentive on total time (TT, (RT + MT)) was assessed in 16 healthy participants using four paradigms: warned simple RT (wSRT), unwarned simple RT (uSRT), uncued choice RT (uCRT), and precued choice RT (pCRT). wSRT, uSRT, and pCRT tasks all allow advance preparation and preprogramming of the movement, whereas uCRT does not. We found a significant effect of monetary incentive in shortening TTs in wSRT, uSRT, and pCRT tasks, but no effect on the uCRT task. These results demonstrate that monetary incentive can speed up movement initiation and execution in human participants, but only in tasks where preprogramming of the response is possible. This suggests that in reaction time tasks such as these, monetary incentive is having its effect by enhancing preparation of preprogrammed movement, but has little effect when movements cannot be specified in advance. These “RT and reward” tasks provide a useful paradigm for investigation into the effects of monetary incentive on reaction times in man and to study motivational modulation of movement speed in health and disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
From observation of human behavior, we know that speed of movement initiation and execution can be influenced by motivational factors, for example walking faster when in a hurry (sense of urgency) or writing faster during an exam (potential reward of good results). How such motivational factors are linked to behavior (including motor output) has been the subject of enormous interest, particularly in reference to Pavlovian conditioning and instrumental learning tasks (Dayan and Balleine 2002). This interest has produced ideas of dissociable components of reward-related behavior. For example, a “directing effect” is hypothesized to represent the current goals of behavior, while an “energizing effect” sets the force or vigor of these actions (Dickinson and Balleine 2002). In the “actor and critic” models of instrumental learning (Dayan and Balleine 2002; O’Doherty et al. 2004), the “critic” component is hypothesized to be involved in orientation toward a situation where reward occurs, and the perceived saliency of the reward while the “actor” component is involved in learning stimulus–response associations that predict reward. Although neither of these models perhaps captures the complexity of the relationship between motivational factors and behavior (Dayan and Balleine 2002), they do provide a framework to consider how motor output might alter with reward.
It is well established that the outcome of actions, whether they are followed by reward or punishment, influences and shapes future behavior, the so-called law of effect (Thorndike 1911). Experiments probing reward mechanisms often use a stimulus–response learning paradigm to examine the effect of the presentation of a reward-predicting stimulus on, for example, neuronal firing in non-human primates with implanted recording electrodes (Fiorillo et al. 2003) or change in BOLD signal in functional magnetic resonance imaging (fMRI) of the human brain (e.g., Elliott et al. 2004; O’Doherty et al. 2004). Weighing up the value of feasible actions and the desirability of potential outcomes is an inherent feature of value-based decision making (Rangel et al. 2008). There is evidence that the provision of monetary incentive can influence top-down control of attention. For example, the efficacy of visual selective attention can be modulated by provision of variable monetary reward as feedback (Della Libera and Chelazzi 2009a, b). The prospect of winning or losing money affected engagement and disengagement from valid and invalidly cued locations in a covert attention task and differentially activated attention networks and limbic areas (Small et al. 2005). Executive control of conflict in the Eriksen flanker task was influenced by positive or negative monetary reinforcement, and both errors and lateralized readiness potentials were sensitive to motivational context in the presence of incompatible distractors (Seifert et al. 2006). Using fMRI and a visual conditional button pressing task, it has been demonstrated that selection and preparation of actions are influenced by the expectation of reward, with activity in the prestriate visual cortex, premotor cortex, and lateral prefrontal cortex showing significant interaction between reward expectation and preparation (Ramnani and Miall 2003).
While motivational modulation of movement speed in humans is recognized as a phenomenon, there is scant empirical investigation into this topic (Ramnani and Miall 2003; Ballanger et al. 2006; Thobois et al. 2007). Animal experiments have examined reaction times (RTs) in response to reward-predicting stimuli (Hollerman et al. 1998; Hassani et al. 2001). Not surprisingly, these studies (which have mainly assessed precued RT) have found a speeding up of RT in response to reward-predicting stimuli (Hollerman et al. 1998; Hassani et al. 2001). It is not clear at which stage of motor performance: preparation, initiation, or execution, such motivational influences are exerting an effect. Simple RT (SRT) and precued choice RT (pCRT) tasks allow advance preparation or preprogramming of the desired movement, whereas uncued choice RT (uCRT) tasks do not (for review see (Jahanshahi 2003). The aim of the present study was to address two questions. First, can monetary reward be used as an incentive to speed up movement in RT tasks in humans? Second, does monetary incentive have similar or differential effects across RT tasks which do (warned and unwarned SRT, precued CRT) or do not (uncued CRT) allow preprogramming and advance preparation of the response? We hypothesized that monetary incentive would speed up performance in RT tasks. As speeding up of RTs in response to reward-predicting stimuli in animal studies is obtained in precued RT tasks where advance preparation is possible (Hollerman et al. 1998; Hassani et al. 2001), we predicted that monetary incentive would be most likely to have an effect when preprogramming or advance preparation of movements was possible and that speed improvements would be seen for SRT and pCRT tasks but that no effect of monetary incentive would be observed in uCRT tasks.
Methods
Participants
Sixteen healthy volunteers (mean age: 33, range: 22–41 years; 8 men) with no history of neurological, physical or psychiatric illness, head injury, alcohol, or drug abuse were included in the study. The study had the approval of our local ethics committee, and all participants gave written informed consent.
Equipment
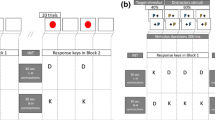
Responses were made on a box with six buttons. The buttons were 2.54 cm in diameter and placed in two vertical rows of three buttons. The buttons were spaced 15.24 cm apart (center to center) horizontally and 10.16 cm vertically. The middle buttons of each row served as the home keys. The other four buttons served as response buttons. The buttons could be covered or left exposed according to the experimental condition. This response box was linked to a computer with a 14-inch monitor on which the stimulus material was displayed. For all conditions, demonstration and practice trials were provided.
Warned and unwarned simple reaction time (wSRT, uSRT)
The participant was seated in front of the computer monitor. Only one home key and one response key were exposed on the button box. The participant was instructed to hold down the home key with the index finger of their right hand. A fixation cross was presented on the screen. On 50% of trials (warned SRT, wSRT), after a variable delay of 1–4 s, a warning signal (S1) was presented in the form of an empty square. After 1,600 ms, this square was filled to become solid white, and this constituted the imperative signal (S2). Participants were then required to release the home key as quickly as possible and press the response key. The screen cleared 500 ms after a response was made. The next trial started when the home key was pressed again. On 50% of trials (unwarned SRT, uSRT), there was no S1 or warning signal and the fixation cross was followed by a filled square immediately, in which case, the participant was required to press the response key straight away. In warned conditions, the participants were instructed to make use of the warning signal and prepare themselves to make a response prior to presentation of S2. Participants were told to respond after presentation of S2 in order to discourage anticipatory responses. In both the warned and unwarned SRT conditions, participants can preprogram the response as its nature is invariant and is known to them before the presentation of the imperative signal.
Uncued choice reaction time (uCRT) and precued choice reaction time (pCRT)
The participant was seated in front of the computer monitor. All four response keys and both home keys were exposed on the response box. The participant was asked to hold down both home keys with the index finger of each hand. A fixation cross was presented on the screen. For precued choice reaction time (pCRT, 50% of trials), after a variable delay of 1–4 s, an unfilled square was presented in one of the four positions on the screen, representing the location of one of the four response buttons. This represented the precue (S1). After 1,600 ms, the square was filled to become solid white. This was the imperative (S2) signal. Participants were then required to release the home key as quickly as possible and press the appropriate response key. The screen cleared 500 ms after a response was made. The next trial started when the home key was pressed again. For uncued choice reaction time (uCRT, 50% of trials), the fixation cross was followed after a delay of 1–4 s by a filled square without presentation of an empty square (i.e., no precue or S1) requiring the participant to press the appropriate response key straight away. In the precued trials, the participants were instructed to make use of the advance information provided by the precue to prepare a response prior to S2. Participants were asked to respond after presentation of S2 in order to discourage anticipatory responses.
Measurement of reaction times, movement times, and errors
The time from presentation of S2 to the release of the home key, i.e., the time between the presentation of the “go” stimulus and the initiation of a response, was measured as the reaction time (RT). The time from release of the home key to pressing the response key, i.e., the time between initiating and executing a button press response, was measured as the movement time (MT). Both RT and MT were recorded by the computer to the nearest millisecond. Three types of error were recorded: anticipation errors (RT less than or equal to 100 ms), decision errors (incorrect response key pressed in choice reaction time conditions), and long responses (RT greater than 2,000 ms). Trials with error data were excluded and replaced.
Experimental design
Each participant attended two sessions of testing separated by at least 1 week. During one session (unrewarded session), participants performed 4 blocks of 100 trials of the SRT tasks (50% wSRT, 50% uSRT randomized) and 4 blocks of 100 trials of the CRT tasks (50% uCRT, 50% pCRT randomized). This session was designed to investigate the effect of repeated task performance on RT in terms of either practice (improved task performance and speeding of RTs across the session) or fatigue (decreased task performance and slowing of RTs across the session). In the second session (rewarded session), participants first performed 2 blocks of 100 trials of the SRT tasks as in the unrewarded session. At the end of the second block, participants were provided feedback and told what their RTs for the second block had been. Prior to commencing the third block, participants were told that for every 10 ms they improved their RTs they would receive a monetary reward of 50 pence. At the end of the third block, they were given feedback on their performance (their exact RTs and whether they had speeded up relative to previous block or not). Prior to the fourth and last block, participants were told that they would receive a monetary reward of 100 pence for every 5 ms and they improved their RTs on the following block. Feedback was also given at the end of this second rewarded block. Participants also performed the CRT task with the first two blocks of 100 trials unrewarded as before, followed by two rewarded blocks as described for the SRT task. The order of SRT and CRT tasks was counterbalanced across participants.
Tridimensional personality questionnaire
Participants also completed the tridimensional personality questionnaire (Cloninger 1987). This questionnaire assesses personality characteristics via answers to 100 questions. Three subscale scores are derived as follows: harm avoidance, reward dependence, and novelty seeking (Cloninger 1987). We predicted a positive correlation between the reward dependence subscores and total reward achieved in the reaction time tasks.
Statistical analysis
Data analysis was performed using SPSS for Windows version 14.0. Because of the nature of the task, requiring participants to lift their index finger from the home key on stimulus presentation (RT) and moving to the response key (MT), it is possible that monetary incentive may influence both movement initiation and execution. Therefore, for the data analysis, we summed RTs and MTs to obtain total time (TT), a combined measure of movement initiation and execution. Our initial analysis of the unrewarded session was performed using TT. Repeated measures ANOVA was used to compare TT across RT tasks and to determine the effect of monetary incentive. Post hoc pairwise comparisons with Bonferroni correction were carried out to further analyze the data shown as significant by the ANOVA.
Results
Unrewarded session—the effect of repeated performance/practice
Figure 1 shows the TT data for each of the four blocks of the unrewarded session for each of the four tasks. We performed a two-way ANOVA with TASK (uSRT, wSRT, uCRT, pCRT) and BLOCK (1–4) as main factors. This analysis revealed a significant effect of TASK [F(3,42) = 124; P < 0.0001], but no effect of BLOCK and no TASK × BLOCK interaction. Corrected pairwise comparisons showed that TTs were significantly faster for the wSRT task and pCRT tasks than either the uSRT or uCRT tasks (P < 0.0001). TTs were significantly faster for the uSRT task than for the uCRT task (P < 0.0001).
Therefore, the above-mentioned analyses showed that in the unrewarded session, TTs only differed as a function of the type of RT task and did not differ across the four blocks and did not show any differential change across the blocks in the four types of RT task.
Errors, anticipation and long responses for SRT tasks, and anticipation, long responses and incorrect responses for CRT tasks were analyzed using Wilcoxon signed ranks tests. There was no difference in number of anticipation or long-response errors between uSRT and wSRT tasks. There was also no difference in long-responses or incorrect response errors between uCRT and pCRT tasks. However, there were significantly more anticipation errors in the pCRT than in the uCRT task (Z = −2.37; P = 0.018). There was no significant change in the number of errors across the four blocks of testing for any of the tasks.
Rewarded session—the effect of monetary incentive
As the rewarded and unrewarded sessions were conducted on different days, we normalized the data for blocks 2–4 to block 1 for both the rewarded and unrewarded sessions.
We examined the effect of reward on TTs for each task. These data are shown in Fig. 2. We performed a three-way ANOVA with TASK (uSRT, wSRT, uCRT, pCRT), BLOCK (2–4), and REWARD (rewarded session, unrewarded session) as main factors. As monetary incentive was only provided for blocks 3 and 4 of the “‘rewarded” session, we would predict a significant BLOCK × REWARD interaction. We found a significant main effect of BLOCK [F(2,28) = 24.4; P < 0.0001] and of REWARD [F(1,14) = 25.3; P < 0.0001]. Post hoc tests showed that the significant main effect of reward was due to overall TTs being faster for the rewarded compared with the unrewarded session. The significant effect of BLOCK was due to significantly shorter TTs in blocks 3 and 4 compared with block 2 (P < 0.0001). As predicted, there was a significant BLOCK × REWARD interaction [F(2,28) = 1,935; P = 0.02]. This was due to a significant shortening of TTs in the rewarded blocks (P < 0.0001). There was also a significant TASK × REWARD interaction [F(3,42) = 1114; P = 0.036]. Corrected pairwise comparisons showed this interaction to be due to a shortening of TTs in blocks 3 and 4 compared with block 2 in the uSRT, wSRT, and pCRT tasks (P < 0.01 for all), but no significant change in TTs in blocks 3 and 4 compared with block 2 for the uCRT task. The main effect of TASK and the TASK × BLOCK or TASK × BLOCK × REWARD interactions were not significant.
Total time (reaction time plus movement time) data for the rewarded (filled squares) and unrewarded (open squares) sessions for each task. a Unwarned simple reaction time (uSRT), b warned simple reaction time (wSRT), c uncued choice reaction time (uCRT), d precued choice reaction time (pCRT). Error bars are standard error of the mean. These data are normalized data: data from blocks 2–4 have been normalized to the data from block 1 for each of the two sessions (unrewarded and rewarded) and are expressed as a percentage of the block 1 value
In sum, while a significant TASK × BLOCK × REWARD would have been the clearest indication of the impact of reward on the RT tasks, nevertheless, the above significant BLOCK × REWARD and TASK × REWARD interactions and subsequent pairwise comparisons indicated that provision of reward shortened TTs relative to the unrewarded block and produced a differential effect on TT across tasks, speeding up TTs for uSRT, wSRT, and pCRT but not in uCRT.
Error data were analyzed as for the unrewarded session using Wilcoxon signed ranks tests. There was no difference between the number of anticipations, long responses, or incorrect responses for any of the RT tasks between unrewarded blocks and rewarded blocks (P > 0.05). Therefore, the speeding up of TTs observed in the uSRT, wSRT, and pCRT tasks was not accompanied by an increased number of errors.
Participants received monetary reward contingent on their performance on blocks 3 and 4 of the rewarded session. Table 1 shows the mean monetary reward received for each of the two rewarded blocks for the combined SRT and combined CRT tasks, as well as the total reward received. As the uSRT and wSRT as well as the uCRT and pCRT tasks were mixed together in a block, it is not possible to separate out the contribution of each task to the overall monetary reward received, but monetary incentive associated with SRT and CRT can be compared. We performed a two-way ANOVA on the TT data with TASK (SRT, CRT) and BLOCK (first rewarded block, second rewarded block) as main factors. This showed no significant effect of TASK, BLOCK, nor any TASK × BLOCK interaction. Thus, the magnitude of reward did not produce any differential effects across tasks.
We calculated a “reward effect” measure for each rewarded block by subtracting the TT in block 2 from that in block 3 of the rewarded session (reward effect for the first rewarded block) and by subtracting the TT in block 3 from that in block 4 of the rewarded session (reward effect for the second rewarded block). The total reward effect was calculated as the sum of the reward effects for the first and second rewarded blocks. This variable, which is a measure of the extent of TT speeding in response to reward, was positively and significantly correlated with the amount of reward received (r = 0.67; P < 0.001).
We also examined correlations between scores on the subscales of the TPQ and the total amount of reward received. There was a positive and significant correlation between the reward dependence subscale of the TPQ and monetary reward received (Spearman’s rho = 0.51; P = 0.019). There were no other significant correlations between other TPQ subscales and the magnitude of reward.
Discussion
We have demonstrated that compared with simple repetition of RT tasks across blocks in the unrewarded session, prospect of monetary incentive significantly speeds up performance in wSRT, uSRT, and pCRT tasks but in contrast has no effect on performance in a uCRT task. Our results indicate that monetary incentive can improve the speed of performance in tasks where preprogramming of the movement is possible, but not when selection and programming of the movement occurs after the go signal as in uCRT. It has been proposed that interval timing is operational in RT tasks, particularly during the preparatory interval, i.e., the period between onset of warning/precue stimuli and go signals (Macdonald and Meck 2004; Praamstra et al. 2006). It is possible that the effect of incentive motivation observed by us in the SRT and precued CRT tasks exerts an influence on this process of interval timing, which participants volitionally altered in anticipation of reward.
In man, monetary incentive has been consistently shown to be an appropriate reward that is associated with activation of reward circuits in the brain (e.g., Elliott et al. 2000; Knutson et al. 2000). There appear to be multiple systems that subserve the response of organisms to reward (for review see Schultz 2000). These systems include reward detection and prediction mechanisms (medial temporal cortex, striatum), reward expectation and processing of relative reward value (orbitofrontal cortex), and goal representation (dorsolateral prefrontal, premotor, parietal cortices, striatum) (Schultz 2000). Cognitive responses to reward such as orientation and attention should not have differed between our tasks, and there is no reason to suspect that a failure to improve in the uCRT task in response to reward was related to differential orienting or attention to reward in this particular task. Likewise, there is no reason for reward expectation, prediction, and detection to have been different for the uCRT compared with the other tasks. The most parsimonious explanation for the results of this study is therefore in terms of the key feature that differentiates the uCRT task from the other RT tasks, namely an inability to plan the required movement prior to presentation of the go signal. Thus, it appears that monetary incentive speeds up movement in RT tasks (uSRT, wSRT, pCRT) where volitional preparation and preprogramming of a response are possible. Our results are consistent with those of Ramnani and Miall (2003) who also found that in their visual conditional button pressing task, in which participants were either able or unable to plan their responses in advance, the influence of expectation of monetary reward was greater at the response planning than at the response execution stage.
Previous work in non-human primates performing rewarded and non-rewarded go/no go RT tasks with an S1/S2 (i.e., pCRT) design (Hassani et al. 2001; Hollerman et al. 1998) provides data relevant to interpretation of the results of the current study. The behavioral data from these studies show, as expected, a shortening of reaction time in rewarded vs. non-rewarded trials and with preferred vs. non-preferred reward (Hassani et al. 2001; Hollerman et al. 1998). Some neurons in the striatum appear to distinguish between the type of reward, some between movement required (left or right arm), and some between trials where movement is required and where it is not. In the striatum, neurons reactive to the spatial requirements of the task were particularly responsive to reward (Hassani et al. 2001; Hollerman et al. 1998). This is in contrast to other studies using similar paradigms, but recording neuronal activity from the orbitofrontal cortex (Tremblay and Schultz 1999). Here, neurons capable of discriminating between rewarded and unrewarded movements or between different rewards show little, if any, response to the spatial requirements of the task (Tremblay and Schultz 1999). This would suggest that certain striatal neurons are particularly tuned to the integration of reward and the movement-related parameters of the reward-predicting task, whereas orbitofrontal neurons are more concerned with the presence or absence of reward itself. Striatal recordings during reward tasks have, however, found very little discrimination between rewarded and unrewarded trials during movement execution itself (Hassani et al. 2001; Hollerman et al. 1998). Therefore, reward appears to have a much smaller influence on neuronal activity related to movement execution compared with earlier components of the behavioral response.
The different reaction time paradigms used in this study specifically probed the interaction between monetary incentive and the ability to preprogram the reward-predicting response. The data presented earlier from non-human primates indicate an important role for the basal ganglia in integrating reward-related and movement-related information. The data from these studies would suggest that the main effect occurs in the phase of movement preparation and that the effect of reward on movement execution itself may be small. This suggestion is supported in our study by the lack of effect of reward on a task where movement preparation is not possible (uCRT). Clearly, our results suggesting that incentive-related speeding is specific to tasks where preprogramming of a movement is possible require replication in future studies with larger samples. It is also possible that provision of reward exerted its influence on movement speed through tuning up attention or a learning mechanism. While the lack of a reward effect in the uCRT task argues against such general effects, nevertheless, in future studies, the potential contributions of attention or learning can be dissociated from a purely motivational effect by, for example, introducing further blocks to determine whether movement speed returns to baseline levels when reward is no longer provided. In the present study, counterbalancing of task presentation was used to overcome any potential order effects. However, in future studies, use of a between-group design, such that participants only perform one type of RT task with unrewarded blocks without any mention of reward followed by rewarded blocks would remove any order effects and the possibility of strategic slowing of RTs on the unrewarded trials.
How are such motivational influences on movement likely to be mediated? The ventral tegmental area (VTA), the nucleus accumbens (NA) and the ventral pallidum (VP), form an appropriate circuit for mediating such motivational modulation of movement, since the medial sections of these structures (NAshell, VPm, VTA) receive limbic input; whereas the lateral sections (NAcore, VPl, substantia nigra) are connected to the motor system. In support of this, a recent imaging study has shown significant activation of the VP in a task where subjects received a monetary reward in proportion to the force generated, regardless of whether or not they were consciously aware of the magnitude of the reward (Pessiglione et al. 2007). Studies of pavlovian instrumental transfer, in which a classically acquired association between a predictor cue and reward subsequently influences the vigor of an instrumental action has been shown to be disrupted following lesions of the VTA or NA in rats (Corbit et al. 2001, 2007) and to be associated with activation of the NA and the amygdale in an fMRI study in man (Talmi et al. 2008). Thus, the VTA and NA form a limbic–motor interface with the VP, which could mediate motivational influences on movement (Mogenson et al. 1980; Mogenson and Yang 1991). A model of the effect of reward on response vigor suggest that in conditions where the average rate of reward is higher, then the vigor or speed of responding will increase and that this is consistent with the effects of dopaminergic pharmacological manipulations on response rates (Niv et al. 2007). A similar effect of reward on movement speed has been modeled by Shadmehr et al. (2010) and linked to slowed movement in Parkinson’s disease. These models deal with movements that are already familiar and can be prepared in advance, so could explain our findings in relation to the uSRt, wSRT, and pCRT conditions where the response was known in advance.
Parkinson’s disease is a dopamine deficiency syndrome in which slowness of movement or bradykinesia is a primary symptom. Bradykinesia has been characterized as “insufficient motor energy” (Hallett 1990). In a recent study of speed of reaching movements in Parkinson’s disease, Mazzoni and colleagues (2007) demonstrated that patients could move faster but ordinarily chose not to do so. They proposed that bradykinesia in Parkinson’s disease represented a shift in the cost/benefit ratio of the energy expenditure necessary to move at normal speed. If this is the case, then provision of monetary incentive for fast movements should alter this cost/benefit ratio and result in patients with Parkinson’s disease producing faster movements. Some indirect evidence for such motivational influences on movement speed in Parkinson’s disease is available. There are reports of ordinarily akinetic patients with Parkinson’s disease being mobilized by the sight of a drowning man or in a fire, with these incidents acting as “motivational” triggers for movement. This so-called paradoxical kinesis is considered to reflect the influence of motivational factors on movement speed. Furthermore, two recent studies used a ball-catching task demonstrated that temporal urgency had a significant effect on movement speed in healthy controls and in patients with Parkinson’s disease (Ballanger et al. 2006; Thobois et al. 2007). Our results indicate that in relation to reaction time tasks, reward has its major effect on task performance by influencing aspects of movement preparation, allowing faster movement initiation in tasks where preprogramming of the movement is possible. In future studies, we plan to use the “RT and reward” paradigm developed here to study motivational influences on movement speed in patients with Parkinson’s disease. It has been proposed that while phasic dopamine mediates action selection through learning, tonic levels of dopamine have an energizing effect and influence response vigor (Niv et al. 2007). If response vigor is modulated by striatal dopamine, then it would be of interest to determine whether the effect of monetary incentive on movement speed in Parkinson’s disease is different on and off dopaminergic medication. Such motivational influences on movement would also be predicted to be particularly altered in patients with Parkinson’s disease with specific motivational deficits such as apathy or depression. Our “RT and Reward” paradigm would also be of value in dissociating motor and motivational components of other negative symptoms such as psychomotor retardation in primary depressive illness or poverty of action in schizophrenia.
References
Ballanger B, Thobois S, Baraduc P, Turner RS, Broussolle E, Desmurget M (2006) “Paradoxical kinesis” is not a hallmark of Parkinson’s disease but a general property of the motor system. Mov Disord 21:1490–1495
Cloninger CR (1987) A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry 44:573–588
Corbit LH, Muir JL, Balleine BW (2001) The role of the nucleus accumbens in instrumental conditioning: evidence of a functional dissociation between accumbens core and shell. J Neurosci 21:3251–3260
Corbit LH, Janak PH, Balleine BW (2007) General and outcome-specific forms of Pavlovian-instrumental transfer: the effect of shifts in motivational state and inactivation of the ventral tegmental area. Eur J Neurosci 26:3141–3149
Dayan P, Balleine BW (2002) Reward, motivation, and reinforcement learning. Neuron 36:285–298
Della Libera C, Chelazzi L (2009a) Visual selective attention and the effects of monetary rewards. Psychol Sci 17:222–227
Della Libera C, Chelazzi L (2009b) Learning to attend and to ignore is a matter of gains and losses. Psychol Sci 20:778–784
Dickinson A, Balleine BW (2002) The role of learning in motivation. In: Gallistel CR (ed) Learning, motivation and emotion, vol 3 of Steven’s handbook of experimental psychology. Wiley, New York
Elliott R, Friston KJ, Dolan RJ (2000) Dissociable neural responses in human reward systems. J Neurosci 20:6159–6165
Elliott R, Newman JL, Longe OA, William Deakin JF (2004) Instrumental responding for rewards is associated with enhanced neuronal response in subcortical reward systems. Neuroimage 21:984–990
Fiorillo CD, Tobler PN, Schultz W (2003) Discrete coding of reward probability and uncertainty by dopamine neurons. Science 299:1898–1902
Hallett M (1990) Clinical neurophysiology of akinesia. Rev Neurol (Paris) 146:585–590
Hassani OK, Cromwell HC, Schultz W (2001) Influence of expectation of different rewards on behavior-related neuronal activity in the striatum. J Neurophysiol 85:2477–2489
Hollerman JR, Tremblay L, Schultz W (1998) Influence of reward expectation on behavior-related neuronal activity in primate striatum. J Neurophysiol 80:947–963
Jahanshahi M (2003) Reaction time as an index of motor preparation/programming and speed of response initiation. In: Hallett M (ed) Handbook of clinical neurophysiology: movement disorders, vol 1. Elsevier, The Netherlands, pp 203–229
Knutson B, Westdorp A, Kaiser E, Hommer D (2000) fMRI visualization of brain activity during a monetary incentive delay task. Neuroimage 12:20–27
Macdonald CJ, Meck WH (2004) Systems-level integration of interval timing and reaction time. Neurosci Biobehav Rev 28:747–769
Mazzoni P, Hristova A, Krakauer JW (2007) Why don’t we move faster? Parkinson’s disease, movement vigor, and implicit motivation. J Neurosci 27:7105–7116
Mogenson GJ, Yang CR (1991) The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol 295:267–290
Mogenson GJ, Jones DL, Yim CY (1980) From motivation to action: functional interface between the limbic system and the motor system. Prog Neurobiol 14:69–97
Niv Y, Daw ND, Joel D, Dayan P (2007) Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology (Berl) 191:507–520
O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ (2004) Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 304:452–454
Pessiglione M, Schmidt L, Draganski B, Kalisch R, Lau H, Dolan RJ, Frith CD (2007) How the brain translates money into force: a neuroimaging study of subliminal motivation. Science 316:904–906
Praamstra P, Kourtis D, Kwok HF, Oostenveld R (2006) Neurophysiology of implicit timing in serial choice reaction-time performance. J Neurosci 26:5448–5455
Ramnani N, Miall RC (2003) Instructed delay activity in the human prefrontal cortex is modulated by monetary reward expectation. Cereb Cortex 13:318–327
Rangel A, Camerer C, Montague PR (2008) A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci 9:545–556
Schultz W (2000) Multiple reward signals in the brain. Nat Rev Neurosci 1:199–207
Seifert J, Naumann E, Hewig J, Hagemann D, Bartussek D (2006) Motivated executive attention–incentives and the noise-compatibility effect. Biol Psychol 71:80–89
Shadmehr R, Orban de Xivry JJ, Xu-Wilson M, Shih TY (2010) Temporal discounting of reward and the cost of time in motor control. J Neurosci 30:10507–10516
Small DM, Gitelman D, Simmons K, Bloise SM, Parrish T, Mesulam MM (2005) Monetary incentives enhance processing in brain regions mediating top–down control of attention. Cereb Cortex 1512:1855–1865
Talmi D, Seymour B, Dayan P, Dolan RJ (2008) Human pavlovian-instrumental transfer. J Neurosci 28:360–368
Thobois S, Ballanger B, Baraduc P, Le BD, Lavenne F, Broussolle E, Desmurget M (2007) Functional anatomy of motor urgency. Neuroimage 37:243–252
Thorndike EL (1911) Animal intelligence: experimental studies. Macmillan, New York
Tremblay L, Schultz W (1999) Relative reward preference in primate orbitofrontal cortex. Nature 398:704–708
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mir, P., Trender-Gerhard, I., Edwards, M.J. et al. Motivation and movement: the effect of monetary incentive on performance speed. Exp Brain Res 209, 551–559 (2011). https://doi.org/10.1007/s00221-011-2583-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-011-2583-5