Abstract
The brain can be considered a dynamical system which is able to oscillate at multiple frequencies. To study the brain’s preferred oscillation frequencies, the resonance frequencies in the frequency response of the system can be assessed by stimulating the brain at various stimulation frequencies. Furthermore, the event-related potential (ERP) can be considered as the brain’s impulse response. For linear dynamical systems, the frequency response should be equivalent to the frequency transform of the impulse response. The present study test whether this fundamental relation is also true for the frequency transform of the ERP and the frequency response of the brain. Results show that the spectral characteristics of both impulse and frequency response in the gamma frequency range are significantly correlated. Thus, we speculate that the resonance frequencies determine the frequency spectrum of the impulse response. This, in turn, implies that both measures are determined by the same, individually specific, neuronal generator mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most physical systems can potentially oscillate—even if this is not their main purpose (Strogatz 2003). For example, a guitar string, a wine glass, and a neuron will all oscillate when stimulated in the appropriate fashion. Interestingly, oscillatory systems usually display resonance behaviour; they oscillate more strongly at their so-called resonance frequency than at other frequencies. This resonance frequency also determines how the systems respond to an input that resembles an impulse, i.e. the impulse response of the system (see below). For this reason, the guitar string, the glass, and the neuron will all display a short oscillatory response when strung, hit, or stimulated, respectively.
These resonance frequencies might also explain the predominance of gamma-band responses in experiments that occur in human electroencephalogram (EEG), magnetoencephalogram, and animal recordings. Cortical gamma-band responses can be evoked by a variety of sensory events and are related to several perceptual and cognitive tasks (Basar-Eroglu et al. 1996). Synchronous oscillations in the gamma-range frame a general principle of functional connectivity (Kaiser and Lutzenberger 2003) and communication (Basar et al. 2001) in the cortex. Evoked gamma-band responses (eGBRs) covering a frequency range of 30–80 Hz and occurring in a time window between 50 and 150 ms constitute the earliest prominent spectral component. This oscillatory activity has been observed consistently in the auditory cortex of animals and humans (Demiralp et al. 1996; Herrmann et al. 2010). The frequency of that eGBR evident in the human scalp electroencephalogram is highly variable across individuals and, at the same time, relatively constant within individuals (Frund et al. 2007). These two phenomena together with the behavioural relevance of the eGBR (Kaiser et al. 2008, 2009) represent the prerequisites for a measure to be considered a personal trait variable (Allport 1927) and have lead to the assumption of an individual gamma-trait (Zaehle et al. 2009).
Gamma-band synchronization emerges in networks of excitatory and inhibitory neurons (Tiesinga et al. 2001; Borgers et al. 2005). The frequency of oscillatory neural activity is mainly determined by the time constant of the shunting inhibition among basket cells (Vida et al. 2006). Thus, the foundation of the intra-individual frequency permanence in the gamma-band response must be related to the neuroanatomical make-up of each individual. If so, also further electrophysiological measurements of the sensory system under investigation should demonstrate response characteristics in an equivalent frequency range.
Generally, the preferred oscillation frequencies of a dynamical system such as the brain are determined by anatomical network properties such as glutamatergic or GABAergic time constants of synaptic connections (Whittington et al. 2000; Traub et al. 1997). Interestingly, even single neuron can show resonance behaviour depending on the parameters of their physiological parameters (Izhikevich et al. 2003; Hutcheon and Yarom 2000; Llinas 1988). A method for studying the brain’s preferred oscillation frequencies is the measurement of its resonance frequencies: the frequency at which the brain shows a stronger response when stimulated when compared to other stimulation frequencies. Since this analysis of resonance frequencies represents the so-called frequency response of linear systems theory, the frequency response with its resonance frequency should also determine which frequencies are present in the impulse response, i.e. the ERP in a physiological system.
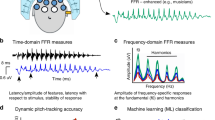
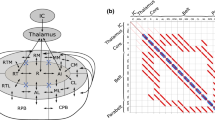
In a linear dynamical system, the input–output behaviour of the system can be inferred by two alternative methods. Either, the system is probed with an impulse and the impulse response is observed, or the system is probed with all frequencies within a specified range and the frequency response (also called transfer function) is observed (cf. Fig. 1 top panel). If the system is linear, the frequency transform of the impulse response must be identical to the frequency response (Oppenheim et al. 1999). It remains to be seen whether this is also true for testing the human brain (cf. Fig. 1 bottom panel). Basar (1980) suggested to use tools of linear systems theory for the analysis of electrophysiological brain responses. This requires to identify which physiological measures most closely represent the parameters of linear systems. One might consider the event-related potential (ERP) as the impulse response, since a short-lasting stimulus is usually used for stimulation. In case of providing oscillations of all frequencies within a specified range as stimuli, we can obtain the frequency response which is composed of as many steady-state responses as there are test frequencies (diagonal profile of the two-dimensional ASS representation in Fig. 2). Based on linear systems theory, our hypothesis was that the frequency response should be similar to the frequency transform of the ERP.
Top panel in a linear dynamical system, the frequency transform of the impulse response must be identical to the frequency response. Bottom panel it remains to be seen whether this is also true for testing the human brain. One might consider the ERP as the impulse response of the brain. The frequency response can be obtained with steady-state experiments. The diagonal profile of the two-dimensional steady-state response (cf. Fig. 2) represents the frequency response of the brain
Top left the two-dimensional ASS response is computed by stimulation the brain with AM oscillations with all frequencies from 20 to 100 Hz and computing the ASS for each frequency. The amplitude spectrum of each ASS is then colour coded as one horizontal line of the diagram. Top right the ERP is computed by stimulating the brain with 100 short-lasting auditory stimuli. Then, the ERP is averaged and its time–frequency representation is computed by means of a wavelet transform. Bottom left the diagonal profile of the ASS shows a close correspondence with the spectrum of the ERP (vertical profile computed within the red vertical lines). Bottom right across individuals, the frequency of the maxima of the peaks within the gamma frequency range correlates significantly, i.e. subjects with high-frequency ASS peaks show high-frequency ERP peaks (top right points in the correlation diagram), whereas subjects with low-frequency peaks in the ASS show low-frequency peaks in their ERP (bottom left points)
To identify the individual resonance frequencies, we applied an auditory steady-state response (ASSR) paradigm. ASSRs are the steady-state components of responses to periodic auditory signals, like amplitude-modulated (AM) tones. The frequency of the ASSR corresponds to the frequency of the input signal. However, the amplitude of the ASSR is not constant but varies with the modulation frequency and shows maxima at its resonance frequencies, typically around 30–80 and 5–20 Hz (Galambos et al. 1981). Thus, the auditory cortical system demonstrates resonance peaks in the system’s output response, indicating system-inherent resonance frequencies. In a former investigation, we characterized the resonance properties of the human visual system by applying a visual steady-state paradigm (Herrmann 2001). Results of this investigation indicated a correlation between transient gamma-band oscillations evoked during perceptual and cognitive tasks (e.g. Herrmann et al. 1999) and oscillatory steady-state activity in the context of repetitive stimulation in the visual domain, i.e. both show peaks in the gamma frequency range.
The present study intended to further characterize the resonance nature of the human brain and relates the transient gamma oscillation in response to acoustic stimulation to the auditory steady-state responses. Here, we test the hypothesis that the underlying mechanism for the brain’s preference to respond with specific frequency components in the gamma range is the resonance of the underlying neural circuitry. For that purpose, we investigated whether inter-individual variations in the transient frequency components of the eGBR are associated with variations in the ASSR resonance frequencies. In a group of 21 subjects, we systematically investigated the ASSR to amplitude-modulated (AM) 1 kHz sine wave tones with modulation frequencies ranging from 20 to 100 Hz in 1-Hz steps. Subsequently, we compared the individual ASSR resonance peak with the individual frequency of the eGBR evoked by a single 1 kHz sine wave tone with 250-ms duration at a middle-central electrode position (Cz).
Results and discussion
In result, all participants exhibited steady-state oscillations corresponding to the range of stimulation frequencies. The averaged ASSR steady-state potentials showed a clear resonance peak at 48 Hz. The averaged spectrum of an event-related potential to a single 1 kHz sine tone exhibited a prominent eGBR with a mean frequency of 46 Hz. Inter-individually, the frequency of the eGBR correlated with the ASSR resonance peak frequency in the gamma range (r = 0.6, P < 0.005) (cf. Fig. 2). This interrelation indicates the existence of stable and system-inherent bioelectric oscillators in the individual human brains. The resonance of the generating neural system seems to determine the frequency of the transient ERP components. This suggests that the brain behaves much like a linear dynamical system, since the frequency transform of the ERP (impulse response) closely resembles the diagonal profile of the steady-state response (frequency response). At first glance, this seems to be a contradiction to the fact that even the output of a single neuron is a non-linear function of its input. However, systems composed of non-linear components can display linear behaviour at larger scales (Freeman 1975).
The hypothesis of the resonance nature of the steady-state responses was first suggested in the nineteen forties (Walter and Walter 1949). In the visual domain, the use of a gradual continuous change in frequency of intermitted photic stimulation (IPS) across the entire EEG frequency range allows the exposure of an individual’s profile of reactivity. This profile displays the individual resonance frequencies and apparently reflects the characteristic EEG oscillators in different bands (Fedotchev et al. 1990; Basar-Eroglu et al. 1992). Basar (1980) suggested that the similar spectra of evoked steady-state brain responses and the spontaneous EEG indicate a close relation of their neural generators. In the same vein, an analogous suggestion has been made regarding the similar spectral characteristics of visually evoked steady-state brain responses and transient gamma-band responses (Herrmann 2001). In the visual domain, visual steady-state responses were located in human visual cortex (Di Russo et al. 2007; Pastor et al. 2003), where also event-related gamma activity was found in intracranial recordings in monkeys (Fries et al. 2001; Rols et al. 2001) and humans (Tallon-Baudry et al. 2005), as well as using surface EEG source localization approaches (Zaehle et al. 2009). Analogously, also for the auditory domain several commonalities between eGBR and ASSR have been identified, strongly suggesting a close relation between both measurements. The sources of both neurophysiological measurements are located in the primary auditory cortex. That has been shown for transient eGBR intracranially in animal (Brosch et al. 2002), and human (Crone et al. 2001) and using surface EEG in human (Schadow et al. 2009), as well as for the ASSR magnetic source (Gutschalk et al. 1999; Pantev et al. 1996; Weisz et al. 2004; Wienbruch et al. 2006; Draganova et al. 2008). Furthermore, both the amplitude of the eGBR and the ASSR can be augmented by attention (eGBR: Busch et al. 2006; Debener et al. 2003; ASSR: Bidet-Caulet et al. 2007; Ross et al. 2004). Additionally, both the ASSR response in the gamma range and the transient eGBR are reduced in patients with schizophrenia (Krishnan et al. 2009; Brenner et al. 2003; Kwon et al. 1999).
In sum, the present study showed that, in line with our hypothesis, the prominent spectral characteristics of the ERP and ASSR profiles correspond, suggesting that the ERP may indeed be determined by the system-inherent resonance frequencies of the brain. Thus, both the transient evoked gamma-band response to impulse stimulation and the ASSR to periodic stimulation seem to be based on the same, individually specific, neuronal mechanism.
Methods
Twenty-one subjects (ten women, mean age of 23.5 ± 5.4) participated in the study. None reported hearing problems or neurological or psychiatric disorders. All of them gave written and informed consent for their participation. The study was approved by the local ethics committee. Recording took place in a sound-attenuated and electrically shielded cabin. EARtone 3A insert-phones were employed being connected to a calibrated attenuator (Programmable Attenuator PA5, Tucker Davis Technologies, Alachua). Prior to the experiment, hearing thresholds for a 1 kHz sine wave tone were determined individually, and the stimuli were presented at 70 dB (HL) above the individual hearing level using Presentation software (http://www.neurobs.com). In the first part of the experiment (ASSR), participants heard 1 kHz sine tones that were amplitude modulated at frequencies between 20 and 100 Hz in steps of 1 Hz. The length of each stimulus depended on the modulation frequency ensuring an exact number of 60 epochs with a length of 500 ms, whereas the onset of each epoch was fixed to the beginning of a full new period. Eighty-one sounds were presented in pseudo-randomized order and were separated by 5-s inter-stimulus-interval (ISI). In the second part of the experiment (ERP), participants heard a single 1 kHz sine wave tone with a length of 250 ms (5 ms rising and falling time). The tone was presented 300 times, each separated by a variable ISI of 1,600–2,500 ms. Participants were asked to passively listen to the auditory stimulation, keep their eyes open and to fixate a cross at the centre of the screen.
EEG was recorded using 31 sintered Ag/AgCl-electrodes mounted in an elastic cap (Easycap GmbH, Herrsching-Breitbrunn, Germany) and placed according to the 10–10 system. Signals were amplified using a BrainAmp system (Brain Products, Munich, Germany) and were referenced to a nose-tip electrode, whereas the ground electrode was placed at AFz. Activity resulting from eye-movements was monitored using an additional electrode placed suborbitally to the right eye. The signals were digitized at 1,000 Hz and analog filtered between 0.016 and 250 Hz. Electrode impedances were kept below 10 kOhm. The analysis of electrophysiological data was conducted using EEProbe (ANT Software, Enschede, The Netherlands). A digital high-pass filter of 0.1 Hz was applied, and data were epoched in the following way: For the ASSR data, averaging epochs lasted from 1,000 ms before until 500 ms after beginning of each data epoch, whereas the first epoch of each stimulus was removed from analysis to avoid contamination with processing of stimulus onset. An automatic artefact rejection excluded the remaining epochs from analysis, if the standard deviation in a moving 200-ms time window exceeded 30 μV. The remaining epochs belonging to the same ASSR stimulus were averaged, and baseline activity was subtracted from each epoch using the time window between −1,000 and 0 ms with respect to the beginning of the averaged epochs. This resulted in one ERP for each stimulation frequency. Further processing of the ERPs was conducted using Matlab 7.6 (http://www.mathworks.com). Frequency spectra were calculated for each ERP at electrode Cz using an autoregressive power spectral density estimation based on the Yule-Walker algorithm with a maximum number of frequency components set to 100. A Hamming window was applied to each ERP before frequency transformation in order to minimize frequency distortions. The estimated spectral values were multiplied with their frequency to correct for the typical 1/f characteristic of the frequency spectrum. The resulting 81 frequency spectra were arranged in a two-dimensional plane (Fig. 2), where the abscissa represented the power of the EEG frequency components (response frequency) caused by stimulation at different stimulus frequencies (stimulation frequency; ordinate). Afterwards, the spectral values of each frequency component in response to the identical stimulation frequency were extracted (and low pass filtered) (e.g. 20 Hz in response to 20 Hz stimulation, 21 Hz in response to 21 Hz stimulation, and so on) allowing the analysis of resonance phenomena. Resonance peaks were revealed as maxima in the resulting spectrum. For the ERP data, averaging epochs lasted from −300 to 500 ms with respect to sound onset, and baseline activity was calculated in a time window between −200 and −100 ms. Analysis of evoked activity was conducted by transforming the averaged single trials (the ERP) into the frequency domain by means of a wavelet transform based on Morlet wavelets. The wavelet transformation was performed for each frequency bin between 1 and 100 Hz in steps of 1 Hz. Individual eGBR frequencies were revealed by detecting the peak frequency in the frequency range of 20–100 Hz and the time interval between 50 and 150 ms of the wavelet transform at electrode Cz. Analysis of ASSR and eGBR measurements was restricted to a middle-central electrode position (Cz), since the dipolar generators of both types of brain activity were demonstrated to lie in auditory cortex and to project most strongly to electrodes near the vertex, i.e. Cz.
Subsequently, for the time–frequency plots the estimated spectral values were multiplied with their frequency to correct for the 1/f characteristic of the frequency spectrum. The frequency values of ASSR resonance frequency and eGBR frequency were inter-individually correlated and statistically verified.
References
Allport GW (1927) Concepts of trait and personality. Psychol Bull 24:284–293
Basar E (1980) EEG-brain dynamics: relation between EEG and brain evoked potentials. Elsevier, Amsterdam
Basar E, Basar-Eroglu C, Karakas S, Schurmann M (2001) Gamma, alpha, delta, and theta oscillations govern cognitive processes. Int J Psychophysiol 39:241–248
Basar-Eroglu C, Basar E, Demiralp T, Schurmann M (1992) P300-response: possible psychophysiological correlates in delta and theta frequency channels. A review. Int J Psychophysiol 13:161–179
Basar-Eroglu C, Struber D, Schurmann M, Stadler M, Basar E (1996) Gamma-band responses in the brain: a short review of psychophysiological correlates and functional significance. Int J Psychophysiol 24:101–112
Bidet-Caulet A, Fischer C, Besle J, Aguera PE, Giard MH, Bertrand O (2007) Effects of selective attention on the electrophysiological representation of concurrent sounds in the human auditory cortex. J Neurosci 27:9252–9261
Borgers C, Epstein S, Kopell NJ (2005) Background gamma rhythmicity and attention in cortical local circuits: a computational study. Proc Natl Acad Sci USA 102:7002–7007
Brenner CA, Sporns O, Lysaker PH, O’Donnell BF (2003) EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry 160:2238–2240
Brosch M, Budinger E, Scheich H (2002) Stimulus-related gamma oscillations in primate auditory cortex. J Neurophysiol 87:2715–2725
Busch NA, Schadow J, Frund I, Herrmann CS (2006) Time-frequency analysis of target detection reveals an early interface between bottom-up and top-down processes in the gamma-band. Neuroimage 29:1106–1116
Crone NE, Boatman D, Gordon B, Hao L (2001) Induced electrocorticographic gamma activity during auditory perception. Clin Neurophysiol 112:565–582
Debener S, Herrmann CS, Kranczioch C, Gembris D, Engel AK (2003) Top-down attentional processing enhances auditory evoked gamma band activity. Neuroreport 14:683–686
Demiralp T, Basar-Eroglu C, Basar E (1996) Distributed gamma band responses in the brain studied in cortex, reticular formation, hippocampus and cerebellum. Int J Neurosci 84:1–13
Di Russo F, Pitzalis S, Aprile T, Spitoni G, Patria F, Stella A, Spinelli D, Hillyard SA (2007) Spatiotemporal analysis of the cortical sources of the steady-state visual evoked potential. Hum Brain Mapp 28:323–334
Draganova R, Ross B, Wollbrink A, Pantev C (2008) Cortical steady-state responses to central and peripheral auditory beats. Cereb Cortex 18:1193–1200
Fedotchev AI, Bondar AT, Konovalov VF (1990) Stability of resonance EEG reactions to flickering light in humans. Int J Psychophysiol 9:189–193
Freeman WJ (1975) Mass action in the nervous system. Academic Press, New York
Fries P, Reynolds JH, Rorie AE, Desimone R (2001) Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291:1560–1563
Frund I, Schadow J, Busch NA, Korner U, Herrmann CS (2007) Evoked gamma oscillations in human scalp EEG are test-retest reliable. Clin Neurophysiol 118:221–227
Galambos R, Makeig S, Talmachoff PJ (1981) A 40-Hz auditory potential recorded from the human scalp. Proc Natl Acad Sci USA 78:2643–2647
Gutschalk A, Mase R, Roth R, Ille N, Rupp A, Hahnel S, Picton TW, Scherg M (1999) Deconvolution of 40 Hz steady-state fields reveals two overlapping source activities of the human auditory cortex. Clin Neurophysiol 110:856–868
Herrmann CS (2001) Human EEG responses to 1–100 Hz flicker: resonance phenomena in visual cortex and their potential correlation to cognitive phenomena. Exp Brain Res 137:346–353
Herrmann CS, Mecklinger A, Pfeifer E (1999) Gamma responses and ERPs in a visual classification task. Clin Neurophysiol 110:636–642
Herrmann CS, Frund I, Lenz D (2010) Human gamma-band activity: a review on cognitive and behavioral correlates and network models. Neurosci Biobehav Rev 34:981–992. doi:10.1016/j.neubiorev.2009.09.001
Hutcheon B, Yarom Y (2000) Resonance, oscillation and the intrinsic frequency preferences of neurons. Trends Neurosci 23:216–222
Izhikevich EM, Desai NS, Walcott EC, Hoppensteadt FC (2003) Bursts as a unit of neural information: selective communication via resonance. Trends Neurosci 26:161–167
Kaiser J, Lutzenberger W (2003) Induced gamma-band activity and human brain function. Neuroscientist 9:475–484
Kaiser J, Heidegger T, Lutzenberger W (2008) Behavioral relevance of gamma-band activity for short-term memory-based auditory decision-making. Eur J Neurosci 27:3322–3328
Kaiser J, Lutzenberger W, Decker C, Wibral M, Rahm B (2009) Task- and performance-related modulation of domain-specific auditory short-term memory representations in the gamma-band. Neuroimage 46:1127–1136
Krishnan GP, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, O’Donnell BF (2009) Steady state and induced auditory gamma deficits in schizophrenia. Neuroimage 47:1711–1719
Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y et al (1999) Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry 56:1001–1005
Llinas RR (1988) The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science 242:1654–1664
Oppenheim AV, Schafer RW, Buck JR (1999) Discrete-time signal processing. Prentice Hall, Upper Saddle River
Pantev C, Roberts LE, Elbert T, Ross B, Wienbruch C (1996) Tonotopic organization of the sources of human auditory steady-state responses. Hear Res 101:62–74
Pastor MA, Artieda J, Arbizu J, Valencia M, Masdeu JC (2003) Human cerebral activation during steady-state visual-evoked responses. J Neurosci 23:11621–11627
Rols G, Tallon-Baudry C, Girard P, Bertrand O, Bullier J (2001) Cortical mapping of gamma oscillations in areas V1 and V4 of the macaque monkey. Vis Neurosci 18:527–540
Ross B, Picton TW, Herdman AT, Pantev C (2004) The effect of attention on the auditory steady-state response. Neurol Clin Neurophysiol 2004:22
Schadow J, Lenz D, Dettler N, Frund I, Herrmann CS (2009) Early gamma-band responses reflect anticipatory top-down modulation in the auditory cortex. Neuroimage 15:651–658
Strogatz S (2003) Sync. The emerging science of spontaneous order. Hyperion, New York
Tallon-Baudry C, Bertrand O, Henaff MA, Isnard J, Fischer C (2005) Attention modulates gamma-band oscillations differently in the human lateral occipital cortex and fusiform gyrus. Cereb Cortex 15:654–662
Tiesinga PH, Fellous JM, Jose JV, Sejnowski TJ (2001) Computational model of carbachol-induced delta, theta, and gamma oscillations in the hippocampus. Hippocampus 11:251–274
Traub RD, Jefferys JGR, Whittington MA (1997) Simulation of gamma rhythms in networks of interneurons and pyramidal cells. J Comput Neurosci 4:141–150
Vida I, Bartos M, Jonas P (2006) Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron 49:107–117
Walter VJ, Walter WG (1949) The central effects of rhythmic sensory stimulation. Electroencephalogr Clin Neurophysiol 1:57–86
Weisz N, Keil A, Wienbruch C, Hoffmeister S, Elbert T (2004) One set of sounds, two tonotopic maps: exploring auditory cortex with amplitude-modulated tones. Clin Neurophysiol 115:1249–1258
Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH (2000) Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol 38:315–336
Wienbruch C, Paul I, Weisz N, Elbert T, Roberts LE (2006) Frequency organization of the 40-Hz auditory steady-state response in normal hearing and in tinnitus. Neuroimage 33:180–194
Zaehle T, Frund I, Schadow J, Tharig S, Schoenfeld MA, Herrmann CS (2009) Inter- and intra-individual covariations of hemodynamic and oscillatory gamma responses in the human cortex. Front Hum Neurosci 3:8
Acknowledgments
This study was supported by the Deutsche Forschungsgemeinschaft (SFB/TRR31-TPA9) and the State of Saxony-Anhalt (excellence cluster C3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaehle, T., Lenz, D., Ohl, F.W. et al. Resonance phenomena in the human auditory cortex: individual resonance frequencies of the cerebral cortex determine electrophysiological responses. Exp Brain Res 203, 629–635 (2010). https://doi.org/10.1007/s00221-010-2265-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-010-2265-8