Abstract
This study examined grip force development in individuals with hemiparesis following unilateral stroke. Eleven patients with chronic stroke with severe hand impairment and five age-matched neurologically intact subjects grasped an instrumented object between the index finger and thumb while fingertip forces, digit posture, and muscle electromyographic activity were recorded. We tested a range of different grip conditions with varying grip sizes, object stability, and grip force level. We found that fingertip force direction in the paretic digits deviated from the direction normal to the grip surface by more than twice as much as for asymptomatic digits. Additionally, the paretic thumb had, on average, 18% greater deviation of grip force direction than the paretic index finger. This large deviation of finger force direction for the paretic digits was consistently observed regardless of grip size, grip force level, and object stability. Due to the large deviation of the force direction from the normal direction, the paretic digits slipped and moved more than 1 cm during 55% of all grasping trials. A regression analysis suggests that this altered grip force direction was associated with altered hand muscle activation patterns, but not with the posture at which the digit made contact with the object. Therapies to redirect the force direction at the digits may improve stroke survivors’ ability to stably grip an object.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Stroke is a leading cause of long-term disability in the United States and the third most frequent cause of death, following diseases of the heart and cancer (Thom et al. 2006). Of the more than 700,000 Americans who experience a stroke each year (Thom et al. 2006), two-thirds survive (Broderick et al. 1998), leading to a current population of more than five million stroke survivors in the United States. (American Heart Association 2005). Many of these individuals have long-term motor and sensory impairments (Woodson 2002), especially in the arm and hand (Parker et al. 1986; Gray et al. 1990; Nakayama et al. 1994b). Studies report that 69% of patients who were admitted to a rehabilitation unit following stroke have mild to severe upper extremity dysfunction (Nakayama et al. 1994a), and only 14–16% of stroke survivors with initial upper extremity hemiparesis regained near-complete motor function (Parker et al. 1986; Nakayama et al. 1994a). Chronic deficits following stroke are especially prevalent in the hand and therefore diminish the capacity to grasp (Trombly 1989; Kamper et al. 2003). As hand grasp has great functional importance for performing activities of daily living, the impairment in grasping significantly lowers stroke survivors’ functional independence and ability to perform activities of daily living.
The difficulty in achieving a stable grasp may be related to a variety of impairments, including reduced grip strength (Boissy et al. 1999), exaggerated grip force fluctuation (Blennerhassett et al. 2006), and deficits in predictive grip force control following stroke (Nowak et al. 2003; Nowak and Hermsdorfer 2005). More importantly, it may result from altered finger force direction during grasp. To grasp an object, properly scaled and directed forces should be generated by the digits (Johansson and Westling 1984; Gordon et al. 1991). In stroke survivors, attempted generation of grasping force with the paretic hand often leads to the object slipping out of the hand. This suggests grip force imbalance and altered finger force direction are interfering with proper grasp of the object. Altered finger force direction can result in reduced normal force and increased shear force, both of which can lead to a higher probability of finger-object slip or grip loss.
Two key factors that affect the development of force direction with respect to the object are digit posture with respect to the object and net force vector direction of the digit tip. Inability to produce proper hand posture with respect to the object (Woodson 2002; Kamper et al. 2003; Lang and Schieber 2004) could lead to object slippage even if the forces produced by the digits were the same as in unimpaired hands. Additionally, abnormal muscle activation patterns of hand muscles (Kamper et al. 2003; Cruz et al. 2005) could result in inappropriately directed force vectors at the digit tips.
Clinical studies have shown that some grasping tasks are consistently easier for stroke survivors to perform than other grasping tasks, depending on grip size and grip type (Woodbury et al. 2007). It may be that the extent of altered finger force direction varies with object size or object stability. Object size variation may change the required hand posture or the required muscle activation patterns (Kamper et al. 2006). Stabilizing the object places additional constraints on the task, possibly making successful grasping much more difficult for stroke survivors.
The main aim of this study was to examine whether digit force vectors from paretic hands are deviated in direction from those observed in asymptomatic digits during pinch grip. This information has important functional implications, since altered finger force direction (e.g., greater ratio of shear force to normal force) may explain the unstable grip observed for stroke survivors. In addition, this study examined whether finger posture relative to the object and muscle activity was associated with the potential change in force direction. These outcomes were measured across objects of different size and stability.
Methods
Subjects
Eleven subjects with chronic hemiparetic stroke and five age-matched neurologically healthy subjects participated in the experiment. The inclusion criteria for stroke subjects were (1) the occurrence of a single stroke at least 9 months prior to the study; (2) severe hand impairment as indicated by a rating of Stage 2 to 3 for the Stage of Hand section of the Chedoke-McMaster Stroke Assessment (Gowland et al. 1995); (3) the ability to produce 5 N pinch force with the paretic hand; (4) no cognitive dysfunction that precluded comprehension of experimental tasks; and (5) no history or clinical signs of concurrent medical problems.
Time since stroke ranged from 15 to 157 months (see Table 1). Eight stroke subjects had lesions in the right hemisphere, whereas three stroke subjects had lesions in the left hemisphere. Seven stroke subjects exhibited sensory deficit for either the paretic thumb or index finger tip pads, determined by the two-point discrimination test (with the threshold distance equal to or greater than 6 mm; Pendleton 2006; Jobe 2007; Magee 2008). Four stroke subjects did not exhibit sensory deficit for the paretic digits (with the threshold distance less than 6 mm; Pendleton 2006; Jobe 2007; Magee 2008). No subject exhibited sensory deficit for the non-paretic digits.
The mean age was 51 (standard deviation, SD, = 8) for stroke subjects and 51 (SD = 3) for control subjects. All subjects signed the Human Consent Form approved by the Institutional Review Board of Northwestern University prior to testing. Both the paretic and non-paretic hands of stroke subjects were tested. For neurologically intact subjects, only the dominant hand was tested.
Procedure
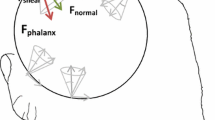
Subjects were seated in a chair with the elbow flexed at 90° and the forearm resting comfortably in the horizontal plane. The wrist was maintained in the neutral posture for all subjects through the use of a splint (Loop Lock Wrist Splint 1299; Tetra Medical Supply Corp., IL). A custom-made fixture was applied to the forearm to fix the forearm and upper arm postures throughout the experiment. An instrumented object was presented to the subjects between the thumb and index finger (see Fig. 1). Using the thumb and index finger, subjects grasped the object for approximately 5 s while three-dimensional (3D) forces at the index finger and thumb, muscle activity, and digit postures were recorded. The 3D forces were measured separately for the thumb and the index finger using miniature load cells (Nano17, Mini40; ATI Industrial Automation, Inc.; Apex, NC; sampling frequency 500 Hz). The grip surfaces for the thumb and index finger were each 3.5 cm wide and 3 cm high. They were covered with a sheet of rubber to increase the coefficient of friction to 0.9 (Seo and Armstrong 2009) and to minimize finger-object slip during grip exertions.
Grip exertions were performed for five grip conditions, simulated using different grip sizes, object stability, and force level. Specifically, three grip sizes—small (0.8 cm), medium (3.8 cm), and large (7.6 cm) distances between the two grip surfaces—were tested (Fig. 1a). In addition to the fixed condition in which the instrumented object position was constrained, an unstable condition in which the object was free to move (Fig. 1b) was tested for the medium-sized object. For all of these trials, subjects were instructed to grip maximally against the grip surfaces.
As maximum grip force levels may be significantly different between the paretic and asymptomatic hands, a 5 N grip exertion condition was added to see if grip force level would affect the dependent variables. The 5 N grip was tested for the medium-sized fixed object. Visual feedback of actual normal force averaged for the two fingers was provided on a computer screen as a bar graph with a mark at the desired level of 5 N. No visual feedback was provided for maximum grip trials.
In sum, a total of five conditions were tested for each limb type (paretic, non-paretic, control) and digit (index finger, thumb): maximum grip for the fixed object of three grip sizes, maximum grip for the unstable object of the medium size, and 5 N grip for the fixed object of the medium size. These five conditions were presented to subjects in a random order. Subjects repeated grip in each condition three times. For stroke subjects, one hand was tested for all conditions, and then the other hand was tested within the same single session. The order of testing for the paretic and non-paretic hands was randomized such that half performed the experiment first with the paretic hand and half with the non-paretic hand.
Along with the 3D forces for the index finger and thumb, digit posture was recorded using a camera-based system (OptoTrak 3020, Northern Digital, Inc., Waterloo, Ontario, Canada; sampling frequency 30 Hz). A total of 9 infrared markers were used, with three markers placed on the thumb (thumb tip, interphalangeal (IP) joint, metacarpophalangeal (MCP) joint), three markers positioned on the index finger (fingertip, distal and proximal IP joints), and three markers attached to the object. Additionally, activity of selected muscles was recorded using bipolar surface electromyography (EMG) electrodes (Delsys, Inc., Boston, MA). Electrodes targeted the flexor digitorum superficialis (FDS), extensor digitorum communis (EDC), first dorsal intersseous (FDI), and thumb thenar eminence (adductor pollicis, abductor pollicis brevis, flexor pollicis brevis, opponens pollicis muscles). The EMG signal was initially low-pass filtered at 225 Hz prior to sampling at 500 Hz.
Analysis
The solid angle (deviation of finger force direction from the normal direction as illustrated in Fig. 2), digit orientation angle, digit contact angle, and muscle activity were computed using force, position, and EMG data recorded during the experiment. The solid angle was computed as the arccosine of the ratio of normal force to resultant force, where resultant force is the Euclidean norm of a force vector in 3D (denoted as “resultant force of the digit” in Fig. 2). Digit orientation angle and contact angle were measured using the digit and object position data. The orientation angle was defined as the angle between the grip surface and the digit plane that is composed of three points (the tip, IP, and MCP joints for the thumb; the tip, DIP, and PIP joints for the index finger; see Fig. 2). For example, the orientation angle for the index finger is normally close to 90° for tip pinch and 0° during lateral pinch. The contact angle was defined as the angle between the grip surface and the long axis of the distal phalanx within the digit plane (see Fig. 2). Thus, the contact angle was independent from the orientation angle. The contact angle is 0° when the distal phalanx is parallel to the grip surface. When the distal phalanx is orthogonal to the grip surface, the contact angle is 90°. The following equations were used to compute the orientation angle and the contact angle:
where v 1 is the vector from the fingertip to the DIP/IP joint, v 2 is the vector from the DIP/IP to PIP/MCP joint for the index finger and thumb, respectively, and n is the vector normal to the grip surface pointing from the thumb toward the index fingertip for the right hand, and in the opposite direction for the left hand, and s is the vector defined by the intersection of the grip surface and the digit plane whose direction is toward the hand.
After sampling, the EMG signal was rectified and low-pass filtered at 10 Hz using MATLAB (The MathWorks, Inc., Natick, MA) to create envelopes that clearly displayed the aggregate muscle activity. The EMGs were expressed as a percentage of maximum voluntary contraction (MVC). The MVC values were recorded at the beginning of the experiment while subjects performed maximum index finger abduction and thumb flexion as well as flexion and extension of all five fingers against resistance. The peak value of the processed EMG for each muscle and each subject during the MVC trials was used to normalize EMG data throughout the experiment. Then, the four EMG values were further normalized to the Euclidean magnitude (defined as the square root of the sum of squares of the elements of the vector) of the vector composed of the four EMG values for each trial, to adjust for different grip force exertion levels and facilitate comparison among the four muscle activities.
After the experiment, all data (solid angle, contact angle, orientation angle, EMGs) were averaged over a period of one-second in which normal force averaged for the thumb and the index finger was the greatest for maximal grip trials or closest to 5 N for 5 N grip trials. This one-second period is referred to as the “hold” period in Fig. 3, which shows solid angle and normal force profiles in a sample trial for a control hand. In some trials during the experiment, the finger slid over the grip surface during grip. Finger “slip” was defined as having occurred when movement of the digit tip with respect to the grip surface exceeded 1 cm. For trials with finger slip, data were analyzed until the time that the finger slid off the grip surface.
In addition to the solid angle, the azimuth angle was computed as the angle between the projection of the resultant force vector onto the grip surface and the projection of the distal phalanx vector onto the grip surface. The azimuth angle of 0° indicates that the projections of the resultant force direction and the distal segment onto the grip surface are aligned with each other. The azimuth angle of 90° indicates that the projection of the resultant force direction onto the grip surface points toward the ulnar direction.
Statistical analysis
As a primary statistical analysis, repeated measures analysis of variance (ANOVA) was performed for solid angle with the between-subject factor of limb type (paretic, non-paretic, control), within-subject factor of digit (thumb vs. index finger), and blocking factor of five grip conditions. Second-order interactions with limb type were included. Once significance was confirmed for the limb type and its interaction with digit, three separate repeated ANOVAs were performed for solid angle to evaluate each effect of grip size, object stability, and force level. First ANOVA was performed for solid angle with two within-subject factors of grip size (small, medium, large) and digit and the between-subject factor of limb type, using data for only the maximum grip and fixed object. Second ANOVA was performed for solid angle with the within-subject factors of object stability (fixed vs. unstable) and digit and between-subject factor limb type, using data for only the maximum grip and medium grip size.
Third ANOVA was performed for solid angle with the within-subject factors of force level (maximum vs. 5 N grip) and digit and between-subject factor limb type, using data for only the medium grip size and fixed object. Second-order interactions with limb type were included. The P-value of 0.017 was considered significant for these three ANOVAs after Bonferroni adjustment. Post hoc Tukey tests were performed to evaluate differences among the three limb types and three grip sizes. The same procedure was repeated for orientation angle, contact angle, and normal force. The same procedure using ANOVA without limb type as a factor was performed for slip occurrence.
For EMG data, repeated measures ANOVA was performed for the between-subject factor of limb type and two within-subject factors of muscles (FDS, EDC, FDI, thenar eminence) and five grip conditions. Second-order interactions were included. Post hoc Tukey tests were performed to evaluate differences among the three limb types and four muscles.
In addition, linear regressions were performed for each digit to determine which of the following variables the solid angle was associated with: contact angle, orientation angle, four EMGs, and normal force. Lastly, the azimuth angles were averaged for each limb type and digit, and the standard deviation was computed using circular statistics (Fisher 1993).
Results
In brief, the solid angle was more than twice greater for the paretic hand than for asymptomatic hands (non-paretic and control). With high solid angles, the paretic fingers slipped, on average, 55% of all trials. Slip occurred only for the paretic hands. The solid angle was significantly associated with muscle activity, but not with the orientation angle and the contact angle. Detailed statistical results are described below.
Solid angle
The mean solid angle during the hold period (denoted in Fig. 3) was 28° (SD = 14) for the paretic hand, 12° (SD = 5) for the non-paretic, and 11° (SD = 6) for the control hand (see Fig. 4a). Solid angle was significantly affected by the limb type (P < .01). Tukey’s post hoc test for limb shows that the solid angle for the paretic hand was significantly greater than those for the non-paretic and control hands (P < .01), whereas the solid angles for the non-paretic and control hands were not significantly different from each other (P > .05).
a Mean ± SE solid angle for three limb types (digits and the 5 grip conditions pooled; n = 11 for paretic and non-paretic, n = 5 for control). Paretic fingers exhibited significantly greater solid angles compared to the asymptomatic fingers during grip. The horizontal line represents the critical angle below which finger slip would not occur for conventional objects with the static coefficient of friction greater than 0.3 (critical angle = 17° = arctan0.3). Mean ± SE solid angles are shown for the two fingers and limb types (b, all grip conditions pooled), and for force levels (c). The paretic thumb had greater solid angles than the paretic index finger (b). Solid angles decreased with increasing grip force for all limb types (c)
The interaction between the limb type and digit was also significant (P < .01). Figure 4b shows mean solid angles for the thumb and index finger separately for the three limb types. The solid angle for the paretic thumb was 18% greater than that for the paretic index finger. Tukey’s post hoc test for limb × digit shows that the solid angle was greater for the thumb than for the index finger for the paretic hand (P < .01), whereas solid angles for the two digits were not significantly different from each other for the non-paretic and control hands (P > .05). The solid angle for the paretic index finger was still greater than that for the non-paretic and control index fingers (P < .01).
Individual ANOVAs performed for the other conditions showed that force level significantly impacted the solid angle (P < .017). The mean solid angle decreased 25% for maximum grip exertions when compared to those for the 5 N grip (Fig. 4c) when averaged for all limb types. The effects of object stability, grip size, and second-order interactions with limb type were not found significant.
Significant variables associated with solid angle
Linear regressions were performed for each digit as follows. For the index finger, linear regression was performed with the solid angle as the response to 6 predictors: orientation angle, contact angle, normal force, FDS, EDC, and FDI EMGs (see Table 2). For the thumb, the solid angle was the response to 5 predictors: orientation angle, contact angle, normal force, and FDI and thenar eminence EMGs (see Table 2). Variation inflation factors ranged from 1.1 to 1.3 for all predictors, indicating little multicollinearity. The solid angle for the index finger was found to be significantly associated with normal force and EDC and FDI EMGs (P < .05). The solid angle for the thumb was found to be significantly associated with normal force and FDI EMG (P < .05). The solid angle was not found to be significantly associated with the orientation angle and the contact angle for both digits. Normal force and EMGs were further examined as follows.
Normal force
Mean normal forces are shown in Fig. 5. Normal force varied significantly with limb type and digit (P < .01). Mean maximal normal force for the paretic hand was only a quarter of that for the asymptomatic hands (12 N vs. 46 N, see Fig. 5a). Tukey’s post hoc test for limb shows that normal force for the paretic hand was significantly less than that for the non-paretic and control hands (P < .01), and normal forces for the non-paretic and control hands were not significantly different from each other (P > .05). Normal force was 8% greater for the thumb than for the index finger, when averaged for all limb types and grip conditions (Fig. 5b). The interaction between limb type and digit was not significant (P > .05).
In addition, normal force was found to significantly vary with grip size, object stability, force level, and the interaction between limb type and force level (P < .017). Interactions between limb type and grip size and between limb type and object stability were not found significant. Mean normal force was 40 N for the large and medium grip sizes, and decreased 19% as the grip size decreased to small. Mean normal force decreased 18% (from 38 to 32 N) for the unstable object compared to the fixed object (averaged for all limb types). Normal force recorded for the 5 N grip was not different among limb types, whereas normal force during maximum grip was different among the three limb types (Fig. 5a).
Muscle activity
Figure 6 shows EMG values for the four muscles (FDS, EDC, FDI, and thenar muscles) and for the three limb types. The EMG values were normalized to the Euclidean magnitude of the vector composed of the four EMG values to adjust for different grip force levels. The ANOVA shows that EMGs were significantly affected by muscle and limb × muscle (P < .01). Tukey’s post hoc test for limb × muscle shows that EMGs were not different among the four muscles for the paretic hand (P > .05), whereas for the non-paretic and control hands, EMG values were different by as much as 163%. Specifically, the EDC, FDI, and thenar eminence EMGs were twice as large as FDS EMG for the non-paretic and control hands (P < .01). The FDS EMG was significantly greater for the paretic hand than for the control and non-paretic hands (P < .01).
Mean ± SE EMG levels for the four channels (FDS flexor digitorum superficialis, FDI first dorsal interosseous, EDC extensor digitorum communis, thenar thenar eminence) and for the three limb types (all 5 grip conditions pooled). The EMG values were expressed as the percentage of the maximum voluntary contraction and then normalized to the Euclidean magnitude of the vector composed of the four muscles’ EMG values. Paretic hands exhibited a lack of discrimination among the four muscles, whereas for non-paretic and control hands, the EDC and FDI activities were twice as large as the FDS activity
Orientation and contact angles
Mean orientation angles were 66° (SD = 25°), 81° (SD = 23°), and 69° (SD = 20°) for the paretic, non-paretic, and control hands, respectively. Limb type, digit, and their interaction were found to be significant for orientation angle (P < .01). Tukey’s post hoc test for the limb type and digit shows that the orientation angle was, on average, 30° greater for the thumb compared to the index finger for the non-paretic and control hands (P < .05), whereas for the paretic hand, the orientation angle did not change with digit (P > .05). Orientation angle did not significantly vary with grip size, object stability, or force level (P > .05).
Mean contact angles were 16° (SD = 26°), 2° (SD = 20°), and 11° (SD = 16°) for the paretic, non-paretic, and control hands, respectively. Contact angle varied significantly with limb type, digit, and object size (P < .01), but not with object stability, force level, or second-order interactions with limb type (limb × digit, limb × size, limb × stability, limb × force). The contact angle for the thumb was, on average, 22° larger than that for the index finger. Contact angle increased, on average, 32° as the grip size decreased from large to small. The contact angle for the non-paretic hand was, on average, 11° less than that for the paretic and control hands (P < .05 from Tukey’s post hoc test for limb type).
Finger force direction in the shear force domain
Mean finger force vectors for each limb and finger are projected onto the grip surface in Fig. 7. The length of the arms represents shear force normalized to normal force (hereafter, normalized shear force). As the solid angle was the greatest for the paretic hand, the magnitude of normalized shear force was also the greatest for the paretic hand, followed by the non-paretic hand and the age-matched control. The mean azimuth angle was −133° for the paretic thumb (standard deviation (SD) = 55°), −134° for the non-paretic thumb (SD = 57°), and −71° for the control thumb (SD = 47°), where −180° indicates the distal direction and −90° indicates the radial direction. The mean azimuth angle for the index finger was 124° for the paretic (SD = 70°), 73° for the non-paretic (SD = 54°), and 133° for the control hand (SD = 56°), where 180° indicates the distal direction and 90° indicates the ulnar direction.
Direction and magnitude of shear force normalized to normal force plotted on the grip surface in the ulnar-radial direction (x-axis) and in the distal-proximal direction (y-axis) at the thumb tip (a) and at the index fingertip (b) as if all fingers are for the right hand. The error bars in the radial direction show + 1 standard error (SE) of normalized shear force magnitude. The error bars in the tangential direction represent ± 1 SE of the azimuth angle. When two lines are drawn from the circle center to the two ends of an error bar (as shown with the segmented lines for the paretic thumb), the angle between the two lines shows ±1 SE of the azimuth angle. The circle represents a safe area in which a finger would not slip while grasping a conventional object with the coefficient of friction greater than 0.3 (Buchholz et al. 1988)
Discussion
Data in the present study demonstrate that the paretic fingers’ grip force at the digits is significantly deviated in direction as well as magnitude in comparison with asymptomatic (non-paretic and control) hands. The deviation was quantified using a solid angle (angle between the grip force and the direction normal to the grip surface). When the solid angle exceeds the arctangent of the static coefficient of friction between the finger and the grip surface, finger slip occurs. Thus, it is critical to keep the solid angle below a threshold level to prevent finger slippage.
Decrease in solid angle, seen with increasing grip force level for all limb types (Fig. 4c), may be indicative of compensatory motor action to reduce the possibility of finger slippage more when gripping at a higher force level than at a lower force level. A similar strategy was seen in safety margins where the magnitude of safety margin was greater for higher force levels than for low force levels during gripping with fingers in unimpaired hands (Johansson and Westling 1984; Seo 2009). Alternatively, the skin coefficient of friction could be higher for 5 N grip exertions than for maximal grip exertions (Bobjer et al. 1993; Sivamani et al. 2003; Seo et al. 2009), thereby affording greater solid angles for 5 N grip exertions.
The solid angle for the paretic fingers was, on average, 2.5 times greater than that for the asymptomatic fingers (28° vs. 11°, see Fig. 4a). This large solid angle for the paretic fingers was consistently observed for all grip conditions. This large solid angle for the paretic fingers can result in finger slip and thus failure to stably grip an object. Indeed, frequent (55% of all trials) finger slip was observed for the paretic grip (when finger slip was determined by fingertip movement greater than 1 cm during grip). The coefficient of friction between the rubber surface of the object and skin is 0.9 (Seo and Armstrong 2009), so the contact surface could accommodate a solid angle up to 42° without finger-object slip. The coefficient of friction of skin on the paretic hand has been shown to not be significantly different from that for asymptomatic fingers (Hermsdorfer et al. 2003). Thus, finger slip may be attributed to altered grip force directions.
Conventional objects that we encounter in daily living may have coefficients of friction as low as 0.3 with skin (Buchholz et al. 1988). To grip these conventional objects without slip, the solid angle needs to be less than the critical angle of 17° (=arctan0.3, see Fig. 4a). This is equivalent to the normalized shear force being less than 30%. This “safe” area in which a finger would not slip while grasping a conventional object with the coefficient of friction greater than 0.3 (Buchholz et al. 1988) is illustrated as a circle in Fig. 7. It can be seen that paretic fingers may fail to grip conventional objects stably due to a large solid angle. For the non-paretic and control fingers, the solid angles are less than the critical angle (in Fig. 4a), and normalized shear forces are within the safe area (in Fig. 7).
The solid angle was found to be significantly associated with muscle activity, but not with the orientation angle and the contact angle in the present study. Although the solid angle was found to be not significantly associated with the orientation and contact angles, it should be noted that in the present study, the subjects’ wrist was maintained in a neutral posture by the splint, and their forearm posture was kept in a comfortable pronation/supination posture by a custom-made fixture. Grip exertions often result in abnormal forearm pronation and wrist flexion for the paretic limb (Brunnstrom 1970). Thus, it is possible that use of the splint and fixture in this study could have stabilized their limb posture, thereby influencing the posture at which the digit made contact with the object and the resultant force directions at the tips of the digits. Grasping would undoubtedly have been further degraded without this support.
The solid angle was found to be significantly associated with muscle activity. Muscle activation patterns are critical for solid angles, because each muscle of the thumb and the index finger can contribute to force generation in a particular direction (Valero-Cuevas 2000; Johanson et al. 2001; Milner and Dhaliwal 2002; Pearlman et al. 2004). While muscle atrophy (Scelsi et al. 1984) and contracture (O’Dwyer et al. 1996) could play a role in causing impairment in finger force coordination, neural factors may best account for finger impairment in persons with hemiparetic stroke (Kamper and Rymer 2000; Kamper et al. 2003). In addition, deficit in individuation of the fingers (Lang and Schieber 2003) and abnormal neural coupling between the thumb and fingers could affect the digit force coordination. In the present study, the four EMG channels were found not to differ among each other for the paretic hand, whereas they were different by as much as 163% among each other for asymptomatic hands. This suggests abnormal co-contraction of hand muscles for the paretic hand, as previously reported (Lang and Schieber 2004; Cruz et al. 2005). Thus, the large solid angle for the paretic fingers may be attributable to the lack of discrimination in muscle activation patterns for the paretic hand.
For the asymptomatic index finger, the EDC and FDI activities were, on average, 2.1 times greater than the FDS activity (Fig. 6; P < .05). In contrast, the paretic hand’s EDC and FDI activities were not different from the FDS activity, suggesting that the EDC and FDI muscles are relatively less activated or that the FDS muscle is over-activated for the paretic hand compared to the asymptomatic hands. This observation is consistent with the previous studies that reported hyperexcitability of FDS, hypoexcitability of EDC, and impaired abduction (Kamper and Rymer 2001; Cruz et al. 2005).
The results from a cadaver study describing the relationship between index finger muscle tensions and force vectors at the digit suggest that disproportionately large FDS activity (Valero-Cuevas et al. 2000) or reduced activation of the EDC and FDI (Valero-Cuevas et al. 2000; Lee et al. 2008) would result in large shear force in the index finger pointing toward the ulnar and distal direction. Indeed, normalized shear force plotted on the grip surface (in Fig. 7b) shows that the paretic index finger force was outside the safe area because it was pointing toward the ulnar and distal direction more so than for the asymptomatic index fingers. Thus, hyperactive FDS muscle or weakened EDC and FDI muscles may contribute to the large solid angle for the paretic index finger. In this case, it is computed, based on the index finger biomechanical model (Valero-Cuevas et al. 2000), that increasing the activity of EDC 140% and FDI 88% may be able to bring the resultant index finger force vector within the safe area.
The paretic thumb’s solid angle was 18% greater than that for the paretic index finger (Fig. 4b). The greater solid angle for the paretic thumb compared to the paretic index finger may be related to greater existence of corticospinal projections to the thumb muscles than to the index finger muscles (Penfield and Boldrey 1937; Penfield and Rasmussen 1950; Woolsey et al. 1952, 1979). Stroke-induced disruption to the corticospinal fibers may preferentially lead to loss of projections to the thumb muscles when compared to the index finger muscles. This could lead to greater impairment in fine motor control for the thumb and may explain greater solid angle for the paretic thumb than for the paretic index finger (Fig. 4b). Based on the cadaver study describing the relationship between thumb muscle tensions and force vectors at the thumb tip (Pearlman et al. 2004), weakness of EPL could contribute to the paretic thumb’s normalized shear force outside the safe area toward the radial and distal directions shown in Fig. 7a. Relative weakness among thumb muscles following stroke, however, has not been empirically demonstrated. In the present study, only the thenar eminence EMG was recorded for the thumb, thus we lack information for the paretic thumb muscle activation pattern. Further investigation using direct measurement of the activity for each thumb muscle may elucidate whether there is abnormal muscle activation pattern for the thumb and whether disproportional weakness among thumb muscles could be related to the large solid angle observed in this study.
Reduced sensation could also contribute to the large solid angle seen in the paretic hand. Tactile feedback is utilized in digit force coordination (Johansson and Westling 1984; Westling and Johansson 1984; Gordon et al. 1991; Monzee et al. 2003). Reduced ability to detect micro-slippage of the skin and changes in shear strain of the skin could have hindered adjustment of force direction, leading to large solid angels for the paretic hand. Reduced proprioception could also hinder adjustment of force direction in response to finger slippage. Indeed, reduced sensation was observed for seven stroke subjects in this study. Conversely, four stroke subjects did not exhibit reduced tactile sensation on their paretic digits and yet produced large solid angles with their paretic digits, implying that not only sensory deficit but also motor deficit plays a role in the altered digit force direction following stroke.
Techniques to promote activation of these under-innervated muscles, such as neuromuscular electrical stimulation, may represent new rehabilitation strategies to redirect the digit force vectors close to the normal direction or within the safe area. Repeated use of neuromuscular electrical stimulation has been shown to increase strength of specific muscles (Chae et al. 1998). Thus, repeated neuromuscular electrical stimulation of under-activated hand muscles could be incorporated into a therapy to correct the digit force direction, possibly under the guidance of biofeedback. Alternatively, reduced activation of over-activated muscles such as FDS for the index finger could lead to decreased solid angle. This, however, may result in decreased force generation capacity. Facilitation of tactile or proprioceptive perception by additional sensory input (Aruin 2005) or tendon vibration (Cordo et al. 1993) may also help better direct digit forces. Visual feedback of force direction to stroke survivors may also help to improve performance, and such a study is currently underway in our laboratory. With improved grip force direction (i.e., reduced solid angle), occurrence of slip can be reduced for the paretic fingers, which then can contribute to improved grip stability and even increased grip strength. As a result, stroke survivors’ hand function may improve.
Conclusion
This study demonstrated that persons with chronic stroke with severe hand impairments generated pinch force significantly deviated from the direction normal to the grip surface with the paretic fingers, compared to asymptomatic fingers. This was seen consistently regardless of grip size, grip force level, and object stability. The paretic thumb had an even greater deviation of grip force direction than the paretic index finger. This altered grip force direction was associated with altered muscle activation patterns, but not with the posture at which the digit made contact with the object. Therapies to redirect the force direction at the digits may improve stroke survivors’ ability to stably grip an object.
References
American Heart Association (2005) Heart disease and stroke statistics—2005 update. In. American Heart Association, Dallas, Tex
Aruin AS (2005) Support-specific modulation of grip force in individuals with hemiparesis. Arch Phys Med Rehabil 86:768–775
Blennerhassett JM, Carey LM, Matyas TA (2006) Grip force regulation during pinch grip lifts under somatosensory guidance: comparison between people with stroke and healthy controls. Arch Phys Med Rehabil 87:418–429
Bobjer O, Johansson SE, Piguet S (1993) Friction between hand and handle. Effects of oil and lard on textured and non-textured surfaces; perception of discomfort. Appl Ergon 24:190–202
Boissy P, Bourbonnais D, Carlotti MM, Gravel D, Arsenault BA (1999) Maximal grip force in chronic stroke subjects and its relationship to global upper extremity function. Clin Rehabil 13:354–362
Broderick J, Brott T, Kothari R, Miller R, Khoury J, Pancioli A, Gebel J, Mills D, Minneci L, Shukla R (1998) The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke 29:415–421
Brunnstrom S (1970) Movement therapy in hemiplegia: a neurophysiological approach. Medical Dept. Harper & Row, New York
Buchholz B, Frederick LJ, Armstrong TJ (1988) An investigation of human palmar skin friction and the effects of materials, pinch force and moisture. Ergonomics 31:317–325
Chae J, Bethoux F, Bohine T, Dobos L, Davis T, Friedl A (1998) Neuromuscular stimulation for upper extremity motor and functional recovery in acute hemiplegia. Stroke 29:975–979
Cordo P, Gandevia SC, Hales JP, Burke D, Laird G (1993) Force and displacement-controlled tendon vibration in humans. Electroencephalogr Clin Neurophysiol 89:45–53
Cruz EG, Waldinger HC, Kamper DG (2005) Kinetic and kinematic workspaces of the index finger following stroke. Brain 128:1112–1121
Fisher NI (1993) Statistical analysis of circular data. Cambridge University Press, Cambridge
Gordon AM, Forssberg H, Johansson RS, Westling G (1991) Integration of sensory information during the programming of precision grip: comments on the contributions of size cues. Exp Brain Res 85:226–229
Gowland C, VanHullenaar S, Torresin W, Moreland J, Vanspall B, Barrecca S, Ward M, Huijbregts M, Stratford P, Barclay-Goddard R (1995) Chedoke-McMaster stroke assessment: development, validation and administration manual. Chedoke-McMaster Hospitals and McMaster University, Hamilton
Gray CS, French JM, Bates D, Cartlidge NE, James OF, Venables G (1990) Motor recovery following acute stroke. Age Ageing 19:179–184
Hermsdorfer J, Hagl E, Nowak DA, Marquardt C (2003) Grip force control during object manipulation in cerebral stroke. Clin Neurophysiol 114:915–929
Jobe MT (2007) Chapter 65—nerve injuries. In: Canale ST, Beaty JH (eds) Campbell’s operative orthopaedics, vol IV. Mosby Elsevier, Philadelphia, pp 3981–3984
Johanson ME, Valero-Cuevas FJ, Hentz VR (2001) Activation patterns of the thumb muscles during stable and unstable pinch tasks. J Hand Surg [Am] 26:698–705
Johansson RS, Westling G (1984) Roles of glabrous skin receptors and sensorimotor memory in automatic control of precision grip when lifting rougher or more slippery objects. Exp Brain Res 56:550–564
Kamper DG, Rymer WZ (2000) Quantitative features of the stretch response of extrinsic finger muscles in hemiparetic stroke. Muscle Nerve 23:954–961
Kamper DG, Rymer WZ (2001) Impairment of voluntary control of finger motion following stroke: role of inappropriate muscle coactivation. Muscle Nerve 24:673–681
Kamper DG, Harvey RL, Suresh S, Rymer WZ (2003) Relative contributions of neural mechanisms versus muscle mechanics in promoting finger extension deficits following stroke. Muscle Nerve 28:309–318
Kamper DG, Fischer HC, Cruz EG (2006) Impact of finger posture on mapping from muscle activation to joint torque. Clin Biomech (Bristol, Avon) 21:361–369
Lang CE, Schieber MH (2003) Differential impairment of individuated finger movements in humans after damage to the motor cortex or the corticospinal tract. J Neurophysiol 90:1160–1170
Lang CE, Schieber MH (2004) Reduced muscle selectivity during individuated finger movements in humans after damage to the motor cortex or corticospinal tract. J Neurophysiol 91:1722–1733
Lee SW, Chen H, Towles JD, Kamper DG (2008) Estimation of the effective static moment arms of the tendons in the index finger extensor mechanism. J Biomech 41:1567–1573
Magee DJ (2008) Chapter 7—forearm, wrist, and hand. In: Magee DJ (ed) Orthopedic physical assessment. Saunders Elsevier, St. Louis, pp 396–470
Milner TE, Dhaliwal SS (2002) Activation of intrinsic and extrinsic finger muscles in relation to the fingertip force vector. Exp Brain Res 146:197–204
Monzee J, Lamarre Y, Smith AM (2003) The effects of digital anesthesia on force control using a precision grip. J Neurophysiol 89:672–683
Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS (1994a) Compensation in recovery of upper extremity function after stroke: the Copenhagen Stroke Study. Arch Phys Med Rehabil 75:852–857
Nakayama H, Jorgensen HS, Raaschou HO, Olsen TS (1994b) Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil 75:394–398
Nowak DA, Hermsdorfer J (2005) Grip force behavior during object manipulation in neurological disorders: toward an objective evaluation of manual performance deficits. Mov Disord 20:11–25
Nowak DA, Hermsdorfer J, Topka H (2003) Deficits of predictive grip force control during object manipulation in acute stroke. J Neurol 250:850–860
O’Dwyer NJ, Ada L, Neilson PD (1996) Spasticity and muscle contracture following stroke. Brain 119(Pt 5):1737–1749
Parker VM, Wade DT, Langton Hewer R (1986) Loss of arm function after stroke: measurement, frequency, and recovery. Int Rehabil Med 8:69–73
Pearlman JL, Roach SS, Valero-Cuevas FJ (2004) The fundamental thumb-tip force vectors produced by the muscles of the thumb. J Orthop Res 22:306–312
Pendleton HM (2006) Chapter 22 evaluation of sensation and intervention for sensory dysfunction. In: Pendleton HM, Schultz-Krohn W (eds) Pedretti’s occupational therapy: practice skills for physical dysfunction. Elsevier Health Sciences, Philadelphia
Penfield W, Boldrey E (1937) Somatic motor sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain J Neurol 60:389–443
Penfield W, Rasmussen T (1950) The cerebral cortex of man: a clinical study of localization of function. Macmillan, New York
Scelsi R, Lotta S, Lommi G, Poggi P, Marchetti C (1984) Hemiplegic atrophy. Morphological findings in the anterior tibial muscle of patients with cerebral vascular accidents. Acta Neuropathol 62:324–331
Seo NJ (2009) Dependence of safety margins in grip force on isometric push force levels in lateral pinch. Ergonomics 52:840–847
Seo NJ, Armstrong TJ (2009) Friction coefficients in a longitudinal direction between the finger pad and selected materials for different normal forces and curvatures. Ergonomics 52:609–616
Seo NJ, Armstrong TJ, Drinkaus P (2009) A comparison of two methods of measuring static coefficient of friction at low normal forces: a pilot study. Ergonomics 52:121–135
Sivamani RK, Goodman J, Gitis NV, Maibach HI (2003) Friction coefficient of skin in real-time. Skin Res Technol 9:235–239
Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, Zheng ZJ, Flegal K, O’Donnell C, Kittner S (2006) Heart disease and stroke statistics-2006 update A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. In, vol 113. Am Heart Assoc
Trombly CA (1989) Stroke. In: Occupational therapy for physical dysfunction. Williams & Wilkins, Baltimore, pp 454–471
Valero-Cuevas FJ (2000) Predictive modulation of muscle coordination pattern magnitude scales fingertip force magnitude over the voluntary range. J Neurophysiol 83:1469–1479
Valero-Cuevas FJ, Towles JD, Hentz VR (2000) Quantification of fingertip force reduction in the forefinger following simulated paralysis of extensor and intrinsic muscles. J Biomech 33:1601–1609
Westling G, Johansson RS (1984) Factors influencing the force control during precision grip. Exp Brain Res 53:277–284
Woodbury ML, Velozo CA, Richards LG, Duncan PW, Studenski S, Lai SM (2007) Dimensionality and construct validity of the Fugl-Meyer assessment of the upper extremity. Arch Phys Med Rehabil 88:715–723
Woodson AM (2002) Stroke. In: Trombly CA (ed) Occupational therapy for physical dysfunction. Lippincott, Williams & Wilkins, Philadelphia, pp 817–853
Woolsey CN, Settlage PH, Meyer DR, Sencer W, Hamuy TP, Travis AM (1952) Patterns of localization in precentral and “supplementary” motor areas and their relation to the concept of a premotor area. Res Publ Assoc Res Nerv Ment Dis 30:238–264
Woolsey CN, Erickson TC, Gilson WE (1979) Localization in somatic sensory and motor areas of human cerebral cortex as determined by direct recording of evoked potentials and electrical stimulation. J Neurosurg 51:476–506
Acknowledgments
This work was supported by the Galvin Scientist Fellowship, the Coleman Foundation, and the American Heart Association 0920067G to N. J. Seo.
Author information
Authors and Affiliations
Corresponding author
Additional information
The work was performed in the Sensory Motor Performance Program, Rehabilitation Institute of Chicago.
Rights and permissions
About this article
Cite this article
Seo, N.J., Rymer, W.Z. & Kamper, D.G. Altered digit force direction during pinch grip following stroke. Exp Brain Res 202, 891–901 (2010). https://doi.org/10.1007/s00221-010-2193-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-010-2193-7