Abstract
This study investigated the effects of walking in an elastic force field (FF) for varying durations (49–1,629 strides) on: (a) the magnitude and duration of aftereffects, (b) performance in the FF on the next day. On day 1 in the FF, subjects (n = 17) showed an initial large error in peak toe velocity during swing (9–61% above baseline) that was largely reduced within the first 40 strides. After FF removal, subjects (16/17) showed aftereffects: (1) reduction in toe velocity (9–38% below baseline), (2) increase in hamstrings muscle activation. While the magnitude of aftereffects did not correlate to FF exposure duration, aftereffects duration did (p < 0.05). During FF exposure on day 2, initial toe velocity error was smaller than on day 1 (13/17 subjects, p < 0.001) regardless of day 1 exposure duration. This was associated with an earlier effective onset in hamstring activation. These results suggest that during walking, even short daily exposures to a FF (≥49 strides) lead to significant retention of the new movement parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In healthy humans, walking results from a tightly controlled muscle activation pattern generated by interactions between voluntary commands, an autonomous rhythmic pattern generator and sensory feedback (reviewed in Patla 1996). While this pattern is extremely well organized across muscles, it can also be remodeled. Over the last 15 years, the adaptive capacity of the neural control of walking has been well demonstrated by altering the walking environment and observing how and to what extent subjects compensated (Dietz et al. 1994; Prokop et al. 1995; Gordon et al. 1995; Layne et al. 1997; Reisman et al. 2005; Lam et al. 2006; Noble and Prentice 2006; Emken and Reinkensmeyer 2005; Choi and Bastian 2007; Blanchette and Bouyer 2009). An important finding of these studies is that upon returning to a normal environment, aftereffects were present in the walking pattern as documented in several variables such as foot fall parameters, kinematics, kinetics, and/or muscle activation patterns. The presence of aftereffects suggests that a central reorganization was involved in the adaptive process, a phenomenon compatible with motor learning.
At the moment, there is still very little understanding of the dynamics of adaptation to/recovery from altered walking environments. An important question is to find out for how long subjects have to be exposed to such environments in order to have a good retention of the modified motor pattern over time, i.e., to evaluate the influence of exposure duration on adaptation characteristics. In particular, two complementary measures are of interest in relation to exposure duration: aftereffects dynamics, and next day performance.
Regarding aftereffect dynamics, data from the different walking studies cannot easily be pooled and compared due to the variety of environmental perturbations used, from minutes in a force field (e.g., Blanchette and Bouyer 2009) to days in spaceflight (Layne et al. 1997). In the upper limb literature, adaptation to altered environments has been more extensively studied, from the very influential early work of Lackner and DiZio (1994) and Shadmehr and Mussa-Ivaldi (1994) to recent computational models of adaptation dynamics (e.g., Smith et al. 2006). In the work of Smith et al. (2006), short-term motor adaptation is described using a two-state, gain independent, multi-rate model. In this model, two “modules”, one with a fast learning time constant, and the other with a slow learning time constant, are used to explain several aspects of motor adaptation dynamics such as savings, anterograde interference, spontaneous recovery, and rapid unlearning. According to the model, aftereffects duration is expected to increase with increasing exposure duration.
Regarding next day performance, the important issue here is to find out the effects of exposure duration on long-term learning processes such as retention. What happens to next day performance if practice is continued after adaptation to the force field has reached a plateau? According to classical motor learning theories, such as “overlearning”, should lead to additional improvement in performance (Ebbinghaus 1913; Krueger 1929; Luh 1922). In addition, as the second exposure to the altered environment is performed after a latent period, the time between these tests allows for processes like consolidation of motor memory to begin. Generally, consolidation refers to neural processes leading to a progressive stabilization in long-term memory and resistance to interference, after a task is practiced (Stickgold and Walker 2007; Brashers-Krug et al. 1996; Dudai 2004; Krakauer and Shadmehr 2006; Doyon and Benali 2005; but see also Caithness et al. 2004). Aftereffects dynamics and next day performance may therefore have quite different relationships with exposure duration.
In the field of human walking, although a few examples of retention of locomotor adaptation have been published (Prokop et al. 1995; Gordon and Ferris 2007), none has specifically addressed this issue. Returning to the reaching literature again, Joiner and Smith (2008) recently tested Smith’s 2006 model (Smith et al. 2006) in a 24-h retention test. Their experiment shows that the longer the initial adaptation period, the higher the retention at 24 h. However, the extent of retention was predicted by the “slow learning module” of their two-state model, not by overall performance.
While quantitatively testing Smith’s model in the context of locomotion was beyond the scope of the present study, we hypothesized that despite the differences in neural control between reaching and walking, the process of adaptation to an external force field would qualitatively follow similar learning rules. Specifically, the aims of the present study were to investigate the effects of exposure duration to an elastic force field on: (a) the magnitude and duration of aftereffects, (b) motor performance during a second exposure to the force field on the next day. In previous experiments (Blanchette and Bouyer 2009), we have developed a simple walking adaptation protocol that consists of walking with an elastic attached between the foot and the front of a motorized treadmill. This paradigm will be repeated here to test our hypothesis. According to Smith’s model, varying force field exposure duration should lead to: (1) aftereffects duration proportional to exposure duration, (2) performance on the retention test proportional to exposure duration. However, as presented below, while locomotor aftereffects followed model predictions, day 2 performance was good regardless of day 1 exposure duration. Preliminary results have appeared in abstract form (Fortin et al. 2007).
Methods
Subjects
Eigthteen subjects (8 females and 10 males, 23–49 years old) participated in this study, after providing informed consent to the protocol approved by the local ethics committee. All subjects were naive to the task and had no self-reported history of neurological or orthopedic disorders that could interfere with the study.
Protocol
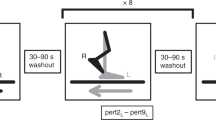
The experiment was carried out on two consecutive days. Each day, subjects walked for three periods: before (control, 5 min), during (adaptation, variable duration across subjects), and after (post-elastic, 10 min) exposure to a force field applied to the foot using an elastic tubing.
To vary exposure duration, a very simple method was used. Subjects were randomly assigned to one of six exposure durations (1, 3, 5, 10, 20, 30 min; 3 subjects/duration), but were allowed to chose their own cadence and comfortable walking speed. The latter was defined on day 1 during a familiarization session prior to the onset of data collection, and then kept constant for the whole experiment. Walking speeds for all participants are presented in Table 1 and ranged from 3.9 to 4.4 km/h. We took advantage of the fact that cadence and walking speed varied slightly across subjects to spread out the amount of exposure experienced by each participant. As a result, each subject actually walked for a different number of strides in the presence of the force field. Therefore, in the present experiment, the term “exposure duration” will refer to the number of strides during which subjects were exposed to the force field.
In order to keep the sample size low, a regression analysis approach was used to evaluate the effects of varying duration of force field exposure (i.e., number of strides) on the control of locomotion. Statistically, this means that all subjects were always considered together (regression analysis, n = 17), thereby increasing statistical power.
Force field generation
To create the force field, an elastic tubing (Thera-Band® Silver) was attached between the dorsal aspect of the right foot and the front of the treadmill (anchor point located 72.5 cm above the moving belt). This arrangement produced a force that pulled the foot forward and up during the swing phase while having minimal effects during stance due to weight bearing on the experimental leg [see Blanchette and Bouyer (2009) for a schematic representation of the experimental setup].
To normalize the level of difficulty of our task considering that each participant had a different maximal lower limb force output (MFO), the elastic force intensity was calibrated to impose a perturbation that represented ~40% of MFO at toe-off (Blanchette and Bouyer 2009). This force intensity was large enough to provide a movement perturbation while leaving force reserve for adaptation. MFO was measured prior to the onset of data collection. A detailed procedure for measuring individual MFOs and calculating elastic tubing length has been previously published (Blanchette and Bouyer 2009). Briefly, while standing up on the treadmill with the belt stationary, subjects pulled backwards continuously for 3 s on a steel cable attached between the foot and the treadmill frame with their right lower limb from a midline limb position. The knee was kept slightly flexed (~10°) during the procedure. A load cell located in series with this cable measured force output. MFO was defined as the average force maintained over the 3 s of the test.
Individual elastic lengths were defined using the force-stretching equation for this tubing (Eq. 1; taken from Blanchette and Bouyer 2009), MFO, and the distance between the foot at toe-off and the anchor point on the treadmill.
In Eq. 1, F is the elastic force in Newtons and x is the percentage elastic stretching [100 × (length at toe-off − rest length)/rest length]. Individual elastic tubing lengths are given in Table 1, and ranged from 27.9 to 59.5 cm. Theraband tubing was chosen for its ability to produce a very stable force output for >5,700 stretch cycles (Patterson et al. 2001), and therefore not changing mechanical properties over the duration of the experiment.
The treadmill belt was not stopped between walking periods, instead, subjects stepped aside (one foot on each side of the moving belt) to allow the experimenter to quickly connect or disconnect the elastic tubing from the foot. This procedure avoided differences in walking speed at the onset/end of each walking period. The instruction given to the subjects was “to resist the force and try to walk normally”.
In order to limit trunk anterior/posterior displacement on the treadmill (and thereby force intensity) during data collection, subjects lightly touched the front handle bar of the treadmill with the tip of their fingers. Despite this precaution, one of the subjects moved substantially forward on the treadmill during FF exposure, thereby reducing elastic elongation. He was therefore excluded from further analysis, bringing the number of subjects analyzed in this study down to 17.
Recordings
Pelvis and right lower limb kinematics were recorded using two Optotrak® cameras (NDI Inc, model 3020). Triads of markers (infrared light emitting diodes) were placed on the right lower limb segments (foot/shank/thigh) and on the pelvis at the level of posterior superior spinous process. Each marker was sampled at 100 Hz. The 12 following anatomical landmarks were also digitized for offline analysis: big toe, heel, fifth metatarsal head, medial and lateral malleoli, medial and lateral femoral condyles, greater trochanter, right and left anterior superior spinous processes, and iliac crests.
Surface electromyography was used to record activity of the following seven muscles: tibialis anterior (TA, ankle dorsiflexor), medial gastrocnemius (MG, ankle plantarflexor/knee flexor), soleus (SOL, ankle plantarflexor), rectus femoris (RF, knee extensor/hip flexor), vastus lateralis (VL, knee extensor), lateral and medial hamstrings (LH and MH, knee flexors/hip extensors). Electrodes (Medi-Trace 200, Ag/Ag–Cl, bipolar configuration, 2 cm apart) were placed on the muscle belly, in parallel with the muscle fibers in accordance with the SENIAM project recommendations (Freriks et al. 1999). To allow EMG comparisons across days, day 1 electrode positions were carefully marked with ink, to allow exact repositioning on day 2 (Winter and Yack 1987; Kadaba et al. 1985, 1989). EMG signals were amplified, band-pass filtered (10–500 Hz), and sampled at 1,000 Hz/channel. EMG and kinematic data were synchronized using custom software.
Custom-made foot switches were used to collect foot fall parameters. A load cell (MLP50, Transducer Techniques) located in series with the elastic tubing measured the forces applied to the foot. A video camera (Sony, DCRSR40) was used to record the general scene in order to provide further qualitative information following the experiment.
Data analysis
Analyzed epochs
Data analysis focussed primarily on the following 5 epochs: (1) the mean of the last 50 strides of control walking (control late, “CL”), (2) the first stride of elastic walking (elastic early, “EE”), (3) the mean of the last 10 strides of elastic walking (elastic late, “EL”), (4) the first stride of post-elastic walking (post-early, “PE”), and (5) the mean of the last 50 strides of post-elastic walking (post-late, “PL”).
Data processing
For kinematics, marker and associated anatomical landmark coordinates were filtered offline using a zero lag digital filter (fourth order Butterworth) at a cut-off frequency of 30 Hz with a custom-made program. Antero-posterior and vertical positions of the big toe were used to calculate a resultant toe velocity. The resultant peak toe velocity during the swing phase of each stride (Blanchette and Bouyer 2009) was identified and expressed in percentage of the difference with control values (‘absolute velocity error’). In order to eliminate offset, EMG activity was digitally band-pass filtered (20–450 Hz, zero lag, Butterworth, 4th order) before rectification. Each stride cycle was identified using the foot switch signals. Strides were time-normalized to 60% for the stance phase and 40% for the swing phase. To allow for comparison across days and subjects, EMG amplitudes for each muscle were normalized to the peak of the ensemble average of control walking (“CL”) obtained on the same day (Yang and Winter 1984).
As the main change in muscle activity occurred around stance-to-swing transition, mean amplitude of rectified EMG activity was calculated in the “zone of interest” (from 20% before to 20% after toe-off; Blanchette and Bouyer 2009). Finally, to study the effective onset of muscle activation, weighted averages of the EMG burst were calculated in this zone of interest and compared across days.
Aftereffects time course measurement
A 95% confidence interval was calculated around the mean of the last 50 strides of the post-elastic condition (“PL”). Using an 11-point moving average as a visual reference, the number of strides before reaching the confidence interval was counted and used to measure aftereffects duration.
Statistics
As force field intensity was calibrated according to individual MFO to normalize the level of difficulty across subjects, different force magnitudes were applied to the foot during FF exposure. Therefore, when walking in the presence of the elastic force, each subject had to learn to produce a specific amount of absolute force. As a result, aftereffects may be influenced by absolute force intensity. To take into account this second variable, a two-factor (exposure duration and absolute force intensity) regression analysis was performed on the selected variables. It must be noted that regression analysis was only performed if the data conformed to the three criteria for this test, i.e., (1) linearity between x and y, (2) for any given value of x, the corresponding value of y is normally distributed about α + βx with the same variance α2 for any x, (3) for any two data points (x1, y1), (x2, y2), the error terms e1, e2 are independent of each other. Such criteria ensure data homogeneity across the spectrum of exposure durations, and represent a necessary step to support the validity of the statistical test.
Repeated-measures ANOVA (non-parametric, Friedman test and Tukey test for multiple comparisons) were used to identify muscle activation patterns that were significantly changed from baseline and to evaluate differences between epochs. Wilcoxon signed-rank tests were used to evaluate differences between days. Statistically significant level was set to 0.05.
Results
Day 1: global effects of walking in the force field
When subjects walked in the presence of the force field, the elastic tubing was maximally stretched at toe-off. As a result, the foot was pulled forward and up during swing. In a previous study from our laboratory (Blanchette and Bouyer 2009), it was found that peak toe velocity during swing was a good marker to describe the effects of the elastic force on lower limb kinematics during locomotion. Figure 1a presents a stride-by-stride time course of peak toe velocity (day 1) for a representative subject (S7) who walked for 241 strides in the force field. First, it can be seen that during control walking, toe velocity was extremely stable from one stride to the next, even over extended walking periods (>200 strides). Secondly, when the force field was present, the subject initially showed a large increase in peak toe velocity due to the pulling action of the elastic tubing. This effect was much larger than interstride variability during control walking. Gradually, toe velocity returned toward baseline, as the subject adapted to the presence of the force. Finally, when the elastic was removed, an initial drop in peak foot velocity was observed, again gradually returning to baseline over several strides. Overall, in the presence of the elastic force, the group of subjects that participated in this study (n = 17) showed an initial increase from baseline in toe velocity that ranged between 9 and 61%. When the elastic was removed, 16/17 subjects initially showed a 9–38% reduction from baseline in toe velocity.
a Time course of peak toe velocity during the swing phase for subject S7 (day 1) for the 3 walking conditions. Each dot represents toe velocity for a single stride. b Aftereffects duration (number of strides) of toe velocity on day 1 in relation to absolute force field intensity and to the number of strides exposed to the elastic force field. Each point represents a single subject. c Aftereffect magnitude (first stride) of toe velocity on day 1 for each subject in relation to absolute elastic force intensity and to the number of strides exposed to the elastic force field. Toe velocity error of the first stride is expressed as a percentage of deviation from CL. Each point represents a single subject
In addition to lower limb kinematics, the muscle activation pattern was also recorded during force field adaptation. Figure 2a shows surface EMG recordings from six muscles in a subject (S10) exposed for 454 strides. Rectified averages from “CL” and “EL” are superimposed. It can be seen that the largest changes in muscle activity occurred in hamstrings (MH and LH), and more specifically between 20% before and 20% after toe-off. Across subjects, a systematic modification in mean amplitude during “EL” was seen in MH and LH (p < 0.05). For VL, a significant change was sometimes present, but was variable across subjects, with no consistent pattern. Further analysis therefore focused only on MH and LH, in a zone from 20% before to 20% after toe-off.
a Filtered and rectified EMG profile (μV) of six recorded muscles at CL (gray area) and EL (dark line) for subject S10 (day 1). b Time course of medial hamstrings (MH) mean amplitude around toe-off (20% before to 20% after) for the same subject. Each dot represents a single stride. Amplitude was normalized to peak locomotor EMG of control (CL). c Day 1 aftereffect duration (number of strides) of MH in relation to absolute force field intensity and to the number of strides exposed to the elastic force field. Each point represents a single subject
Figure 2b shows a stride-by-stride time course of mean MH amplitude in the zone of interest (same subject as on Fig 2a). The presence of the elastic produced elevated activity that was beyond stride-to-stride variability during control walking. Similar increases in activity levels were observed in MH and LH in all subjects during elastic walking. After elastic removal, part of the increased activity remained for several strides in 16/17 subjects. Co-activation between VL and MH/LH was only seen in two subjects (including the one presented in Fig. 2a) within a short portion of the zone of interest.
Day 1: aftereffects versus exposure duration
Overall, exposure duration to the force field ranged from 49 to 1,629 strides. Individual exposure durations are shown in Table 1. Subject distribution was well balanced, as mean weight and height in the six “time assignations” varied by less than 10% (Table 1).
The effect of varying force field exposure duration on aftereffects was quantified by comparing the first stride of post-elastic walking across subjects. As mentioned in the “Methods”, the fact that elastic tubings were calibrated to a fixed percentage of individual MFOs caused absolute elastic force to differ across subjects. As aftereffects are proportional to absolute force compensation, a two dimensional regression analysis was performed. In order to visualize this multiple regression, 3D graphs were constructed (Figs. 1b, c, and 2c), where each point represents a single subject, expressing aftereffects as a function both of exposure duration and absolute force field intensity. The resulting linear regression plane is also shown in the figures. The orientation of this plane takes into account both exposure duration and absolute force field intensity.
Regarding the duration of toe velocity aftereffects (Fig. 1b), the multiple linear regression analysis (aftereffects duration vs. absolute force field intensity and exposure duration) showed that subjects exposed for longer durations took significantly more strides to return to the baseline after force field removal (R 2 = 0.40, p < 0.05). However, this relationship was independent of absolute force field intensity. This effect can be appreciated graphically on Fig. 1b as a larger tilt in the regression plane along the exposure duration axis than along the force field intensity axis.
Regarding the magnitude of toe velocity aftereffects, the multiple linear regression analysis did not reach statistical significance. Unidimensional linear regressions were then performed separately for each factor (factor 1: absolute force intensity, factor 2: exposure duration). In this second analysis, although the subjects with greater absolute force intensity showed larger initial aftereffects magnitude (R 2 = 0.34, p < 0.05), the exposure duration did not affect the magnitude of these aftereffects. This can be appreciated graphically on Fig. 1c as a larger tilt in the regression plane along the force field intensity axis than along the exposure duration axis.
For muscle activations, aftereffects duration in MH was also significantly positively correlated to exposure duration (R 2 = 0.50, p < 0.05). Again, absolute force field intensity did not influence aftereffects duration. This effect can be appreciated graphically on Fig. 2c as a larger tilt in the regression plane along exposure duration axis than along the force field intensity axis. For LH, despite a similar increase in muscle activation during elastic walking as for MH, aftereffects duration was not significantly correlated to exposure duration. Differences in MH and LH behavior have been previously reported (Blanchette and Bouyer 2009). Regarding aftereffects magnitude in MH and LH, regression analysis could not be performed as the data did not conform to the criteria for this test (see Methods). Furthermore, data could not be transformed using standard linearization methods. The muscle activation analysis is thus limited to the effects of varying force field exposure duration on duration of aftereffects.
Day 1 versus day 2: initial performance during elastic walking
Another objective of the present study was to compare performance in the force field between the first exposure and a retest on the following day. Figure 3 presents absolute toe velocity error during force field exposure on these 2 days for all subjects. Figures 3a and 3b show peak toe velocity errors separately for each subject after specific number of strides in the FF on days 1 and 2, respectively. The first stride is represented by the first data point. Each subsequent data point corresponds to an average of 5 strides. The reduction in toe velocity error between days 1 and 2 was statistically significant as early as on the first stride in the FF (Wilcoxon signed-rank test, p < 0.001). Figure 3c presents the day 1 and day 2 initial velocity error as a function of exposure duration. In this case, to visually appreciate absolute force intensity distribution across exposure durations, force classes were arbitrarily constructed. In general, different absolute force intensities were found inside each range of exposure durations. In addition, subjects with the same range of absolute force intensities did not necessarily show the same amount of initial velocity error. On day 2, the majority of subjects (13/17) improved their performance, as measured by a reduction in initial velocity error. Multiple linear regression analysis showed that while exposure duration did not affect this improvement, absolute elastic force intensity did (R 2 = 0.44, p < 0.05): the larger the absolute force intensity, the greater the amount of error reduction on day 2.
Toe velocity error (absolute value) during elastic walking for every subject at selected intervals for day 1 (a) and day 2 (b). Only the first stride is plotted for t = 1; every other point represents an average of 5 strides. Toe velocity error is expressed as a percentage deviation from CL. c Initial velocity error (EE, first stride) of peak toe velocity for elastic walking on days 1 (d1) and 2 (d2). Each point represents a single subject. Each subject is plotted as a function of the number of strides exposed to the force field on day 1. The different symbols represent classes of absolute elastic force intensities (see inset)
Mean EMG amplitude around toe-off on the first stride of elastic walking was also compared between day 1 and day 2. No significant difference was found for MH or LH, in part due to inter-subject differences in activation strategies: some subjects used an earlier burst onset with low mean amplitude, while others presented a burst onset similar to baseline but with a modification in the timing of the peak amplitude (data not shown). As these strategies could all affect the effective onset of muscle activity between days, a weighted average was calculated in the zone of interest (20% before to 20% after toe-off), to find the “center of mass” of the muscle activation burst and look for changes between days (see Methods). Figure 4a, b presents examples of rectified EMG activation patterns around toe-off in MH and LH for the first elastic walking stride of each day in a subject (S4) that walked 135 strides in the FF. An earlier onset of activity is clearly seen in both muscles for this subject. Now considering all subjects, a significant change was measured between days in the weighted average of EMG activity (effective onset) for both muscles (Wilcoxon signed-rank test, p < 0.05): on day 2, the majority of subjects (12/16 for MH and LH) activated their hamstrings earlier than on day 1. Figures 4c, d presents data for all subjects, using the force class format introduced in Fig. 3c. Multiple linear regression analysis showed that absolute force intensity had a significant effect on changes observed in the effective onset of muscle activity between days for LH. Subjects exposed to greater absolute force intensity showed larger changes in activation onset on day 2 (R 2 = 0.46, p < 0.05). However, exposure duration did not influence EMG onset changes between days (p > 0.05). A similar trend was observed for MH but statistical significance was not reached (R 2 = 0.30, p = 0.10).
Days 1 and 2 filtered and rectified EMG activation pattern (μV) around toe-off for the first stride of elastic walking (EE) for LH (a) and MH (b) in subject S4. Onset of activation of LH (c) and MH (d), respectively, for days 1 (d1) and 2 (d2), expressed as a percent of stride. Each subject is represented according to the number of strides exposed to the elastic on day 1. Symbols represent classes of absolute elastic force intensity (see inset). TO Toe-off
Discussion
The aim of this study was to evaluate the effects of varying FF exposure duration during walking on immediate aftereffects and on performance during a delayed second exposure.
Magnitude of immediate aftereffects is independent of exposure duration (≥49 strides)
On day 1, subjects showed an initial error in their peak toe velocity during elastic walking that gradually returned toward control levels. The muscle activation pattern was also gradually modified with an increase in lateral and medial hamstrings burst duration. When the elastic was removed, subjects showed aftereffects in toe velocity as well as in muscle activation pattern (16/17 subjects). The presence of these aftereffects is consistent with a central reorganization of the control of movement, as described in previous studies looking at adaptations to modified environments (Lackner and DiZio 1994; Shadmehr and Mussa-Ivaldi 1994; Emken and Reinkensmeyer 2005; Reisman et al. 2005; Lam et al. 2006; Noble and Prentice 2006; Blanchette and Bouyer 2009). Results presented here show that walking in the elastic force field for 49 strides (smallest exposure duration, ~1 min) was sufficient to elicit kinematic aftereffects. The magnitude of these aftereffects, however, was independent of exposure duration to the force field in the range of 49–1,629 strides. This effect could be explained by a very rapid recalibration of the motor program to the force field environment (Lackner and DiZio 2000). Although an accurate recalibration time course could not be measured in the present study due to intersubject variability in initial effects and adaptation strategies, Fig. 3a nevertheless, clearly shows that most of the initial velocity error was removed by the 40th stride of elastic walking. This rapid recalibration is compatible with adaptation time courses obtained in similar experiments where subjects were adapted to velocity or acceleration dependent force fields (Noble and Prentice 2006; Emken and Reinkensmeyer 2005; Lam et al. 2006. These authors obtained adaptations within 45–50, ~10, and ~12 strides, respectively.
Magnitude of immediate aftereffects is related to the intensity of the force field
In the present study, the intensity of the force field was set to ~40% (Table 1) of MFO for each individual in order to normalize the level of difficulty of the task while making sure enough force reserve was available to allow for adaptation. However, this normalization to MFO resulted in different absolute force intensities across subjects due to individual strength differences. Therefore, our analysis also considered absolute elastic force field intensity as a second independent variable. Although exposure duration did not influence kinematic aftereffects magnitude, absolute elastic force intensity did. When subjects adapted to the perturbation, they had to resist an absolute force. In the present study, absolute intensity of the perturbation varied across subjects from 25 to 90 N. The central nervous system had to gauge the absolute force intensity delivered by the elastic in order to produce appropriate counteracting forces to reduce the movement error. When the elastic was removed, the modified motor command for the given force field persisted temporarily, explaining why differences occurred in the magnitude of kinematic aftereffects according to absolute force field intensity.
Duration of immediate aftereffects is related to exposure duration
In contrast to aftereffect magnitude, the duration of these effects was positively correlated to exposure duration, independently of absolute force field intensity. This is compatible with motor learning: after a rapid recalibration, making additional movements (strides) in the presence of the force, a phenomenon called “overlearning”, could improve the immediate consolidation process. During the learning process, several cellular mechanisms are initiated. This overlearning may have improved synaptic efficacy, in a way described by Hebb (1949), explaining the longer time to recovery once the force is removed for toe velocity and MH activation.
In the upper limb literature, Smith et al. (2006) recently proposed a mathematical model to describe force field adaptation dynamics for reaching movements. This model is composed of two elements: a fast and a slower “module”. As no equivalent model exists for walking, and knowing that the neural control of reaching and walking are quite different, it is nevertheless interesting to consider Smith’s model in the context of locomotion. This model predicts that aftereffects duration should be proportional to exposure duration. This prediction is remarkably compatible with our kinematic results, suggesting that two systems (that may or may not be the same as for reaching) could also be acting during locomotor adaptation.
Regarding the muscle activation pattern, adaptation and aftereffects were observed both in LH and MH. The fact that MH aftereffects duration was correlated to exposure duration supports the notion that the transfer of the adapted motor program to normal walking was more robust with overlearning. In LH, while muscle activity was also increased in the presence of the force field, aftereffect duration was not correlated to exposure duration. Differences in behavior between LH and MH have also been reported previously using this force field (Blanchette and Bouyer 2009), and may point out to different functional roles during adaptation for these agonists. Further studies focusing on LH/MH differences will be required to clarify this difference in aftereffects.
Day 2 performance is independent of day 1 exposure duration (≥49 strides)
On day 2 during force field exposure, substantial retention of the newly acquired locomotor program was observed, as shown by a reduction in initial velocity error. On the first stride in the presence of the force, this error reduction occurred regardless of first day exposure duration. These results agree with Savion-Lemieux and Penhune (2005) who showed for a timed motor sequence task, that delay was more important for retention than the amount of practice. It is possible that with consolidation, the new movement parameters became separately stored from the original walking movement. This would make them more readily accessible, thereby explaining the lower initial errors on day 2 in the presence of the force field.
Here again, it is interesting to compare our results to the predictions of Smith’s model for reaching adaptation. In a very recent study, Joiner and Smith (2008) have shown that for reaching, performance at 24 h is related to initial exposure duration, more specifically to the parameters of the slow learning process of their two-state model (Fig. 3 in Joiner and Smith 2008). Applying their model to our data, the prediction was therefore that the locomotor performance on day 2 would be proportional to exposure duration on day 1. This prediction was not confirmed by our findings, suggesting that contrary to same-day aftereffects, reaching and walking may use different mechanisms for next day retention. During learning, a set of cellular and molecular mechanisms are triggered (Bailey and Kandel 1993). Some of these learning mechanisms continue to operate after practice has stopped (Seeds et al. 1995). Moreover, it has been suggested that the mechanisms involved in short-term and in long-term memory are not the same. For example, animal studies have shown that short-term memory processes involve modifications of pre-existing proteins while long-term memory processes require activation of gene expression, new protein synthesis and/or the formation of new neural connections (Kandel 2001; Bailey et al. 1996). Thus, long-term memory processes could be related not only to the repeated usage of a synapse but also to the state of the transcriptional machinery (Kandel 2001). It is possible that in the present task, some of the long-term mechanisms involved were initiated during the 24-h delay between the two walking tests in the force field, explaining why exposure duration did not have the same effect on day 1 aftereffects and day 2 initial errors. Of course, these explanations remain theoretical, as cellular aspects of learning could not be addressed in the present experiment.
Regarding the motor pattern, inter-subject differences in muscle activation strategy were observed during force field adaptation. Some subjects used an earlier onset of muscle activity, while others changed the shape of the activation burst without necessarily advancing burst onset. Using a “center of mass” analysis on the rectified EMG activity, it was possible to show that as a group, subjects on day 2 had an earlier effective onset of activation of both MH and LH. These results likely represent anticipation of the force perturbation. This mechanism could explain in part the reduction in initial velocity error on day 2, through a preloading of hamstrings in preparation for the upcoming pulling action of the elastic force arriving near toe-off. Other studies have shown a modification in the onset of activation of muscles after adaptation (Thoroughman and Shadmehr 1999; Hinder and Milner 2005). Similar to the improvement observed in kinematic performance, this change in the onset of muscle activation on day 2 could be explained by the initiation of long-term learning mechanisms (Kandel 2001).
Conclusion
The present study shows that exposure duration has an impact on immediate transfer of motor patterns (aftereffects). However, retention of the new movement parameters, when tested as initial performance during elastic exposure 24 h later, was apparently not influenced by exposure duration, at least for exposures greater than 49 strides, with the majority of participants showing an initial lower error in their peak toe velocity and an earlier hamstrings activation. These results suggest that in healthy subjects, even very short daily exposures to a force field leads to the retention of a newly learned locomotor pattern. Our future work will address how much of this capacity remains after disease/injury to the central nervous system affecting the control of walking. If adaptive capacity is conserved, force field could then be considered as a tool to train people with locomotor disabilities.
References
Bailey CH, Kandel ER (1993) Structural changes accompanying memory storage. Annu Rev Physiol 55:397–426
Bailey CH, Bartsch D, Kandel ER (1996) Toward a molecular definition of long-term memory storage. Proc Natl Acad Sci USA 93:13445–13452
Blanchette A, Bouyer LJ (2009) Timing-specific transfer of adapted muscle activity after walking in an elastic force field. J Neurophysiol. 102:568–577
Brashers-Krug T, Shadmehr R, Bizzi E (1996) Consolidation in human motor memory. Nature 382:252–255
Caithness G, Osu R, Bays P, Chase H, Klassen J, Kawato M, Wolpert DM, Flanagan JR (2004) Failure to consolidate the consolidation theory of learning for sensorimotor adaptation tasks. J Neurosci 24:8662–8671
Choi JT, Bastian AJ (2007) Adaptation reveals independent control networks for human walking. Nat Neurosci 10:1055–1062
Dietz V, Zijlstra W, Duysens J (1994) Human neuronal interlimb coordination during split-belt locomotion. Exp Brain Res 101:513–520
Doyon J, Benali H (2005) Reorganization and plasticity in the adult brain during learning of motor skills. Curr Opin Neurobiol 15:161–167
Dudai Y (2004) The neurobiology of consolidations, or, how stable is the engram? Annu Rev Psychol 55:51–86
Ebbinghaus H (1913) Memory: a contribution to experimental psychology. Dover Publications, New York
Emken JL, Reinkensmeyer DJ (2005) Robot-enhanced motor learning: accelerating internal model formation during locomotion by transient dynamic amplification. IEEE Trans Neural Syst Rehabil Eng 13:33–39
Fortin K, Blanchette A, McFadyen BJ, Bouyer LJ (2007) Effects of exposure duration to an elastic force field during human walking. Soc Neurosci (abstract)
Freriks B, Hermens H, Disselhorst-Klug C, Rau G (1999) The recommendations for sensors and sensor placement procedures for surface electromyography. In: Hermens H, Freriks L, Merletti R, Hagg G, Stegeman D, Blok J, Rau G, Enschede Disselhorst-KlugsC (eds) SENIAM 8: European recommendations for surface electromyography. Roessingh Research and Development BV, The Netherlands, pp 15–53
Gordon KE, Ferris DP (2007) Learning to walk with a robotic ankle exoskeleton. J Biomech 40:2636–2644
Gordon CR, Fletcher WA, Melvill JG, Block EW (1995) Adaptive plasticity in the control of locomotor trajectory. Exp Brain Res 102:540–545
Hebb DO (1949) The organization of behavior: a neurophsychological theory. Wiley, New York
Hinder MR, Milner TE (2005) Novel strategies in feedforward adaptation to a position-dependent perturbation. Exp Brain Res 165:239–249
Joiner WM, Smith MA (2008) Long-term retention explained by a model of short-term learning in the adaptive control of reaching. J Neurophysiol 100:2948–2955
Kadaba MP, Wootten ME, Gainey J, Cochran GV (1985) Repeatability of phasic muscle activity: performance of surface and intramuscular wire electrodes in gait analysis. J Orthop Res 3:350–359
Kadaba MP, Ramakrishnan HK, Wootten ME, Gainey J, Gorton G, Cochran GV (1989) Repeatability of kinematic, kinetic, and electromyographic data in normal adult gait. J Orthop Res 7:849–860
Kandel ER (2001) The molecular biology of memory storage: a dialogue between genes and synapses. Science 294:1030–1038
Krakauer JW, Shadmehr R (2006) Consolidation of motor memory. Trends Neurosci 29:58–64
Krueger WCF (1929) The effect of overlearning on retention. J Exp Psychol 12:71–78
Lackner JR, DiZio P (1994) Rapid adaptation to Coriolis force perturbations of arm trajectory. J Neurophysiol 72:299–313
Lackner JR, DiZio P (2000) Aspects of body self-calibration. Trends Cogn Sci 4:279–288
Lam T, Anderschitz M, Dietz V (2006) Contribution of feedback and feedforward strategies to locomotor adaptations. J Neurophysiol 95:766–773
Layne CS, McDonald PV, Bloomberg JJ (1997) Neuromuscular activation patterns during treadmill walking after space flight. Exp Brain Res 113:104–116
Luh CW (1922) The conditions of retention. Psychol Monogr 31:1–87
Noble JW, Prentice SD (2006) Adaptation to unilateral change in lower limb mechanical properties during human walking. Exp Brain Res 169:482–495
Patla AE (1996) Neurobiomechanical bases for the control of human locomotion. In: Bronstein A, Brandt T, Wolloncott MH (eds) Clinical aspects of balance and gait disorders. Arnold, London, pp 19–40
Patterson RM, Stegink Jansen CW, Hogan HA, Nassif MD (2001) Material properties of thera-band tubing. Phys Ther 81:1437–1445
Prokop T, Berger W, Zijlstra W, Dietz V (1995) Adaptational and learning processes during human split-belt locomotion: interaction between central mechanisms and afferent input. Exp Brain Res 106:449–456
Reisman DS, Block HJ, Bastian AJ (2005) Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol 94:2403–2415
Savion-Lemieux T, Penhune VB (2005) The effects of practice and delay on motor skill learning and retention. Exp Brain Res 161:423–431
Seeds NW, Williams BL, Bickford PC (1995) Tissue plasminogen activator induction in Purkinje neurons after cerebellar motor learning. Science 270:1992–1994
Shadmehr R, Mussa-Ivaldi FA (1994) Adaptive representation of dynamics during learning of a motor task. J Neurosci 14:3208–3224
Smith MA, Ghazizadeh A, Shadmehr R (2006) Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol 4:e179
Stickgold R, Walker MP (2007) Sleep-dependent memory consolidation and reconsolidation. Sleep Med 8:331–343
Thoroughman KA, Shadmehr R (1999) Electromyographic correlates of learning an internal model of reaching movements. J Neurosci 19:8573–8588
Winter DA, Yack HJ (1987) EMG profiles during normal walking: stride-to-stride and inter-subject variability. Electroenceph Clin Neurophysiol 67:402–411
Yang JF, Winter DA (1984) Electromyographic amplitude normalization methods: improving their sensitivity as diagnostic tools in gait analysis. Arch Phys Med Rehab 65:517–521
Ackowledgments
The authors would like to thank subjects for participating in this project, M. Denis Côté for statistical support, M. Simon Lebel for data collection and M. Guy St-Vincent for his technical support. Financial support was provided by an operating grant from the Canadian Institutes of Health Research (CIHR). Karine Fortin and Laurent J. Bouyer were supported by the Fonds de la Recherche en Santé du Québec (FRSQ).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fortin, K., Blanchette, A., McFadyen, B.J. et al. Effects of walking in a force field for varying durations on aftereffects and on next day performance. Exp Brain Res 199, 145–155 (2009). https://doi.org/10.1007/s00221-009-1989-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-009-1989-9