Abstract
The acoustic startle reflex in rats can be inhibited if a prepulse stimulus is presented just before the startle stimulus (prepulse inhibition; PPI). When postnatal day 7 (P7) rats are exposed to agents that block the NMDA receptor (NMDAR), robust apoptosis is observed within hours and is thought to be followed at later ages by a significant loss of PPI. To understand these observations further, we exposed rat pups to vehicle or the NMDAR antagonist MK801 (1 mg/kg) at P6, P8, and P10. We then examined animals for PPI at P28 and P56. Compared to vehicle controls, we found no evidence for PPI deficits in the MK801-treated group, although we did observe prepulse-induced delay in response time at P56 (but not at P28). In a parallel study, we also performed histological analysis of brain sections for evidence of the pro-apoptotic marker activated caspase-3, 8 h after vehicle or MK801 injection into P6 animals. We found that there was a robust increase in this marker of cell death in the inferior colliculus of MK801 compared to vehicle-treated animals. Thus, transient blockade of the NMDAR during the postnatal period not only promotes early apoptosis in a brain region critical for acoustic processing but also leads to auditory deficits at a later age, suggesting that injury-induced loss of collicular neurons leads to network reorganization in the auditory system that is progressive in nature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 1999, Ikonomidou and colleagues showed that dizocilpine (MK801), an N-methyl-d-aspartate receptor (NMDAR) antagonist, caused age-dependent apoptosis in the neonatal rat brain (Ikonomidou et al. 1999). Thus, at postnatal day 7 (P7), induction of the pro-apoptotic enzyme, activated caspase-3 (AC3), can be observed as early as 2–4 h after NMDAR blockade in many cortical and subcortical structures (Johnson et al. 1998; Turner et al. 2002, 2007; Jevtovic-Todorovic et al. 2003; Wang and Johnson 2007). Peak AC3 induction is thought to occur at 8–16 h and is followed by a peak in DNA damage at 24 h (Ikonomidou et al. 1999; Turner et al. 2007). In addition to apoptosis, there are potent changes in proteins associated with synapses, cytoarchitecture, and plasticity (Wang et al. 2004; Lema Tomé et al. 2006b; Lu et al. 2006; Kaindl et al. 2008; Ringler et al. 2008), indicating that the CNS undergoes profound structural alteration during a period within which many developmentally regulated changes are already in progress (Rice and Barone 2000).
Such profound damage at an early stage in life would be expected to lead to further structural and, ultimately, behavioral alterations at later ages. To address the latter possibility, several groups have employed the robust and reliable behavioral assay known as prepulse inhibition (PPI). When presented with a brief (50 ms) and intense (95–120 dB) acoustic stimulus, an acoustic startle response (eye-blink in humans, body-jerk in rodents) is generated, which can be measured with great fidelity and accuracy (Blumenthal and Berg 1986; Swerdlow et al. 2001). However, when a prepulse stimulus is delivered just prior (30–240 ms) to the startle stimulus, the magnitude of the startle response is inhibited, thus illustrating PPI (Blumenthal 1999; Swerdlow et al. 2001). Compared to control animals, rat pups exposed to NMDAR antagonists at P7 have been reported to display less pronounced PPI when they reach maturity (P56 or older) (Wang et al. 2001, 2003; Harris et al. 2003), suggesting that NMDAR blockade-induced apoptosis at P7 leads to acoustic deficits in mature animals. Given that the PPI response is present across species (Swerdlow et al. 2001) and that PPI deficits are observed in schizophrenia (Geyer et al. 1990; Swerdlow et al. 1994), the P7-NMDAR blockade model is attractive to researchers wishing to model this disorder, especially as schizophrenia is now thought to have developmental origins (Remschmidt 2002; Eastwood 2004); though alternative interpretations exists (Weinberger 1996).
In order to connect these early cell death and structural changes with behavioral deficits later in life, we used the measurement of acoustic startle and PPI as a behavioral end-point in our own studies. Thus, at P6, P8, and P10, rat pups were injected with vehicle or the NMDAR antagonist MK801 and later examined for PPI changes at P28 and P56. For comparison, we also examined the effects of another NMDAR antagonist, the dissociative anesthetic, ketamine, on PPI. Further, because the inferior colliculus is thought to be essential for PPI (Leitner and Cohen 1985; Li et al. 1998; Silva et al. 2005), we examined brain stem sections for apoptosis 8 h after exposure to vehicle, MK801, or ketamine. Our findings are discussed with respect to both earlier reports as well as clinical implications.
Methods
Animal groups and drug injection protocol
All animals were treated according to the guidelines described by NIH and the Wake Forest University School of Medicine Animals Care and Use Committee. At postnatal day 6 (P6), rat pups (Sprague–Dawley; Charles Rivers, Charlotte, NC, USA) were injected with vehicle (sterile PBS; N = 12; 8 males), MK801 [1 mg/kg in sterile PBS; N = 10; 6 males; previous work from our lab has shown that this dose of MK801 promotes robust, widespread injury within hours (Turner et al. 2002, 2007)], or ketamine [50 mg/kg; N = 14; 12 males; dose based on that shown to induce apoptosis (Fredriksson et al. 2004)]. Injections were performed subcutaneously (a skin-fold was formed at the nape of the neck and the syringe needle inserted into this fold). These injections were repeated on P8 and P10. The 3-injection regimen was chosen because it has been shown that MK801-induced apoptosis occurs during the period spanning P4–P10, with a peak at P7 that rapidly diminishes thereafter (Ikonomidou et al. 1999; Turner et al. 2007) and that other NMDAR blockers are thought to promote PPI deficits at later ages when used in a similar fashion (Wang et al. 2001, 2003). After each injection, the pups were returned to their mother and monitored for recovery (all pups completely recovered from their injections). Pups were then allowed to mature and later tested for prepulse inhibition (PPI; see below). In a parallel study, P6 pups were injected with vehicle (PBS; N = 5), MK801 (1 mg/kg; N = 5), or ketamine (50 mg/kg; N = 3) and after 8 h brain sections were examined for evidence of apoptosis (see below).
PPI (acoustic startle) behavioral assay
Following P6–P10 injections (see above) animals were returned to the mother and given ad libitum access to food and water, and were handled at regular intervals (every 2–3 days) for short periods (1–2 min) by the investigator prior to PPI assessment. The first PPI test age was performed at P28. The second test age was performed at P56. Thus, at P28, and again at P56, animals were transported to the test area at least 1 h prior to testing in order to acclimate to the testing room. Every attempt was made to maintain the same conditions under which the tests were performed (time of testing, routine of procedure, housing prior to testing, pre-cleaning all components of the startle chamber). Animals were housed in individual holding cages for 15 min, and were then placed in a small chamber within the acoustic startle box (SDI, San Diego, CA, USA). The chamber rested on the displacement stage, which produced an output voltage relative to changes in the animal’s movement. Once the animal was inside the startle chamber, the door was closed and animals were allowed to acclimate for 5 min (background noise level was 77 dB). After this period, 39 trials were presented, with each trial including a broadband noise pulse (40 ms duration, instantaneous rise time, 120 dB), with these pulses preceded by a broadband noise prepulse (20 ms duration, instantaneous rise time, 5, 10, or 15 dB above background; delivered 100 ms prior (onset to onset) to the startle pulse) on 75% of trials, in random order. Startle responses were recorded by measuring stage displacement in the startle chamber for 100 ms after onset of the startle pulse. Dependent variables included the average voltage over the entire scoring window, the maximal voltage (peak) during the scoring window, and the time at which that peak occurred (peak latency). Averages for each of these dependent variables were calculated across the 15 control (pulse alone) trials, and across the eight trials at each prepulse intensity. PPI was calculated for each prepulse intensity condition for average voltage and peak voltage with the proportion of difference method [(prepulse condition minus control condition) divided by control condition] (Blumenthal et al. 2004). PPI for peak latency was calculated by subtracting peak latency in the control condition from that in each prepulse condition. We performed analyses to determine if there were gender differences but found males and females performed similarly within their treatment group, and we therefore collapsed all data for each group. A similar study did find gender differences under similar experimental and test conditions but the differences were very small despite a much larger sample size for both genders (Harris et al. 2003).
Immunohistochemistry (IHC)
Because PPI requires a functional inferior colliculus (Leitner and Cohen 1985; Li et al. 1998; Silva et al. 2005), we examined brain stem sections for apoptosis (AC3 induction) in pups treated with vehicle (PBS; N = 5), MK801 (1 mg/kg; N = 5), or ketamine (50 mg/kg; N = 3). Thus, following injections, animals were anesthetized 8 h later with isoflurane (2%; Webster Veterinary, Sterling, MA, USA), perfused with PBS followed by 4% paraformaldehyde in PBS (PFA; Sigma, St Louis, MO, USA), and brains were then dissected. Tissue was post-fixed in 4% PFA for 24 h, equilibrated in 10, 20, and 30% sucrose, parasagittally cut into 60 μm sections, and stored in PBS at 4°C. Sections were later incubated with a rabbit anti-AC3 polyclonal primary (1:2,000; Cell Signaling, Beverly, MA, USA) for 24 h in IHC buffer (1% bovine serum albumin and 0.1% Triton X-100 in PBS; Sigma, St Louis, MO, USA). Sections were then washed in PBS (×3), and incubated with a biotinylated, goat-anti-rabbit, secondary antibody (1:200 in IHC buffer at room temperature; Vector Labs, Burlingame, CA, USA). After PBS washes (×3), sections were incubated in ABC Elite solution according to instructions (Vector Labs), washed again in PBS (×3), and exposed to VIP chromagen for approximately 5 min (Vector Labs). The VIP reaction was terminated with 2–3 PBS washes and sections were then mounted onto SuperFrost glass slides (Fisher, Pittsburgh, PA, USA). After drying, slides were passed through 70, 95, and 100% alcohols, then CitraSolve, and coverslipped using Depex resin (Fisher).
AC3 counts
Images were captured at 10× magnification, using an Olympus IX70 Inverted fluorescent microscope (Olympus, Melville, NY, USA), an Orca 238 digital camera (Hamamatsu, Bridgewater, NJ, USA), and IPLab software (v3.65a, Scanalytics, Billerica, MA, USA). Images were taken from parasagittal sections corresponding to approximately lateral 2.90 mm to lateral 0.40, according to Paxinos (Paxinos and Watson 1998). Images were imported into ImagePro 5.0 (Media Cybernetics, Baltimore, MD, USA) for semi-quantitative analysis (see below). Similar to previously described methods (Lema Tomé et al. 2006a; Turner et al. 2007; Turner et al. 2009a), we counted the number of AC3-positive cells within the inferior colliculus of brains sections from vehicle, MK801 or ketamine-treated animals. Within the image frame (1.35 × 1.05 mm), we set the size and intensity thresholds to exclude background or artifact labeling (size was set to exclude anything less than 5 μm or greater than 100 μm; intensity was set to exclude any object with a value of less than 50 (twice background value), using a scale of 0 = no signal, 255 = highest intensity). Once these parameters had been set, counting was performed automatically by ImagePro 5.0, independent of the observer (counting performed by investigator blind to the treatment group). We sampled from 4 to 6 sections per animal.
Statistics
For our PPI data, a repeated-measures ANOVA was employed for each dependent variable, with age and prepulse intensity as within-participant factors and drug condition (vehicle, ketamine, MK801) as a between-groups condition. Analysis was performed using SPSS software (version 16.0; Chicago, IL, USA). For cell counts, the mean (±SE) AC3 profiles was estimated for each treatment group and differences between the groups determined by a one-way ANOVA with a Bonferroni post-test comparison of means (version 4.0; GraphPad, San Diego, CA, USA).
Results
MK801 has age-dependent effects on the startle response
Prepulse inhibition deficits have been observed in mature animals (P56 or later) when NMDARs are blocked at an earlier age (Wang et al. 2001, 2003; Harris et al. 2003). Thus, we examined PPI at P28 and P56 in animals treated with vehicle (sterile PBS) or MK801 (1 mg/kg) at P6, P8, and P10 (see “Methods”). For comparison, we also included a third group that was treated with ketamine (50 mg/kg) at P6, P8, and P10.
Control (no prepulse) startle reactivity was evaluated in ANOVA with age as a within-animal factor and drug group as a between-groups factor (see Table 1). Peak voltage increased significantly with age, F(1,15) = 18.54, P < 0.001, as did average voltage, F(1,15) = 18.96, P < 0.001, but peak latency was not affected by age. None of these dependent variables were affected by drug group, and no interactions reached significance. Clearly, there were predictable changes in the startle response that reflect normal, ongoing development, but these changes were not affected by administration of the drugs in this study.
Prepulses caused robust PPI, and the PPI of peak voltage increased as prepulse intensity increased, F (2,30) = 45.80, P < 0.001, ε = 0.789, and as age increased, F (1, 15) = 26.46, P < 0.001 (see Fig. 1). PPI of average voltage also increased as prepulse intensity increased, F (2,30) = 42.19, P < 0.001, ε = 0.893, and as age increased, F (1,15) = 23.51, P < 0.001. PPI of average voltage is not presented in a figure, since the values for average and peak voltage are virtually identical, never varying more than 3.5%. No interactions were significant for either response magnitude or average voltage, and drug condition had no effect on either measure (for the drug group factor, no F value exceeded 1.20 and no P value was less than 0.33, for any main effect or interaction). Thus, neither MK801 nor ketamine were able to influence PPI at P28 or P56, an outcome that contrasts markedly with that found by other groups using similar agents or paradigms (Wang et al. 2001, 2003; Harris et al. 2003).
Proportional PPI of peak magnitude. Rats pups were injected with vehicle (sterile PBS), MK801 (1 mg/kg), or ketamine (50 mg/kg) at P6, P8, and P10 as described in the “Methods”. At P28 and P56, animals were tested for PPI. The inhibition of the startle response in each of the prepulse intensity conditions (5, 10, or 15 dB above background) was related to the peak voltage recorded. Because we did not observe drug-induced changes in PPI, the data in this figure represent mean values (±SE) from all treatment groups at each prepulse level. PPI increased as age increased, and as prepulse intensity increased (P < 0.05, repeated measures ANOVA)
Prepulse inhibition of peak latency increased as prepulse intensity increased, F (2,30) = 4.90, P < 0.025, ε = 0.860, and as age increased, F(1,15) = 11.67, P < 0.01. Significant interactions were found between prepulse intensity and drug group, F (4, 30) = 3.27, P < 0.05, and between age and drug group, F(2,15) = 4.62, P < 0.05. These interactions were tested further by ANOVA of prepulse intensity and drug group within age, revealing an interaction between prepulse intensity and drug group at P56, F(4,30) = 2.92, P < 0.05, but no significant interaction at P28. Thus, at 56 but not 28 days of age, increasing prepulse intensity caused an increasing delay of peak voltage in the MK801 group, but not in the vehicle or ketamine animals (Fig. 2).
Differences in peak latency between prepulse and startle, pulse-only trials. Rats pups were injected with vehicle (sterile PBS), MK801 (1 mg/kg), or ketamine (50 mg/kg) at P6, P8, and P10 as described in the “Methods”. At P28 (a) and at P56 (b), animals were tested for PPI and the delay in response time to the startle stimulus in each of the prepulse intensity conditions (5, 10, or 15 dB above background) was determined by measuring the mean (±SE) peak latency differences (in ms) between the prepulse and the control condition (startle only). At P56 (but not P28) a significant interaction was found between prepulse intensity and drug group (P < 0.05, repeated measures ANOVA)
MK801 induces AC3 in the inferior colliculus
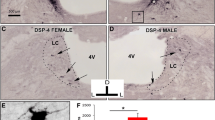
Next we examined if NMDAR blockade could induce injury in the inferior colliculus, an area that is thought to be crucial for inhibition of the acoustic startle response (Leitner and Cohen 1985; Li et al. 1998; Silva et al. 2005). Thus, P6 animals were injected with vehicle or MK801 (1 mg/kg) and 8 h later animals were processed for expression of the apoptotic marker AC3 (a time when induction of this enzyme is thought to be maximal) (Turner et al. 2007). In parasagittal sections from vehicle-treated animals, there were isolated cells that displayed AC3-ir in the inferior colliculus and surrounding brain stem structures (Fig. 3a). In contrast, in sections from MK801-treated animals, dense staining was found within the inferior colliculus but not in the surrounding brain stem regions (Fig. 3b). We quantified AC3-postiive cells in both groups and found that there was a robust increase in AC3-positive profiles in brain stem sections from MK801-treated animals compared to vehicle controls (Fig. 3c; P < 0.001; one-way ANOVA). When we examined brain stem sections from ketamine-injected animals, we found that they were mostly indistinguishable from vehicle controls, which was confirmed by our semi-quantitative analysis (Fig. 3c). Thus, our histological study revealed that, 8 h after MK801 exposure, robust apoptosis was observed in a brain stem structure critical for PPI. In contrast, using the present dose and injection protocol, ketamine did not appear to induce such injury.
MK801 induces activated caspase-3 in the inferior colliculus. Rat pups were injected with vehicle (sterile PBS), MK801 (1 mg/kg), or ketamine (50 mg/kg) at P6 and after 8 h processed for activated caspase-3 (AC3) expression within the inferior colliculus as described in the “Methods”. a In sections from vehicle-treated or ketamine-treated animals, AC3 expression (arrow) was relatively low (only vehicle is shown). Inset (dashed lines) shows close-up of area in a′. b In contrast, in sections from MK801-treated animals, AC3 expression was robust (arrows). Inset (dashed lines) shows close-up of area in b′. Note the higher number of intensely stained AC3-positive cells compared to vehicle (see a′). (c) Semi-quantitative analysis of AC3 profiles revealed that the increase in AC3 expression in MK801-treated animals was highly significant compared to vehicle controls (***P < 0.001; ANOVA, Bonferroni post-test comparison of means). There was no statistical difference between the means for the vehicle and ketamine groups (NS–not significant; ANOVA, Bonferroni post-test comparison of means). d Low power image of dorsal brain stem from an MK801-treated animal. Dashed line indicates the boundary of the inferior colliculus. Scale bar indicates 500 μm (a and b), 120 μm (insets), and 2 mm (d)
Discussion
In P7 or older animals, we have observed numerous molecular changes taking place in many forebrain structures hours to days after MK801-induced AC3 expression (Turner et al. 2002; Lema Tomé et al. 2006b; Turner et al. 2007). Our data from the inferior colliculus demonstrate that such changes also occur at the level of the brain stem (see Fig. 3). In order to correlate these molecular changes with behavioral deficits, we examined the effects of early exposure (P6–P10) of MK801 on PPI in older animals (P28 or P56). Choosing PPI as a behavioral end-point seemed a reasonable approach, given that other groups have demonstrated PPI deficits in older animals that were exposed to NMDAR antagonists at or around P7 (Wang et al. 2001, 2003; Harris et al. 2003).
In contrast to expected outcomes, we were unable to demonstrate any drug-induced changes in PPI of startle magnitude at P28 or P56. However, we did find evidence of a more subtle effect on startle behavior following NMDAR blockade during the postnatal period. Thus, whereas PPI of response magnitude in animals treated with MK801 from P6 to P10 was indistinguishable from that of animals treated with vehicle (at P28 or P56), there was a longer response time on prepulse trials in MK801-treated animals compared to vehicle controls (at P56 only; see Fig. 2b), with the delay of peak startle reactivity increasing as prepulse intensity increased. To our knowledge this is an entirely novel finding and suggests that researchers should look beyond PPI of startle magnitude and consider prepulse modulation of startle latency as worthy of investigation.
To complement our behavioral studies, we also examined effects of NMDAR blockade at the histological level. Thus, MK801 was found to induce robust AC3 expression in the inferior colliculus 8 h after injection into P6 animals. Clearly, not only does NMDAR blockade induce robust apoptosis in the inferior colliculus at P6, but a behavioral output that this brain stem structure regulates (PPI) is significantly altered in an age-dependent manner. It should be recalled that, during this postnatal period, auditory-specific input to the inferior colliculus is still being refined (Friauf and Lohmann 1999). Further, PPI is thought to be developmentally regulated and responses to acoustic startle stimuli continue to evolve postnatally (Martinez et al. 2000). Thus, a series of earlier events at the cellular level (cell death, synaptic reorganization, changes in network activity) could lead to behavioral alterations as animals mature, suggesting that the pathologies we describe here are progressive in nature.
Given that we observed AC3 induction in the inferior colliculus of P6 animals 8 h after MK801 injection, why was it that we did not observe deficits of PPI of startle magnitude at later ages? The acoustic startle reflex requires an intact brain stem circuitry that includes the lateral lemniscus, cochlear nucleus, and reticular formation (Davis et al. 1982). However, a functional inferior colliculus appears crucial for prepulse inhibition (Leitner and Cohen 1985; Li et al. 1998; Silva et al. 2005). Induction of AC3 in vulnerable inferior colliculus neurons at P6 may alter the inhibitory role of this structure at later ages. Thus, our histological evidence dovetails well with the behavioral data and suggests that previous studies may have overlooked crucial evidence that the acoustic startle reflex is indeed influenced by early exposure to NMDAR antagonists, just not in the manner previously documented (Harris et al. 2003; Wang et al. 2003). The recent reports that other groups have also been unable to replicate earlier findings of deficits in PPI of response magnitude (Rasmussen et al. 2007; Boctor and Ferguson 2009) suggest that claims which state that PPI deficits in adults are to be expected following NMDAR blockade at P7 are no longer tenable.
In contrast to an absence of a PPI deficit in response magnitude, we did find that MK801 induced prepulse-dependent slowing of the startle response. However, it is unclear why this should only be observed at P56 and not P28, although this may be related to the fact that the startle response itself increased in magnitude as the animals matured. Perhaps later maturation of the startle response (at P56) makes it more amenable to latency inhibition by a prepulse in this drug condition. Regardless, these outcomes suggest an evolving pathology, perhaps reflecting slowly occurring synaptic and/or network reorganization following injury at P6–P10. Indeed, we plan to investigate if the P28 and P56 brains differ with respect to a number of neuronal markers such as NeuN, calcium binding proteins, cytoarchitectural or synapse associated proteins, to determine if there is a histological basis for this outcome. Indeed, previous data suggest we should expect to see changes in many classes of neuronal markers (Lema Tomé et al. 2006b; Turner et al. 2007, 2009b).
The absence of an effect following ketamine injections would suggest that NMDAR blockade per se cannot explain MK801 action. However, we used ketamine in an identical manner to MK801 (a single injection at P6, P8, and P10) and so the much shorter-acting ketamine may not have had sufficient time to block the NMDAR and promote MK801-like toxicity, as other groups appear to have also recognized (Boctor et al. 2008). Although disappointing from an experimental viewpoint, the clinical implication is that a window may exist wherein surgical anesthesia may still be achieved without compromising otherwise vulnerable neurons. Future studies should shed significant light on this issue.
Although our own data, and that of others, may raise questions about the utility of PPI as a behavioral end-point in models of neonatal brain injury, it is nevertheless a well-established and reliable procedure for measuring drug-induced changes in sensorimotor gating, and the response is present across species. Thus, with greater precision, the PPI assay still has a role to play in determining the effects of perinatal brain injury, making it favorable for translational research. Indeed, the novel observations we describe here suggest that we are well-positioned to close the gap in knowledge between molecular pathologies occurring during the postnatal period and the behavioral pathologies occurring in adults.
References
Blumenthal TD (1999) Short lead interval modification. In: Dawson ME, Schell AM, Boehmelt AH (eds) Startle modification: implications for neuroscience cognitive science and clinical science. Cambridge University Press, Cambridge, pp 51–77
Blumenthal TD, Berg WK (1986) Stimulus rise time, intensity, and bandwidth effects on acoustic startle amplitude and probability. Psychophysiology 23:635–641
Blumenthal TD, Elden A, Flaten MA (2004) A comparison of several methods used to quantify prepulse inhibition of eyeblink responding. Psychophysiology 41:326–332
Boctor SY, Ferguson SA (2009) Neonatal NMDA receptor antagonist treatments have no effects on prepulse inhibition of postnatal day 25 Sprague–Dawley rats. Neurotoxicology 30:151–154
Boctor SY, Wang C, Ferguson SA (2008) Neonatal PCP is more potent than ketamine at modifying preweaning behaviors of Sprague–Dawley rats. Toxicol Sci 106:172–179
Davis M, Gendelman DS, Tischler MD, Gendelman PM (1982) A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci 2:791–805
Eastwood SL (2004) The synaptic pathology of schizophrenia: is aberrant neurodevelopment and plasticity to blame? Int Rev Neurobiol 59:47–72
Fredriksson A, Archer T, Alm H, Gordh T, Eriksson P (2004) Neurofunctional deficits and potentiated apoptosis by neonatal NMDA antagonist administration. Behav Brain Res 153:367–376
Friauf E, Lohmann C (1999) Development of auditory brainstem circuitry activity-dependent and activity-independent processes. Cell Tissue Res 297:187–195
Geyer MA, Swerdlow NR, Mansbach RS, Braff DL (1990) Startle response models of sensorimotor gating and habituation deficits in schizophrenia. Brain Res Bull 25:485–498
Harris LW, Sharp T, Gartlon J, Jones DN, Harrison PJ (2003) Long-term behavioural, molecular and morphological effects of neonatal NMDA receptor antagonism. Eur J Neurosci 18:1706–1710
Ikonomidou C, Bosch F, Miksa M, Bittigau P, Vockler J, Dikranian K, Tenkova TI, Stefovska V, Turski L, Olney JW (1999) Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 283:70–74
Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF (2003) Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci 23:876–882
Johnson KM, Phillips M, Wang C, Kevetter GA (1998) Chronic phencyclidine induces behavioral sensitization and apoptotic cell death in the olfactory and piriform cortex. J Neurosci Res 52:709–722
Kaindl AM, Koppelstaetter A, Nebrich G, Stuwe J, Sifringer M, Zabel C, Klose J, Ikonomidou C (2008) Brief alteration of NMDA or GABAA receptor-mediated neurotransmission has long term effects on the developing cerebral cortex. Mol Cell Proteomics 7:2293–2310
Leitner DS, Cohen ME (1985) Role of the inferior colliculus in the inhibition of acoustic startle in the rat. Physiol Behav 34:65–70
Lema Tomé CM, Bauer C, Nottingham C, Smith C, Blackstone K, Brown L, Hlavaty C, Nelson C, Daker R, Sola R, Miller R, Bryan R, Turner CP (2006a) MK801-induced caspase-3 in the postnatal brain: inverse relationship with calcium binding proteins. Neuroscience 141:1351–1363
Lema Tomé CM, Nottingham CU, Smith CM, Beauchamp AS, Leung PW, Turner CP (2006b) Neonatal exposure to MK801 induces structural reorganization of the central nervous system. Neuroreport 17:779–783
Li L, Korngut LM, Frost BJ, Beninger RJ (1998) Prepulse inhibition following lesions of the inferior colliculus: prepulse intensity functions. Physiol Behav 65:133–139
Lu LX, Yon JH, Carter LB, Jevtovic-Todorovic V (2006) General anesthesia activates BDNF-dependent neuroapoptosis in the developing rat brain. Apoptosis 11:1603–1615
Martinez ZA, Halim ND, Oostwegel JL, Geyer MA, Swerdlow NR (2000) Ontogeny of phencyclidine and apomorphine-induced startle gating deficits in rats. Pharmacol Biochem Behav 65:449–457
Paxinos G, Watson A (1998) The rat brain in stereotaxic coordinates. Academic Press, San Diego
Rasmussen BA, O’Neil J, Manaye KF, Perry DC, Tizabi Y (2007) Long-term effects of developmental PCP administration on sensorimotor gating in male and female rats. Psychopharmacology (Berl) 190:43–49
Remschmidt H (2002) Early-onset schizophrenia as a progressive-deteriorating developmental disorder: evidence from child psychiatry. J Neural Transm 109:101–117
Rice D, Barone S Jr (2000) Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 108(Suppl 3):511–533
Ringler SL, Aye J, Byrne E, Anderson M, Turner CP (2008) Effects of disrupting calcium homeostasis on neuronal maturation: early inhibition and later recovery. Cell Mol Neurobiol 28:389–409
Silva RC, Sandner G, Brandao ML (2005) Unilateral electrical stimulation of the inferior colliculus of rats modifies the prepulse modulation of the startle response (PPI): effects of ketamine and diazepam. Behav Brain Res 160:323–330
Swerdlow NR, Braff DL, Taaid N, Geyer MA (1994) Assessing the validity of an animal model of deficient sensorimotor gating in schizophrenic patients. Arch Gen Psychiatry 51:139–154
Swerdlow NR, Geyer MA, Braff DL (2001) Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl) 156:194–215
Turner CP, Pulciani D, Rivkees SA (2002) Reduction in intracellular calcium levels induces injury in developing neurons. Exp Neurol 178:21–32
Turner CP, Miller R, Smith C, Brown L, Blackstone K, Dunham SR, Strehlow R, Manfredi M, Slocum P, Iverson K, West M, Ringler SL, Berry ZC (2007) Widespread neonatal brain damage following calcium channel blockade. Dev Neurosci 29:213–231
Turner CP, Debenedetto D, Liu C (2009a) NMDAR blockade-induced neonatal brain injury: reversal by the calcium channel agonist BayK 8644. Neurosci Lett 450:292–295
Turner CP, Debenedetto D, Ware E, Stowe R, Lee A, Swanson J, Walburg C, Lambert A, Lyle M, Desai P, Liu C (2009b) Postnatal exposure to MK801 induces selective changes in GAD67 or parvalbumin. Unpublished observations
Wang CZ, Johnson KM (2007) The role of caspase-3 activation in phencyclidine-induced neuronal death in postnatal rats. Neuropsychopharmacology 32:1178–1194
Wang C, McInnis J, Ross-Sanchez M, Shinnick-Gallagher P, Wiley JL, Johnson KM (2001) Long-term behavioral and neurodegenerative effects of perinatal phencyclidine administration: implications for schizophrenia. Neuroscience 107:535–550
Wang C, McInnis J, West JB, Bao J, Anastasio N, Guidry JA, Ye Y, Salvemini D, Johnson KM (2003) Blockade of phencyclidine-induced cortical apoptosis and deficits in prepulse inhibition by M40403, a superoxide dismutase mimetic. J Pharmacol Exp Ther 304:266–271
Wang C, Anastasio N, Popov V, Leday A, Johnson KM (2004) Blockade of N-methyl-d-aspartate receptors by phencyclidine causes the loss of corticostriatal neurons. Neuroscience 125:473–483
Weinberger DR (1996) On the plausibility of “the neurodevelopmental hypothesis” of schizophrenia. Neuropsychopharmacology 14:1S–11S
Acknowledgments
The authors are indebted to Jody Roberts for her technical support and training efforts in establishing the PPI behavioral assay. These studies were supported by NIH RO1 051632 and a Wake Forest University Cross Campus Collaboration Research Support Fund grant to CPT and TDB. Immunohistochemistry performed by CL, image analysis and cell counts by CL and CPT, acoustic startle (PPI) assay performed by AL and JS, and data analysis by AL, JS, TDB, and CPT. Manuscript preparation by AL, TDB, and CPT.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Lyall and J. Swanson are co-first authors.
Rights and permissions
About this article
Cite this article
Lyall, A., Swanson, J., Liu, C. et al. Neonatal exposure to MK801 promotes prepulse-induced delay in startle response time in adult rats. Exp Brain Res 197, 215–222 (2009). https://doi.org/10.1007/s00221-009-1906-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-009-1906-2