Abstract
All movements are accompanied by postural reactions which ensure that the balance of the body is maintained. It has not been resolved that to what extent the primary motor cortex and corticospinal tract are involved in the control of these reactions. Here, we investigated the contribution of the corticospinal tract to the activation of the soleus (SOL) muscle in standing human subjects (n = 10) in relation to voluntary heel raise, anticipatory postural activation of the soleus muscle when the subject pulled a handle and to reflex activation of the soleus muscle when the subject was suddenly pulled forward by an external perturbation. SOL motor evoked potentials (MEPs) elicited by transcranial magnetic stimulation (TMS) increased significantly in relation to rest −75 ms prior to the onset of EMG in the heel-raise and handle-pull tasks. The short-latency facilitation of the soleus H-reflex evoked by TMS increased similarly, suggesting that the increased MEP size prior to movement was caused at least partly by increased excitability of corticospinal tract cells with monosynaptic projections to SOL motoneurones. Changes in spinal motoneuronal excitability could be ruled out since there was no significant increase of the SOL H-reflex until immediately prior to EMG onset for any of the tasks. Tibialis anterior MEPs were unaltered prior to the onset of SOL EMG activity in the handle-pull task, suggesting that the MEP facilitation was specific for the SOL muscle. No significant increase of the MEPs was observed prior to EMG onset for the external perturbation. These data suggest that the primary motor cortex is involved in activating the SOL muscle as part of an anticipatory postural reaction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anticipatory postural reactions are necessary in order to counteract the change in centre of gravity, when we make an arm movement while standing (Belen’kii et al. 1967; Cordo and Nashner 1982; Pal’tsev and El’ner 1967); one example is whenever we try to open a heavy door by pulling the handle towards us. Without anticipatory contractions in the lower extremity muscles, such as the triceps surae, we would pull our body forward, rather than open the door. These unconscious anticipatory postural reactions are appropriately scaled movements, which precede the voluntary activation of the prime mover by some 20–40 ms, and may be seen as early as 110 ms in the EMG from the triceps surae muscle group following a starter signal when compared to 120–130 ms for a voluntary activation of the same muscle group (Cordo and Nashner 1982). The anticipatory postural reactions are, on the other hand, slower than long-loop stretch reflexes, which have latencies around 90–100 ms (Christensen et al. 2000). Evidence suggests that the supplementary motor area is important for at least the anticipatory postural reactions that take place in the contralateral arm during tasks, which involve loading or unloading of one arm by the other (Massion et al. 1989; Viallet et al. 1992). It has been suggested that the descending drive for these reactions comes from the contralateral primary motor cortex, since the reactions are abolished or greatly diminished in patients with hemiparesis of the postural arm (Bennis et al. 1996; Palmer et al. 1996; Diedrichsen et al. 2005; Viallet et al. 1992) and transcranial magnetic stimulation (TMS) over the contralateral, but not the ipsilateral primary motor cortex, may delay the postural reactions (Palmer et al. 1994; Taylor 2005). Furthermore, a reduction in the short-interval intracortical inhibition (SICI) of soleus MEPs is seen during postural activity when compared to rest (Soto et al. 2006), which also indicates that primary motor cortex may be involved in postural activity. However, it cannot be fully excluded that subcortical mechanisms also contribute to these findings and the extent to which the primary motor cortex contributes to the generation of the anticipatory reactions has, therefore, not been fully clarified. The purpose of the present study was to address this issue. We compared changes in soleus (SOL) motor evoked potentials (MEPs) elicited by TMS with changes in soleus H-reflexes prior to the onset of a voluntary activation of the soleus muscle (heel raise), an anticipatory postural activation of the soleus muscle (handle pull) or an external postural perturbation resulting in soleus activation (sudden forward pull). In two additional experiments, we also investigated facilitation of soleus H-reflexes using subthreshold TMS and changes in tibialis anterior (TA) MEPs prior to the onset of EMG in the handle-pull task.
Methods
Participants
The first experiment involving measurement of SOL MEP’s and H-reflexes during three different motor tasks (described in details below) were performed in ten healthy subjects (three women, seven men; age range 23–39 years). The second experiment involving measurement of TA MEPs were performed in ten healthy subjects (four women, six men; age range 21–32 years), The third experiment involving sub-threshold TMS facilitation of SOL H-reflexes were performed in eight healthy men (age range 21–39 years). None of the subjects participated in more than one of the experiments. All subjects gave their written informed consent to the experimental procedures, which were approved by the local ethics committee. The study was performed in accordance with the declaration of Helsinki. None of the subjects had any history of neurological disease.
Experimental procedures
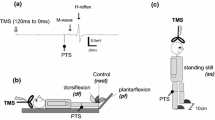
Soleus MEPs following TMS and SOL H-reflexes evoked by tibial nerve stimulation were measured in two otherwise identical sessions on separate days. In each experimental session, measurements were obtained prior to and during three different motor tasks: (1) heel raise, (2) voluntary pull of a handle and (3) external perturbation (described in details below). All three tasks involved activation of the ankle plantar flexors either as part of a voluntary activation (heel raise), an anticipatory postural reaction (pull of handle) or an external perturbation. In all three tasks, the subject was standing in an upright position on a force platform. Prior to the actual experiment subjects were given 10–20 trials of each task to familiarize them to the experimental procedure and to measure the onset of SOL muscle activity (EMG onset). Trials with and without stimulation (MEP or H-reflex) were randomly alternated in all three motor tasks. Measurements were also obtained in a control situation without movement. The control measurements were randomly alternated with measurements during movement. The subjects were warned that the subsequent measurement would not require any movement by two warning signals in rapid succession. In the control measurements, the subjects were instructed to stand upright, leaning slightly forward with their hands on the bar (heel raise) or holding the handle in front of them (handle-pull and external perturbation). The stimuli were applied 3 s after the two beeps. A total of 110 trials of each task were performed.
-
1.
Heel raise
Subjects were asked to do voluntary heel raises using the ankle plantar flexors. To exclude postural sway the subjects were leaning slightly forward, with their hands placed on the bar in front of them. The subjects were given an acoustic “ready” cue followed by a second acoustic “go” cue. The “go” cue was delivered randomly at intervals of 4–7 s in relation to the “ready” cue in order to prevent the subjects from anticipating the exact time of the stimuli.
-
2.
Handle pull
Subjects were asked to pull a handle towards them in response to an acoustic cue, using mainly the biceps brachii (BB) muscle. This also produced a burst of activity in the SOL muscle. This pattern of EMG activity has previously been described for the lateral gastrocnemius muscle (Fig. 2; Cordo and Nashner 1982). The handle was attached to a load of 6–8 kg depending on the subjects with the wire running through a winch system, which allowed the subjects to pull the weight in one direction, and prevented the weight from pulling in the other direction, when the subjects were standing passively with the handle in their hand (Fig. 1). As in the previous task, the subjects were given an acoustic “ready” cue followed by an acoustic “go” cue in a random manner at intervals of 4–7 s.
Experimental setup. Subjects were standing on a force platform (a), holding the handle bar (b), the bar was attached to a wire which passed through a screen (c), preventing visual feedback of the weight (e), the wire was running through a release mechanism (d) allowing the experimenter to perform unexpected perturbations where the subject was pulled forward. A safety bar was placed in front of the subject for security reasons (f)
-
3.
External perturbation
Subjects were asked to stand with the handle in their hand in front of them as in the previous task. An acoustic “ready” cue was then given at random intervals (4–7 s) followed by release of the weight by a custom-build release system (Fig. 1). The system was designed to release the weight by opening the winch system following a trigger pulse delivered from a personal computer. No acoustic cue were given at the time of the weight release and a screen prevented the subject from seeing the weight and the release system and thereby preventing the use of acoustic or visual cues to anticipate the time of the release. The subjects were instructed to resist the forward pull of the body produced by the drop of the weight, which therefore resulted in activation of both the BB and triceps surae muscles (Fig. 2; Cordo and Nashner 1982).
Soleus and biceps brachii (BB) EMG and vertical ground reaction force in relation to heel raise (a), handle pull (b) and external perturbation (c) in a single subject. The traces in a–c are from top to bottom: 1 the position of the head in the forward–backward plane, 2 the elbow joint angle, 3 the ankle joint angle, 4 lowpass (10 Hz) filtered (filt.) SOL EMG imposed on 5 the rectified (rect.) SOL EMG, 6 lowpass (10 Hz) filtered (filt.) BB EMG imposed on 7 the BB rectified EMG and 8 the vertical ground reaction force (Fz). The scales for the different traces are given in the right part of the figure. Horizontal dashed lines represents the base line for the filtered EMG. The scale for the EMG traces (SOL and BB) are given for the filtered EMG (0.035 mV) and for the rectified EMG (0.2 mV). Vertical dashed lines represents EMG onset in all three motor tasks. For the heel-raise (a) and handle-pull (b) tasks traces begin at the time of the “go” signal, whereas for the perturbation task (c) the traces starts when the trigger was delivered to the winch system. The onset latencies are 185, 165 and 200 ms for the heel-raise, handle-pull and perturbation tasks, respectively. All traces are from one single trial
EMG measurements
Surface electrodes were used for recording of electromyographic activity (EMG). EMG activity was recorded from the left TA muscle, the BB muscle of the left arm and the SOL muscle of the left leg by nonpolarizable bipolar Ag–AgCl electrodes (1 cm2, interelectrode distance 2 cm.) The amplified EMG signal was filtered (band-pass 25 Hz to 1 KHz) and sampled at 2 KHz on a personal computer (using Micro1401 and signal software from Cambridge Electronic Design; Cambridge, England) for offline analysis.
The average delay between the acoustic “go” cue and the EMG onset was obtained from 10 to 20 trials for each task (heel-raise, handle-pull and external perturbation) in the beginning of each experiment and is used during the experiment to set different intervals of the stimulations in relation to the “go” cue. For this purpose, the signal was rectified and averaged. Identification of the EMG onset was done by visual inspection of the averaged EMG traces at high resolution. EMG onset was taken as the time point where the EMG signal increased more than twofold over the background noise.
During the actual experiment, the latency of EMG onset in trials without stimulation was monitored in each task. Following the experiments, the latency between the “go” cue and the onset of EMG activity was measured in trials without stimulation. To investigate whether any learning effects took place over time, which could lead to improvement in reaction times we compared the initial 10–20 reaction times with the reaction times measured throughout the three different tasks. No significant difference between the two sets of reaction times was observed for the heel-raise task (P = 0.303) 183 ± 8 versus 189 ± 10 ms, for the handle-pull task (P = 0.747) 157 ± 5 versus 161 ± 6 ms and for the perturbation task (P = 0.946) 168 ± 8 versus 169 ± 11 ms. In fact, some subjects became slightly slower in the heel-raise and handle-pull tasks as the study progressed probably due to fatigue. It is our belief that the two motor tasks (heel raise and handle pull) performed in this study are movements performed in everyday life and, therefore, probably have been over-learned already.
Mmax recording
In the beginning of each experimental session, the maximal M-wave (Mmax) was elicited in the SOL muscle for normalisation of subsequent MEP and H-reflex recordings. Mmax was elicited by electrical stimulation of the tibial nerve in the popliteal fossa. In the experiment involving measurement of TA MEPs, Mmax was elicited in the TA muscle for normalisation of MEPs. Mmax in the TA was elicited by electrical stimulation of the common peroneal nerve distal to caput fibulae. The intensity of the stimulation in both experiments was increased from a subliminal level until no further increase in the peak-to-peak amplitude of the M-wave was observed.
TMS
On the first experimental day, MEPs were elicited by TMS over the right (contra-lateral) leg motor cortical area at the hot spot for activation of SOL by a magnetic stimulator (Magstim 200, Magstim Company Ltd). A magnetic field of maximum 2 T for 100 μs could be delivered through the figure of eight coil (loop diameter, 9 cm; type no. 8106) The MEPs were recorded from the soleus muscle via surface electrodes. The hotspot was identified as the spot in which the lowest intensity of magnetic stimulation was required to evoke a MEP of 50 μV peak-to-peak amplitude in at least three of five consecutive trials in the standing subject without background EMG activity in the SOL muscle. The coil was then fixed to the scalp using a custom-build rigid plastic harness. The harness allowed the subject to stand freely without the coil moving. The coil was oriented and positioned with the handle pointing backwards. The stimulation intensity was then adjusted to produce a clear MEPs at rest. Stimulation intensity varied between subjects from 80 to 100% of maximal stimulator output. Based on the onset latencies for SOL EMG, TMS stimulations were applied in a random fashion at different times relative to EMG onset. Single pulses were delivered at control condition, −125, −100, −75, −50, −25, 0, 25, 50, 150 ms. Ten pulses were delivered at each time relative to EMG onset. Each trial lasted 11 s allowing the subject to return to normal position before the next stimulation was applied. Recordings were monitored online, and the trial was discarded if the subject failed to respond. An online trend-plot of the amplitude of responses evoked in the control situation was used to check whether the control MEP amplitude was held constant.
To investigate whether the observed premovement increase of SOL MEPs was caused by a general arousal effect or related to this specific muscle alone, we performed a second experiment. The purpose of this experiment was to investigate the modulation of TA MEPs in the handle-pull task only. Since TA MEPs generally have a lower threshold and reach larger maximal amplitudes than SOL MEPs, it is usually not possible to obtain MEPs in the two muscles of a similar size when using the same stimulus intensity (Morita et al. 2000). We, therefore, chose to use lower stimulation intensities to make a direct comparison of TA MEP amplitudes to the observed premovement increase of SOL MEPs in the handle-pull task. Stimulations were delivered in the same fashion as in the main experiment with an intensity that would elicit MEPs in the TA of around 5–6% of Mmax in the control situation. We chose to time the stimulations relative to SOL EMG onset, since no EMG was observed in the TA muscle in this task. It was ensured in all trials in all three tasks that there was no EMG activity in the soleus muscle prior to the elicitation of the MEP.
H-reflex recordings
On the second experimental day, the same protocol as the first day was used. Instead of MEPs, H-reflexes in the SOL muscle were elicited by stimulation of the tibial nerve in the popliteal fossa. The cathode was a ball which was placed in the distal part of the popliteal fossa and secured to the limb by velcro-bands strapped around the leg just below the knee joint. The anode was a plate which was placed just above the knee and secured by the same velcro-bands. The peak-to-peak amplitude of the H-reflex at rest was expressed relative to Mmax. The lowest intensity of stimulation that produced an H-reflex of a similar size as the control MEP obtained on the first experimental day was used. The purpose of this was to make it possible to compare the size of the H-reflexes and the MEPs at different time points relative to EMG onset. The size of the control H-reflex was monitored throughout the session to keep the size constant. At each active trial two pulses were delivered. The first pulse served as the test stimulus and was adjusted as described above; the second pulse was elicited 50 ms after the first pulse and served as a control stimulus. The intensity of this pulse was adjusted to evoke a clear M-response. The control stimulus ensured that changes in the H-reflex prior to the contraction could not be attributed to changes in the efficiency of the stimulus or the recording conditions (i.e. position of recording electrode). The control stimuli as well as H-reflex amplitudes were monitored online during the experiment, to ensure constant recording conditions. It was ensured in all trials in all three tasks that there was no EMG activity in the soleus muscle prior to the H-reflex elicitation.
The effect of subthreshold TMS on SOL H-reflexes
Motor-evoked potentials reflect the direct and indirect (transsynaptic) activation of corticospinal cells, which in turn have both direct and indirect (via interneurones) projections to the spinal motoneurones (Petersen et al. 2003). The size of the MEPs is, therefore, influenced by excitability changes in corticospinal tract cells, cortical interneurones, spinal interneurones and especially spinal motoneurones (Nielsen et al. 1999). If TMS is decreased to an intensity where it does not evoke an MEP, but still evoke descending activity in direct and indirect projections to the motoneuornes, it is possible to demonstrate the evoked EPSPs and IPSPs in the spinal motoneurones with the use of H-reflex testing (Nielsen et al. 1993; Nielsen et al. 1999; Petersen et al. 2003). In this way, a time course of the effect of TMS on the spinal motoneuronal excitability may be obtained, where the earliest occurring facilitation may be ascribed to activation of the fastest conducting corticospinal tract cells with monosynaptic projections to the SOL motoneurones (Nielsen et al. 1993). The size of this short-latency facilitation has been shown in several studies to be selectively sensitive to changes in the excitability of the corticomotoneuronal cells, whereas changes in spinal motoneuronal or interneuronal excitability has no effect (Nielsen et al. 1993; 1995; Petersen et al. 1998; 2003). To obtain further evidence of changes in corticospinal excitability, we consequently also investigated the size of this short-latency facilitation of the H-reflex prior to the onset of the SOL EMG in the handle-pull task. First, we tested the facilitation of the H-reflex using interstimulus intervals between electrical nerve stimulation and TMS from −5 to 0 ms in steps of 1 ms during a tonic-plantar flexion, the negative values indicate that the conditioning stimulation (TMS) was applied after the test stimulus (the H-reflex). Since it is important to measure the facilitation within the initial 0.5–1.0 ms after its onset to avoid contamination from non-monosynaptic corticospinal pathways (Nielsen et al. 1993), we then confirmed the onset of the short-latency facilitation in interstimulus steps of 0.5 ms. An interval within the initial 0.5–1.0 ms after the onset of facilitation was then used for the subsequent measurements in relation to the handle-pull task. Measurements were made at −125 and −75 ms prior to SOL EMG onset.
A minimum of ten conditioned and ten unconditioned reflexes were averaged for each time point. The control H-reflex size was maintained at around 20–25% of Mmax, where the H-reflex is most sensitive to changes in motoneurone excitability (Crone et al. 1990). For the TMS stimulation, an intensity corresponding to 95% of active-motor threshold during the tonic-plantar flexion was used (Nielsen et al. 1993). H-reflexes without and with TMS stimulation (unconditioned and conditioned reflexes, respectively) were delivered in a random fashion.
Kinematic analysis
Kinematic analysis of the movements at different joints was obtained in all three tasks using the Qualisys motion capture system (Qualisys, Gothenburg, Sweden). Three infrared cameras were placed at different angles to the left of the subject and reflecting markers were placed on (1) the base of the fifth metatarsus, (2) the malleolus, (3) the head of the fibula, (4) the crista iliaca, (5) the acromion, (6) the olecranon, (7) the processus styloideus ulnae and (8) in front of the outer ear canal on the left side of the subject’s body. The sampling rate was 120 Hz. The kinematic measurements were synchronized with the EMG measurements by the “go” cue (see above).
Force recordings
The vertical ground reaction force (Fz) was obtained using an AMTI force platform (model nr. OR6-6-1000). The signal was feed through a MINI AMP Amplifier (AMTI) and recorded with the EMG at 2 kHz on a personal computer (using Micro1401 and signal v 3.09 software).
Data analysis
The EMG data were analysed using Cambridge electronic design (CED) software, signal v 3.09. For each stimulation time point relative to EMG onset, an average of the peak-to-peak amplitude of the MEP or H-reflex was produced. The averages consisted of ten trials each. This amplitude was then expressed as % of the maximal M-wave for that subject.
Statistics
Statistical analysis was done using Sigmastat 2.03 (SPSS Inc.). Before statistical comparison, all data sets were tested for normal distribution by a Kolmogorov–Smirnov test. All data are presented as mean ± SEM unless reported otherwise. Changes in EMG onset latency between muscles were tested by Student’s t test for pairwise comparison. Changes in MEP and H-wave amplitudes between the different stimulation time points in each task and differences between tasks were investigated using one-way ANOVA tests for repeated measurements with stimulation time relative to EMG onset as a factor. Differences in MEP or H-reflex amplitudes between the three tasks within each stimulation time point were investigated using two-way ANOVA tests with task and stimulation time as factors.
To investigate the differences between H-wave amplitudes and TMS amplitudes within each task we used two-way ANOVA tests on data normalized to control with stimulation time relative to EMG onset and stimulation type (i.e. TMS and H-reflex) as factors. Bonferroni correction was used in all cases for multiple pairwise comparisons.
Results
Latency of EMG activity
Figure 2 shows kinematic and EMG recordings during the three tasks for a single subject. In all three tasks, activation of the plantar flexors and a subsequent increase in the ground reaction force were seen. The onset latency of SOL EMG (as indicated by the vertical lines) was shorter in the handle-pull task (Fig. 2b; 165 ms) than in the other two tasks (Fig. 2a, c; 185 and 200 ms, respectively). A similar trend was observed in all subjects and when comparing the average latencies for the population of subjects, a statistically significant difference was found in the latency of SOL EMG between heel-raise and handle-pull tasks; 183 ± 8 and 157 ± 5 ms, respectively, Table 1, (P < 0.01). In the external-perturbation task, the latency from the trigger to the onset of SOL EMG was 168 ± 8 ms. Statistical analysis revealed no significant difference between SOL EMG onset in the perturbation task compared to the heel-raise task (P = 0.260) or between the perturbation task and handle-pull task (P = 0.359). Most of the delay in SOL EMG onset in the perturbation task is explained by the opening of the winch system and the drop of the weight. Furthermore, the latency for the BB EMG was 158 ± 17 ms. There was no significant difference (P = 0.662) between onset of BB EMG and SOL EMG in the perturbation task. It is also seen from Fig. 2c that the subject was not pulled forward until immediately prior to the onset of the SOL EMG activity (forward movement head; upper trace and elbow extension, second trace; Fig. 2c). The forward pull caused an increase in the vertical ground reaction force (bottom trace; Fig. 2c) and a stretch of the ankle plantar flexors immediately prior to the EMG onset (third trace from above; Fig. 2c).
There was no BB EMG activity in the heel-raise task (Fig. 2a), but it should be noted that the latency of the BB EMG was longer than that of the SOL EMG in the handle-pull task (Fig. 2b). For the population of subjects this difference was statistically significant (215 ± 11 and 157 ± 5 ms, respectively; P < 0.01; Table 1). This finding thus confirms the observation by Cordo and Nashner (1982) that anticipatory postural reactions in the SOL muscle precedes the activation of the prime mover (the BB) in relation to arm movements while standing.
Modulation of MEP and H-reflex amplitude
The modulation of the MEP in relation to the onset of SOL EMG in the three tasks is shown for a single subject in Fig. 3a–c. The size of the MEP was 3.5% of Mmax in the control situation. At −75 ms prior to onset of EMG a clear increase of the MEP was observed for the heel-raise and handle-pull tasks, but not for the external perturbation. At EMG onset a clear increase was observed in all tasks. Similar observations were made in all subjects. The average SOL control MEP sizes were 4.6 ± 0.8, 4.8 ± 1 and 3.8 ± 0.5% of Mmax in the heel-raise, handle-pull and perturbation tasks, respectively. For the population of subjects, a statistically significant increase of the MEP in relation to the control MEP was observed in the heel-raise task (F = 6.209) and handle-pull task (F = 6.084). Pairwise comparison revealed that there was a significant increase in MEP amplitude at an interval of −75 ms prior to EMG onset in the heel-raise (P = 0.018) and handle-pull tasks (P = 0.015), but as can be seen from Fig. 3d a tendency for an increase was observed already −125 ms prior to EMG onset. A significant difference in MEP size relative to control (F = 8.339) was also observed following the external perturbation. However, the pairwise comparison revealed a significant difference only at onset of EMG (0 ms) compared to control. Comparison of the MEP measurements prior to EMG onset in the three tasks revealed a significant difference in the modulation (F = 2.121). Pair-wise comparisons revealed significant differences between the handle-pull task and the perturbation task at −100 and −75 ms before EMG onset (P = 0.024 and P = 0.020, respectively) and between heel-raise and perturbation task at −75 ms before EMG onset (P = 0.027). There was no statistically significant difference of the MEP amplitude in the control situation between the three tasks (P = 0.93). It should be noted that the MEP was of a similar amplitude in measurements following EMG onset during the handle-pull task as during the heel-raise task, although a significantly lower amplitude of the background EMG activity was observed in the handle-pull task than in the heel-raise task (P < 0.001; see Table 1).
Modulation of SOL MEPs in relation to the onset of SOL EMG activity. a–c Data from a single subject. The traces are the average of ten MEP’s recorded from the SOL muscle at three different stimulation time points relative to EMG onset (control −75 and 0 ms). a Heel-raise task, b handle-pull task, c external-perturbation task. d The population average of the MEPs recorded at each interval in relation to SOL EMG onset. The amplitude of the MEPs is expressed as a percentage of Mmax. Measurements from the heel-raise, the handle-pull and the external-perturbation tasks are shown as black, white and grey columns, respectively. Mean ± SE values (n = 10) are shown, *P < 0.05
The size of the H-reflex was adjusted in the control situation to the same size as the MEP in the control situation, The average control H-reflex sizes were 5.1 ± 1, 5.1 ± 0.8 and 5.0 ± 1% of Mmax in the heel-raise, handle-pull and perturbation tasks, respectively. No statistical difference (P = 0.991) between the control reflex sizes was found and no statistical difference was found between control H-reflexes sizes and control MEPs at the two different test days (P = 0.19). Figure 4a–c shows the modulation of the H-reflex in a single subject in the three tasks. A significant difference in H-reflex amplitudes over stimulation time prior to EMG onset were found in the heel-raise (F = 17.207), handle-pull (F = 7.771) and perturbation tasks (F = 10.744). However, no clear change in the H-reflex size is seen until around the time of the EMG onset and pairwise comparisons revealed that an increase in the H-reflex in relation to the control measurement was not observed until −25 ms prior to the onset of EMG for all three tasks (Fig. 4d; P < 0.05 for all three tasks). There was a statistically significant difference in the modulation between the three tasks (F = 2.221), but this difference was only present at −25 ms before onset of EMG, where H-reflex amplitude was significantly larger in the perturbation task compared to the handle-pull task (P = 0.004) and at EMG onset where the heel-raise and perturbation tasks was significantly different from the handle-pull task (P = 0.008 and P = 0.01, respectively).
Modulation of SOL H-reflexes in relation to onset of soleus EMG activity. a–c Data from a single subject. The traces are the average of ten H-reflexes recorded from the SOL muscle at three different stimulation time points relative to EMG onset (control −75 and 0 ms). a Heel-raise task, b handle-pull task, c external-perturbation task. d The population average of the SOL H-reflexes recorded at each interval in relation to soleus EMG onset. The amplitude of the H-reflexes is expressed as a percentage of Mmax. Measurements from the heel-raise, the handle-pull and the external-perturbation tasks are shown as black, white and grey columns, respectively. The scaling of a–c is different than that of Fig. 3a–c. Mean ± SE values (n = 10) are shown, *P < 0.05
When comparing the modulation of the MEP and the H-reflex prior to the onset of EMG, a statistically significant difference was observed for the heel-raise (F = 7.974), handle-pull (F = 3.433) and external-perturbation tasks (F = 4.764) (Fig. 5). The difference between the facilitation of the MEP and H-reflex in the heel-raise and handle-pull tasks became statistically significant −75 ms prior to EMG onset (P = 0.002 and P = 0.01, respectively). However, a clear tendency for a difference was present already at −125 and −100 ms prior to EMG onset in both tasks. In the perturbation task, a difference was observed only at −25 ms where H-reflexes were much larger than MEPs (P = 0.001).
Comparison between MEP (closed circles) and H-reflex amplitudes (open circles) in the three different tasks. The size of both responses is normalized to their respective size in the control measurement without movement (and expressed as % of control). All data points are the population average for each time point in relation to the onset of EMG activity in the soleus muscle. a Heel-raise task, b handle-pull task, c perturbation task. The scaling of c is different since H-reflex amplitudes were larger in the pertubation task. Mean + SE values are shown, *P < 0.05
Modulation of TA MEPs
The modulation of TA MEPs is shown in Fig. 6. It is evident that the amplitude of the TA MEPs did not increase during the handle-pull task (F = 1.687). No statistical difference (P = 0.106) between control and different stimulation times relative to SOL EMG onset was observed.
The effect of subthreshold TMS on SOL H-reflexes
Figure 7a shows a typical time course of the effect of subthreshold TMS on soleus H-reflexes during tonic-plantar flexion in a single subject. In this subject, the short-latency facilitation had an onset at a conditioning-test interval of −4.5 ms. A conditioning-test interval of 4 ms (indicated by arrow) was then selected for the subsequent measurements in relation to the handle-pull task. At this interval the facilitation is likely to selectively reflect activation of the fastest conducting corticospinal cells with monosynaptic projections to the SOL motoneurones and to be selectively sensitive to changes in their excitability (Nielsen et al. 1993; Petersen et al. 2003). The onset of the facilitation varied between −5 and −0.5 ms in the different subjects and in all of them an interval within 0.5–1.0 ms after this onset was selected for the measurements in relation to the handle-pull task. Figure 7b shows the population average of the short-latency facilitation at this interval at −125 and −75 ms prior to EMG onset in the handle-pull task. The ANOVA test revealed a significant difference among the different stimulation time points (F = 8.117). Pairwise comparison between the unconditioned H-reflex at −125 ms (22 ± 4% of Mmax) and the conditioned H-reflex at −125 ms (24 ± 3% of Mmax) and the unconditioned H-reflex at −75 ms revealed no statistical difference (22 ± 4 and 24 ± 4 of Mmax, respectively, P > 0.05), suggesting that there was no change in spinal motoneuronal excitability at these intervals. The conditioned reflex on the other hand increased significantly at −75 ms prior to EMG onset (33 ± 5% of Mmax, P < 0.001) compared to the unconditioned H-reflex at −125 ms, which indicates an increased excitability of the fastest conducting corticospinal projections with monosynaptic projections to the spinal motoneurones.
TMS conditioning of the SOL H-reflex during the handle-pull task (a). Time course for the facilitation of the H-reflex in a typical subject using subthreshold (0.95 × MT) TMS during a tonic-plantar flexion. In this subject, the short-latency facilitation is observed at an interstimulus interval of −4 ms (arrow). The dashed line represents the size of the control reflex. b The population average H-reflex size in % of Mmax in the conditioned and unconditioned state at −125 and −75 ms prior the EMG onset in SOL in the handle-pull task. At −75 ms prior to EMG onset a clear facilitation of the H-reflex is observed, mean ± SE (n = 8) values are shown **P < 0.01)
Discussion
We have demonstrated in this study that SOL MEPs evoked by TMS begin to increase around −75 ms prior to EMG onset in relation to both voluntary activation (i.e. heel raise) and an anticipatory postural reaction (i.e. handle pull), but not in relation to an external perturbation. SOL H-reflexes were in contrast not increased until just prior to EMG onset in all three tasks. The short-latency, presumed monosynaptic, corticospinal facilitation of the SOL H-reflex also increased around −75 ms prior to the onset of SOL EMG activity in the handle-pull task. These findings suggest that the primary motor cortex is involved in anticipatory postural reactions.
Involvement of motor cortex in the initiation of anticipatory postural reactions
The increase of the SOL MEPs and the short-latency TMS-induced facilitation of the SOL H-reflex prior to the onset of EMG in the voluntary heel-raise and the handle-pull tasks is similar to that observed prior to the onset of other voluntary movements (Nielsen and Petersen 1992; Chen et al. 1998; Schneider et al. 2004). This increase was not caused by a general arousal reaction or by increased attention in relation to the “ready” cue, since no increase in TA MEPs were observed in the handle-pull task and since the increase in the SOL MEPs was not observed until −75 ms before the onset of EMG activity. For both tasks, the increased size of the SOL MEPs thus more likely reflect the increased excitability of the corticospinal cells as they become activated by inputs to the primary motor cortex prior to the movement as also evidenced from the increase of the short-latency TMS-induced facilitation of the SOL H-reflex. This facilitation is in all likelihood caused by activation of corticospinal cells with direct monosynaptic projections to SOL motoneurones and its size selectively reflects changes in the excitability of these corticospinal cells (Nielsen et al. 1993).
Although the onset of discharge of corticospinal cells varies considerably, most of the cells begin to discharge around 100 ms prior to onset of movement (Cheney and Fetz 1980), thus not very different from the onset of the MEP facilitation in this and other studies (Nielsen and Petersen 1992).
The increase of the H-reflex around 25–50 ms prior to the onset of EMG activity in this and other studies (Nielsen and Petersen 1992; Nielsen and Kagamihara 1993; Crone and Nielsen 1989; Pierrot-Deseilligny et al. 1971) similarly reflects at least partly the increased excitability of the spinal motoneurones as they become activated by the descending commands. The lack of increase of the MEPs prior to EMG onset for the external perturbation and the similar onset times for the facilitation of the H-reflex in all three tasks, is thus evidence that the primary motor cortex and corticospinal tract are only activated prior to the onset of EMG activity in the heel-raise and handle-pull tasks, but, unsurprisingly, not prior to the external perturbation. Although MEPs and H-reflexes do not necessarily activate exactly the same population of motoneurones (Nielsen et al. 1999), this is unlikely to explain their different modulation prior to EMG onset.
It has been demonstrated that TMS activates spinal interneurones and likely also other subcortical neurones, which influence the size of the MEPs (Iles and Pisini 1992; Nielsen and Kagamihara 1993) and it could be argued that some of the changes in the MEPs prior to the onset of contraction are caused by changes in the excitability of such subcortical neurones. We cannot fully exclude this possibility, since control experiments demonstrating lack of modulation of MEPs evoked by transmastoid stimulation of the corticospinal tract (Ugawa et al. 1991) are not feasible in relation to these postural tasks. However, based on the similarity between the MEP modulation prior to EMG onset for the handle pull and the heel raise there is no reason to assume a different contribution from subcortical mechanisms in the two tasks. The increase of the short-latency facilitation of the SOL H-reflex also provides evidence that the increase of the MEP around −75 ms prior to EMG onset in the two tasks was mainly due to increased cortical excitability. It, therefore, seems reasonable to argue that the anticipatory postural reaction in the SOL muscle involves the primary motor cortex and the corticospinal tract to the same extent as when the SOL muscle is used as the prime mover. The findings are thus in line with the idea that the anticipatory reactions (of the SOL muscle in the handle-pull task) are an integrated part of the motor programme for the activation of the prime mover (the TA muscle in the handle-pull task) as has also been suggested in previous lesion and TMS studies for other anticipatory postural reactions (Palmer et al. 1994; 1996; Bennis et al. 1996; Taylor 2005). Since anticipatory postural reactions during a bimanual unloading task are impaired in patients with lesions in the contralateral supplementary motor cortex (Viallet et al. 1992), it seems likely that this area and possibly also other circuitries are involved in motor planning and hence responsible for ensuring the activation of the leg area of the motor cortex shortly prior to the activation of the arm area in the handle-pull task. Whether the same population of corticospinal tract cells are used for conveying the signal for the anticipatory postural reaction and the signal for a voluntary movement involving the SOL muscle as prime mover, is difficult to conclude from the experiments in the present study, but the similar behaviour of the MEPs in relation to the handle-pull and heel-raise tasks suggests that this is a possibility.
SOL activation following external perturbation
It is not surprising that there were no changes in MEPs until −25 ms prior to EMG onset in the external-perturbation task. The subject had no way of anticipating the time of the perturbation and also had no visual information about the drop of the weight. Therefore, changes in cortical excitability at earlier intervals would have been surprising. The activation of the SOL muscle thus must have been initiated as a reaction to the forward pull of the body when the weight was dropped. It is not possible from our data to make any firm conclusions regarding the underlying mechanisms for this. The forward movement of the head, elbow extension, the increase in the vertical ground reaction force and the stretch of the ankle plantar flexors evoked by the perturbation all occurred around the time of the SOL EMG onset (Fig. 2c) and it was not possible from the delay to disclose whether the activation was consistent with activation of either the vestibular system or stretch reflexes. Since both the H-reflex and MEP were facilitated at the same time in relation to EMG activity in the external-perturbation task it is also not possible to make any conclusions from that comparison of whether cortical mechanisms, possibly through long-loop transcortical reflexes (Christensen et al. 2000), contributes to the activation of the muscle. The larger facilitation of the H-reflex in all three tasks just prior to and in the beginning of the SOL activation may be an indication of reduced presynaptic inhibition of the Ia afferents as has been demonstrated in previous studies (Crone and Nielsen 1989; Nielsen and Kagamihara 1993). However, any firm conclusions regarding this would require specific measurement of presynaptic inhibition, which would be difficult to achieve in the investigated tasks.
Functional interpretation
The findings in the study confirm the well-established feed-forward nature of the anticipatory postural reactions and emphasizes that they are an integrated part of the motor programme controlling any voluntary movement that require postural adjustments. By integrating the reactions into the motor program at a stage before the activation of primary motor cortex it is ensured that the activation of muscles in different limbs are optimally coordinated and that the anticipatory postural reaction is optimally scaled and timed in relation to the activation of the prime mover.
Conclusion
We have found evidence that anticipatory postural reactions involve a coordination of activity in arm and leg muscles at a cortical level and that the motor cortex is in all likelihood involved in the initiation of anticipatory postural reactions.
References
Belen’kii VE, Gurfinkel VS, Pal’tsev EI (1967) Control elements of voluntary movements. Biofizika 12:135–141
Bennis N, Roby-Brami A, Dufosse M, Bussel B (1996) Anticipatory responses to a self-applied load in normal subjects and hemiparetic patients. J Physiol Paris 90:27–42
Chen R, Yaseen Z, Cohen LG, Hallett M (1998) Time course of corticospinal excitability in reaction time and self-paced movements. Ann Neurol 44(3):317–25
Cheney PD, Fetz EE (1980) Functional classes of primate corticomotoneuronal cells and their relation to active force. J Neurophysiol 44:773–791
Christensen LO, Petersen N, Andersen JB, Sinkjaer T, Nielsen JB (2000) Evidence for transcortical reflex pathways in the lower limb of man. Prog Neurobiol 62:251–272
Cordo PJ, Nashner LM (1982) Properties of postural adjustments associated with rapid arm movements. J Neurophysiol 47:287–302
Crone C, Nielsen J (1989) Spinal mechanisms in man contributing to reciprocal inhibition during voluntary dorsiflexion of the foot. J Physiol 416:255–272
Crone C, Hultborn H, Mazieres L, Morin C, Nielsen J, Pierrot-Deseilligny E (1990) Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: a study in man and the cat. Exp Brain Res 81:35–45
Diedrichsen J, Verstynen T, Lehman SL, Ivry RB (2005) Cerebellar involvement in anticipating the consequences of self-produced actions during bimanual movements. J Neurophysiol 93:801–812
Iles JF, Pisini JV (1992) Cortical modulation of transmission in spinal reflex pathways of man. J Physiol 455:425–446
Massion J, Viallet F, Massarino R, Khalil R (1989) The supplementary motor area is implicated in the coordination between posture and movement in man. C R Acad Sci III 308:417–423
Morita H, Olivier E, Baumgarten J, Petersen NT, Christensen LOD, Nielsen JB (2000) Differential changes in corticospinal and Ia input to tibialis anterior and soleus motor neurones during voluntary contraction in man. Acta Physiol Scand 170(1):65–76
Nielsen J, Kagamihara Y (1993) The regulation of presynaptic inhibition during co-contraction of antagonistic muscles in man. J Physiol 464:575–593
Nielsen J, Morita H, Baumgarten J, Petersen N, Christensen LO (1999) On the comparability of H-reflexes and MEPs. Electroencephalogr Clin Neurophysiol Suppl 51:93–101
Nielsen J, Petersen N (1992) Changes in motor cortex excitability preceding voluntary ramp-and-hold plantarflexion in man. Acta Physiol Scand 146:399–400
Nielsen J, Petersen N, Deuschl G, Ballegaard M (1993) Task-related changes in the effect of magnetic brain stimulation on spinal neurones in man. J. Physiol 471:223–243
Nielsen J, Petersen N, Ballegaard M (1995) Latency of effects evoked by electrical and magnetic brain stimulation in lower limb motoneurones in man. J Physiol 484:791–802
Palmer E, Cafarelli E, Ashby P (1994) The processing of human ballistic movements explored by stimulation over the cortex. J Physiol 481(pt 2):509–520
Palmer E, Downes L, Ashby P (1996) Associated postural adjustments are impaired by a lesion of the cortex. Neurology 46:471–475
Pal’tsev YI, El’ner AN (1967) Preparatory and compensatory period during voluntary movement in patients with involvement of the brain of different localizations. Biophysics 12:161–168
Petersen N, Christensen LO, Nielsen J (1998) The effect of transcranial magnetic stimulation on the soleus H reflex during human walking. J Physiol 513(pt 2):599–610
Petersen NT, Pyndt HS, Nielsen JB (2003) Investigating human motor control by transcranial magnetic stimulation. Exp Brain Res 152:1–16
Pierrot-Deseilligny E, Lacert P, Cathala HP (1971) Amplitude and variability of monosynaptic reflexes before a voluntary movement. Physiol Behav 7:495–508
Soto O, Valls Solé J, Shanahan P, Rothwell J (2006) Reduction of intracortical inhibition in soleus muscle during postural activity. J Neurophysiol 96:1711–1717
Schneider C, Lavoie BA, Barbeau H, Capaday C (2004) Timing of cortical excitability changes during the reaction time of movements superimposed on tonic motoractivity. J Appl Physiol 97:2220–2227
Taylor JL (2005) Independent control of voluntary movements and associated anticipatory postural responses in a bimanual task. Clin Neurophysiol 116:2083–2090
Ugawa Y, Rothwell JC, Day BL, Thompson PD, Marsden CD (1991) Percutaneous electrical stimulation of corticospinal pathways at the level of the pyramidal decussation in humans. Ann Neurol 29:418–427
Viallet F, Massion J, Massarino R, Khalil R (1992) Coordination between posture and movement in a bimanual load lifting task: putative role of a medial frontal region including the supplementary motor area. Exp Brain Res 88:674–684
Acknowledgments
This study was supported by grants from the Danish Research Council, The Elsass foundation, The Novo Nordisk Foundation and The Danish Society of Multiple Sclerosis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petersen, T.H., Rosenberg, K., Petersen, N.C. et al. Cortical involvement in anticipatory postural reactions in man. Exp Brain Res 193, 161–171 (2009). https://doi.org/10.1007/s00221-008-1603-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-008-1603-6