Abstract
As a result of intrafusal thixotropy, muscle contraction at a short length followed by passive lengthening enhances the subsequent tonic vibration reflex (TVR). We studied the effects of muscle vibration, contraction, and their combination on the subsequent TVR in the left biceps in 20 healthy men. The preceding vibration (20 or 80 Hz) conditioning at a short or long length was applied to the muscle belly with and without a contraction. After conditioning, distal tendon vibration (80 Hz) was used to elicit the TVR at the test length. The strength of the TVR was measured by surface electromyography. Conditioning with 80-Hz vibration at a short length followed by passive lengthening enhanced the subsequent TVR, which was greater in the presence than in the absence of a conditioning contraction. These results suggest that vibration and contraction work synergistically to develop intrafusal thixotropy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Both extra- and intrafusal muscle fibers have a resting tension that is dependent on the history of muscle contraction (Proske et al. 1993; Campbell and Lakie 1998). Passive stiffness of a muscle after contraction depends on whether the muscle was contracted immediately beforehand at a short length (hold-short conditioning with a contraction) or at a long length (hold-long conditioning with a contraction) (Gregory et al. 1988; Proske et al. 2000). Hold-short conditioning with a contraction followed by passive lengthening results in increased stiffness of the muscle owing to removal of any preexisting slack, whereas hold-long conditioning with a contraction followed by passive shortening deliberately introduces slack in muscle fibers. This property, known as thixotropy, is affected by the detachment and formation of long-term stable cross-bridges between actin and myosin filaments (Hill 1968; Whitehead et al. 2001). Muscle contraction promotes the detachment of these stable cross-bridges (Gregory et al. 1998; Hagbarth and Nordin 1998), and the muscle needs to be held at the length where the contraction occurred for a few seconds to promote the reformation of cross-bridges (Hagbarth and Nordin 1998).
This history-dependent property of intrafusal muscle fibers, intrafusal thixotropy, affects the sensitivity of muscle spindles (Hagbarth et al. 1985; Gregory et al. 1988; Proske et al. 1993). Hold-short conditioning with a contraction followed by stretching the muscle increases the afferent discharge from the spindle, while hold-long conditioning with a contraction reduces the discharge (Gregory et al. 1988; Morgan et al. 1991; Proske et al. 1992). These history-dependent afferent discharges from muscle spindles are responsible for limb position-sense errors following conditioning (Gregory et al. 1988; Gooey et al. 2000). After hold-short conditioning with a contraction of the biceps muscle, subjects perceive that their conditioned forearms are placed in a more extended position than they actually are (Gregory et al. 1988; Ishihara et al. 2004; Yasuda et al. 2006; Sekihara et al. 2007).
Intrafusal thixotropy also affects the vibration sensitivity of muscle spindle primary endings in humans and cats (Morgan et al. 1991; Burke and Gandevia 1995; Nordin and Hagbarth 1996; Proske and Gregory 1999; Gooey et al. 2000). Mechanical vibration applied to the tendon or belly of a skeletal muscle induces an involuntary tonic reflex contraction in the muscle, which is referred to as the tonic vibration reflex (TVR) (Eklund and Hagbarth 1966). It has been reported that the magnitude of the TVR is altered by intrafusal thixotropy. Nordin and Hagbarth (1996) demonstrated in humans that hold-short conditioning with a contraction results in a greater TVR response compared with hold-long conditioning with a contraction.
Muscle vibration by itself helps introduce muscle thixotropy by means of its shaking effects on muscles (Gregory et al. 1988). A human study by Eklund and Hagbarth (1966) reported residual facilitation of the TVR following muscle vibration. However, in that paper, the muscle was not systematically preconditioned before each test. Since the sensitivity of muscle spindles depends on muscle history, this sensitivity can be misinterpreted if the muscle is not systemically conditioned beforehand (Gooey et al. 2000). As a result, the role of muscle spindles in the residual facilitation of muscle vibration is unclear. The results of limb position-sense error experiments by Gregory et al. (1988) suggest that muscle vibration acts directly on intrafusal muscles to stimulate the release of cross-bridges and promote the thixotropic process. Accordingly, if muscle vibration has thixotropic aftereffects on the TVR, as does the conditioning contraction, the aftereffects of muscle vibration followed by a period in which reformation of stable cross-bridges occurs on the TVR should depend on whether the muscle was vibrated and relaxed beforehand at a short length or at a long length.
The aftereffects of muscle vibration may be cooperative with those of muscle contraction in thixotropically changing the intrafusal stiffness. Gregory et al. (1998) demonstrated that a voluntary contraction as weak as 10% of maximum effort was sufficient for the full thixotropic aftereffects of contraction on the subsequent stretch reflex. The amplitude of the reflex is not increased further by larger voluntary conditioning contractions. However, in our previous study, we found that limb position-sense errors caused by preceding hold-short conditioning with a forceful contraction are increased further by muscle vibration applied during the forceful contraction (Ishihara et al. 2004). This suggests that vibration and muscle contraction work together to change intrafusal stiffness.
The primary aim of the present study was to identify the aftereffects of muscle vibration at a short length on the TVR and to explore whether muscle vibration during a conditioning contraction at a short length provides further aftereffects on TVRs. We hypothesized that (1) muscle vibration at a short length followed by passive lengthening increases the subsequent TVR and (2) muscle vibration during muscle contraction at a short length increases the TVR further, even though the muscle is fully contracted. If muscle vibration acts in a thixotropic manner, muscle vibration at a long length conversely will not increase the TVR, which may support the idea that a peripheral mechanism of action is involved in the residual facilitation of muscle vibration. In the present study, we compared the aftereffects of muscle vibration on the TVR between hold-short conditioning and hold-long conditioning.
Methods
Participants
The study was performed on 20 healthy men (aged 21–30 years) and was approved by the ethics committee of Showa University. The subjects were aware of the purpose of the study, and all signed an informed consent form.
Mechanical arrangement
All subjects were blindfolded during the experiments. Each subject was seated comfortably on a chair with both elbows positioned in fixed places on a metal table apparatus (Yasuhisa device; Yasuhisa Koki, Tokyo, Japan), which consisted of two identical parallel tables. The height of the apparatus was adjusted so that the subject’s upper arms were both entirely resting on wooden triangular blocks, which sloped at a 30° angle (Fig. 1a). The left forearm was set with the palm facing upward on another block sloping at 30°, which was placed on the metal table. The left biceps was used for all experiments.
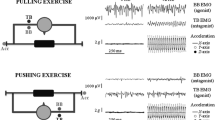
a Schematic representation of the experimental set-up. Each subject was comfortably seated on a chair in the upright position, as described in the “Methods”. The left biceps was used in all studies. b Surface electromyography (EMG) and mechanical vibration. The magnitude of the tonic vibration reflex (TVR) was estimated by surface EMG. Mechanical vibration was applied to the belly of the left biceps during the conditioning period and to the distal tendon during the subsequent TVR period. c Schema of the experiments designed to test the combined effects of preceding muscle vibration and muscle contraction on the TVR. At the start of each trial, the root mean squared (RMS) EMG activity of the left biceps during maximal voluntary contraction (MVC) was measured to normalize the RMS EMG activity during the following period. Preconditioning was performed at an elbow angle of 180° prior to each trial. After preconditioning, the left forearm was returned to the test position (120°) by an experimenter. The biceps was then shortened to 60° (hold-short conditioning) or stretched to 180° (hold-long conditioning) for the muscle belly to undergo vibration (20 or 80 Hz) with or without muscle contraction. After each maneuver, the muscle was returned to the test position (120°). Mechanical vibration, applied to the distal tendon (80 Hz), was started to elicit the TVR with the left forearm fixed at the test position (120°) by the experimenter. The vibration was continued for 120 s. The strength of the TVR was quantified by the average amplitude of RMS EMG activity during 30–120 s of vibration time. HLC: hold-long conditioning with a contraction; HLV20: hold-long conditioning with vibration at 20 Hz; HLV80: hold-long conditioning with vibration at 80 Hz; HSC: hold-short conditioning with a contraction; HSV20: hold-short conditioning with vibration at 20 Hz; and HSV80: hold-short conditioning with vibration at 80 Hz
Electromyography
Surface electromyography (EMG) was used to determine the magnitude of the TVR. EMG activity was recorded with surface electrodes (Vitrode L, Nihon Kohden, Tokyo, Japan). A pair of electrodes was placed on the belly of the left biceps muscle approximately 60 mm apart (Fig. 1b). An earth electrode was placed over the ulna styloid. The skin was cleaned before EMG measurements. The EMG signal was recorded using a data acquisition system (PowerLab 4/20, ADInstruments, Castle Hill, Australia) with a PowerLab bioamp (ML135; ADInstruments) and analyzed using Chart software (ADInstruments). The PowerLab bioamp was set to apply a 10-Hz high-pass filter and a 500-Hz low-pass filter, with a 50-Hz notch filter. The EMG signal was sampled at 1,000 Hz and smoothed using a root mean squared (RMS, 200 ms running time window) calculation with Chart software (RMS EMG).
At the start of each trial, the RMS EMG activity of the left biceps during maximal voluntary contraction (MVC) was measured to normalize the RMS EMG activity during the following period (Fig. 1c). Subjects were instructed to perform an isometric elbow flexor MVC lasting at least 3 s, with the left forearm locked at an elbow angle of 120°. The 10-s averaged RMS EMG activity immediately after preconditioning (see below) was defined as baseline in each trial (baseline RMG EMG in Fig. 1c). In each trial, the differences in RMS EMG activity from the baseline were normalized to the amplitude of RMS EMG activity during MVC (% MVC).
Vibration
We used an electrical vibrator (Minato Medical Science, Osaka, Japan), which had a motor-driven eccentric disk, and was enclosed in a plastic box measuring 65 × 32 × 27 mm. It produces mechanical oscillations at an amplitude of approximately 1 mm and frequencies of up to 100 Hz. The vibrator was positioned and pressed lightly by an experimenter on the muscle belly between the EMG electrodes or on the distal tendon of the left biceps, so that the vibrator did not interfere with the distal EMG electrode (Fig. 1b).
The sensitivity of muscle proprioceptors to vibration depends on vibration frequency. We used vibration frequencies of 80 and 20 Hz in this study. The 80-Hz vibration frequency was chosen because evidence from microneurography tests indicates that muscle spindle primary endings are optimally stimulated at 80 Hz (Roll et al. 1989). We also included the 20-Hz vibration frequency as a reference. This frequency, which is suboptimal to drive the Ia afferent discharge, optimally excites the secondary endings and Golgi tendon receptors in a one-to-one manner (Roll et al. 1989).
Preconditioning
All trials were preceded by a preconditioning maneuver designed to put elbow flexors into a defined state (Gregory et al. 1998). After the RMS EMG activity during MVC was measured at an elbow angle of 120° (Fig. 1c, MVC), the subject was then directed to exert isometric MVC at an elbow angle of 180° for 8 s (Fig. 1c, preconditioning). Following the contraction at 180°, the muscle was held in this position for 3 s. The left forearm was then slowly returned to 120° by an experimenter and placed on the block.
Hold-short conditioning
After MVC and preconditioning were completed, the left forearm was lifted by the experimenter to an elbow angle of 60° over 3 s. The subject was asked to exert MVC of the left biceps muscle for 8 s at this position while the experimenter held the subject’s wrist, and was then asked to relax the left biceps muscle fully for 3 s while the experimenter kept the forearm in this position. Vibration (20 or 80 Hz) was delivered to the belly of the left biceps while the subject isometrically contracted the muscle. The left forearm was then lowered carefully by the experimenter to an elbow angle of 120° over 3 s (Fig. 1c). In trials to identify the independent effect of vibration at a short length on the TVR, vibration (20 or 80 Hz) was delivered to an uncontracted bicep, which was kept at an elbow angle of 60° for 8 s, after which the muscle was allowed another 3-s relaxation period at this position before being lowered to 120°.
Hold-long conditioning
After MVC and preconditioning were completed, the left elbow was extended by an experimenter so that the left forearm was placed at an elbow angle of 180°. The subject was asked to exert MVC of the left biceps muscle for 8 s at this position against the force the experimenter applied by pressing the subject’s wrist, and then to relax it fully at this position for 3 s (Fig. 1c). Mechanical vibration (20 or 80 Hz) was applied to the belly of the left biceps at the time of the 8-s conditioning contraction. The left forearm was then raised by the experimenter to an angle of 120° over 3 s. In trials to identify the independent effect of vibration at a long length, vibration (20 or 80 Hz) was delivered to an uncontracted bicep, which was kept at an elbow angle of 180° for 8 s, after which the muscle was allowed another 3-s relaxation period at this position before being raised to 120°.
TVR
The TVR of the conditioned muscle was measured isometrically at an elbow angle of 120° (Fig. 1c). Vibration (80 Hz) applied to the distal tendon of the left biceps evoked a TVR after each maneuver. During the TVR, the left forearm was supported by the 30° block at an elbow angle of 120°, and forearm elevation was prevented by the experimenter. The strength of the TVR was quantified by RMS EMG activity (Fig. 1c). In each trial, RMS EMG activity was averaged over 30–120 s of vibration time using Chart software (average RMS EMG in Fig. 1c). Each trial was performed twice, and these two sets of RMS EMG data, standardized by MVC, were averaged for each subject.
Experimental protocol
Tonic vibration reflex was measured after each of the following ten maneuvers, which were performed in a random order: preconditioning followed by (1) hold-long conditioning with a contraction (HLC); (2) hold-long conditioning with vibration at 20 Hz (HLV20); (3) hold-long conditioning with vibration at 80 Hz (HLV80); (4) hold-long conditioning with a contraction and vibration at 20 Hz (HLC + HLV20); (5) hold-long conditioning with a contraction and vibration at 80 Hz (HLC + HLV80); (6) hold-short conditioning with a contraction (HSC); (7) hold-short conditioning with vibration at 20 Hz (HSV20); (8) hold-short conditioning with vibration at 80 Hz (HSV80); (9) hold-short conditioning with a contraction and vibration at 20 Hz (HSC + HSV20); and (10) hold-short conditioning with a contraction and vibration at 80 Hz (HSC + HSV80), as shown schematically in Fig. 1c. Comparisons were made between the magnitudes of RMS EMG activity during the TVR following the ten maneuvers, with RMS EMG activity after the HLC maneuver used as a control.
Statistical analysis
Results are expressed as mean ± SD. RMS EMG activity, standardized by MVC, was analyzed by one-way analysis of variance (ANOVA) for repeated measures followed by Tukey–Kramer multiple comparisons test (InStat; GraphPad, San Diego, USA). Within-factor (vibration and contraction) effects and interaction between the two effects were tested by two-way ANOVA for repeated measures with the Huynh-Feldt correction (SPSS, SPSS Japan, Tokyo, Japan). Significance was set at P < 0.05.
Results
A typical example of RMS EMG signals of the left biceps from one subject is shown in Fig. 2. The application of mechanical vibration (80 Hz) to the distal tendon of the left biceps increased RMS EMG activity, suggesting that the vibration stimulus elicited TVR in the muscle. After hold-long conditioning, the increase in RMS EMG activity during the subsequent TVR period was similar between the maneuvers (Fig. 2a–c). After hold-short conditioning, increases in RMS EMG activity were greater than those after hold-long conditioning maneuvers (Fig. 2d–f), with the exception of the HSV20 maneuver (data not shown). In particular, an increase in RMS EMG activity was noted after the HSC + HSV80 maneuver (Fig. 2f). Hold-short conditioning with vibration at 80 Hz, without a contraction (HSV80 maneuver), also enhanced the effects on RMS EMG activity during a subsequent TVR period (Fig. 2e), whereas vibration at 80 Hz at the long length (HLV80 maneuver) failed to enhance the aftereffects on RMS EMG activity (Fig. 2b).
Examples of RMS EMG activity during the TVR period following six of the ten maneuvers, as performed by a single subject. a–f RMS EMG activity increases in response to the 80-Hz tendon vibration, suggesting that the TVR is elicited. The increase in RMS EMG activity is greatest after conditioning with a contraction and vibration at a frequency of 80 Hz at a short length (HSC + HSV80 maneuver, f)
Figure 3 illustrates the pooled results of RMS EMG activity during the TVR period after the ten maneuvers. Figure 3a shows the mean ± SD RMS EMG activity during the TVR period after the ten maneuvers in each participant (n = 20). Figure 3b shows that the TVR after hold-long conditioning with vibration (HLV20 and HLV80 maneuvers) was similar to that after hold-long conditioning with a contraction (HLC maneuver). Figure 3c shows that muscle vibration during hold-long conditioning contraction provided no additional effects on the TVR. In contrast, Fig. 3d and e confirm the remarkable changes in RMS EMG activity after hold-short conditioning; especially the HSC + HSV80 maneuver compared with the HLC maneuver. There were significant differences in the magnitudes of RMS EMG activity during the TVR period between the HLC maneuver and the HSV80 maneuver (P < 0.05) and between the HSV20 maneuver and the HSV80 maneuver (P < 0.05) (Fig. 3d), suggesting that vibration at 80 Hz by itself increases TVR when the vibration is applied at a short muscle length. Figure 3e shows that the magnitude of RMS EMG during the TVR period was greater after the HSC maneuver than after the HLC maneuver (P < 0.05). Furthermore, the magnitude of RMS EMG activity was greater after the HSC + HSV80 maneuver than after both the HLC and the HSC maneuvers (P < 0.05), suggesting that vibration at 80 Hz during hold-short conditioning contraction further enhances the effects on the TVR.
The pooled results of average amplitude of RMS EMG activity during the TVR period after the ten maneuvers. a 90-s averaged RMS EMG activity during the TVR period (30–120 s) in each participant. Muscle vibration at the long length without (b) or with (c) voluntary muscle contraction (HLV20 or HLV80 maneuver) did not further increase the RMS EMG activity compared with the HLC maneuver. d Muscle vibration at 80 Hz at the short length (HSV80 maneuver) increased RMS EMG activity during the following TVR period compared with muscle contraction at the long length (HLC maneuver). e Muscle contraction at the short length (HSC maneuver) increased RMS EMG activity compared with the HLC maneuver. Muscle vibration at 80 Hz at the short length provided a further increase in RMS EMG activity when combined with muscle contraction (HSC + HSV80 maneuver) compared with muscle contraction alone at the short length (HSC maneuver). RMS EMG activity was normalized to the amplitude of RMS EMG activity during maximal voluntary contraction (% MVC). Values are mean ± SD (n = 20). *P < 0.05: one-way analysis of variance (ANOVA) for repeated measures followed by Tukey–Kramer multiple comparisons test; N.S. not significant by one-way ANOVA
We then analyzed whether the effect of vibration on TVR differs in the absence (Passive) or presence (Contract) of a conditioning contraction (Fig. 4a, b). During hold-long conditioning, there was no contraction effect, vibration effect, or any interaction between the two effects (Fig. 4a). In contrast, during hold-short conditioning, there was a statistically significant interaction between the contraction and vibration effects (Fig. 4b, two-way ANOVA, # P < 0.05), suggesting that vibration effects that enhance TVR are greater in the presence than in the absence of a conditioning contraction.
Differences in vibration effects on the subsequent TVRs in the absence (Passive) and presence (Contract) of muscle contraction when conditioning occurred at elbow angles of 180° (long muscle length) and 60° (short muscle length). a When conditioning occurred at 180°, the magnitude of the TVR after muscle vibration at 80 Hz did not change compared with that after muscle vibration at 20 Hz, regardless of whether the muscle was passive (Passive) or contracted (Contract). Two-way ANOVA shows that there is no vibration effect, contraction effect, or interaction between the two effects. b When conditioning occurred at 60°, the magnitude of the TVR after muscle vibration at 80 Hz (HSV80) was larger than that after muscle vibration at 20 Hz (HSV20). In addition, muscle vibration at 80 Hz elicited a larger increase in the magnitude of the TVR when combined with muscle contraction (Contract) than when muscle was passive (Passive). Two-way ANOVA showed that there is a vibration effect (*P < 0.05), a contraction effect (! P < 0.05), and an interaction between the two effects (# P < 0.05). This interaction suggests that the enhancing effect of muscle vibration at 80 Hz on the subsequent TVR is stronger in the presence than in the absence of muscle contraction at the short length. Values are mean ± SD (n = 20)
Discussion
This series of experiments revealed that (1) muscle vibration at 80 Hz at a short length followed by passive lengthening increased the subsequent TVR and (2) muscle vibration at 80 Hz at a short length during forceful conditioning contraction provided a further increase in the subsequent TVR.
In the present study, all trials were started with the biceps in a slack state. The tested muscle underwent preconditioning at a stretched position (180°) beforehand so that the intrafusal fibers of its spindles were slack. Thus, the spindles in the biceps were in an insensitive state before any conditioning maneuver was performed. The HLC maneuver, which is identical to the preconditioning maneuver, was used as a control to identify the effects of vibration and contraction on the subsequent TVR. Muscle spindle sensitivity after the preconditioning plus the HLC maneuver was presumably identical to that after preconditioning. Under these conditions, secondary endings independent of the muscle history (Proske et al. 1992) may have responded to mechanical vibration during the TVR period. However, it has been reported that secondary endings are less sensitive to mechanical vibration than primary endings (Roll et al. 1989; Proske and Gregory 1999). The TVR after the HLC maneuver was small, but it was not eliminated.
The HSC maneuver enhanced the RMS EMG activity of the muscle during a subsequent TVR period compared with the HLC maneuver. This result confirms earlier observations from other reports of intrafusal muscle thixotropy. Morgan et al. (1991) showed that the responses of muscle spindles to a locally applied vibration depended on the immediate history of contraction and length changes in the cat soleus muscle; the hold-short conditioning with a contraction greatly increased the vibration sensitivity. Nordin and Hagbarth (1996) showed that the TVR elicited by vibration of a human finger extensor was increased when preceded by hold-short conditioning with a contraction, which was consistent with microneurographic recordings (Burke and Gandevia 1995). Gooey et al. (2000) showed that the illusion of muscle lengthening evoked by muscle vibration increases after flexion conditioning (that is, hold-short conditioning with a contraction of the biceps). Proske and colleagues explain this in terms of intrafusal thixotropy as follows. When stable cross-bridges of intrafusal fibers of a muscle are allowed to form while the muscle is held at a short length after a voluntary contraction, this leaves the intrafusal fibers taut on returning the muscle to an intermediate length. The increased stiffness of intrafusal fibers allows more effective transmission of a vibratory stimulus to the sensory endings (Proske and Gregory 1999; Gooey et al. 2000). Therefore, the responsiveness of the Ia afferents to the vibration is increased after the HSC maneuver.
Our study revealed that vibration enhanced the TVR when the muscle was vibrated at a short muscle length (the HSV80 maneuver), which was consistent with the work of Eklund and Hagbarth (1966), who used an unconditioned muscle. However, vibrating the muscle at a long length (the HLV80 maneuver) did not enhance TVR. This discrepancy suggests that the enhancing effects were not simply a result of the vibratory stimulus itself. Hagbarth and Nordin (1998) showed that mechanical vibration of a muscle at a short length was followed by an involuntary aftercontraction, which is believed to be a result of intrafusal thixotropy. Our finding supports the idea by Gregory et al. (1988) that muscle vibration acts directly on intrafusal muscles to stimulate the release of cross-bridges and to promote the thixotropic process.
Our study also demonstrated that muscle vibration at 80 Hz combined with the HSC maneuver (the HSC + HSV80 maneuver) further increased the subsequent TVR. This is consistent with our previous study showing that thixotropic limb position sense errors are increased when muscle vibration is applied in combination with hold-short conditioning contraction (Ishihara et al. 2004). Moreover, our results indicate that the effects of a contraction and mechanical vibration on the TVR are synergistic when hold-short conditioning occurred (Fig. 4b). The enhancing effect of vibration at 80 Hz on the TVR was more obvious in the presence than in the absence of a hold-short conditioning contraction. Intrafusal contractions are the most effective method of removing preexisting slack by detaching stable cross-bridges and allowing them to reform at the short length (Gregory et al. 1988). Our subjects voluntarily contracted the biceps during conditioning. Thus, the intrafusal fibers of the conditioned muscle likely contracted because of the α–γ linkage. Gregory et al. (1998) showed that preexisting slack in intrafusal fibers of human soleus muscle is eliminated by 10% MVC of the muscle at a short length and that a 10% contraction produces effects similar to a 25% contraction. They concluded that a voluntary contraction as weak as 10% MVC is sufficient to remove the slack. We have discussed our findings in terms of passive properties that are attributable to cross-bridge kinetics. However, the increased TVR after muscle vibration and contraction may partly arise through central mechanisms because our subjects contracted the biceps with 100% MVC during conditioning.
Two major steps are required to elicit a TVR (Burke et al. 1976). First, the vibrator activates the muscle spindle to produce an Ia afferent discharge signal. Second, muscle spindle discharges are sent to the spinal cord where they activate monosynaptic and polysynaptic circuits, causing the muscle to contract. Thus, the post-vibration or post-contraction excitation of motoneurons (Sapirstein et al. 1937; Hutton and Suzuki 1979; Enoka et al. 1980) may be factors that change the TVR after conditioning. Spinal motoneurons can exhibit self-sustained depolarization after vibration or contraction, referred to as a plateau potential owing to persistent inward currents (Kiehn and Eken 1997). Plateau potential-like behavior may have contributed to post-conditioning excitability of the motoneurons. Recent animal work has shown that the persistent inward currents are greatly reduced by stretching the antagonist muscle (Hyngstrom et al. 2007). This would mean that the passive movement from a long to an intermediate length of the biceps stretches the triceps and turns off any additional post-conditioning motoneurons excitability. Aside from this, involuntary contraction after conditioning may be partly explained by a persistent excitatory state in cortical motoneurons (Sapirstein et al. 1937). Neural circuits that involve the TVR are also located at a supra-segmental level (Eklund et al. 1982). The measurement of motor evoked potentials of the biceps using transcranial magnetic stimulation would be useful to investigate whether conditioning changes the subsequent corticospinal excitability (Steyvers et al. 2003).
One may raise a question why the vibration that evokes the TVR at the test length does not abolish the thixotropic effects of preceding conditioning. For example, it might be expected that after hold-long conditioning, the TVR is initially depressed and, as the vibration continues, the TVR increases. However, this did not occur. In our study, mechanical vibration was applied to the belly of the left biceps during the conditioning period and to the distal tendon during the TVR period. We speculate that pressure generated by muscle belly vibration is more directly transmitted to stable cross-bridges and more efficiently detaches these cross-bridges compared with tendon vibration.
It is important to consider whether limitations in our methodology influenced our results. There is a postural aftercontraction that occurs after muscle contraction at a short length or mechanical vibration (Gilhodes et al. 1992; Hagbarth and Nordin 1998). Although we have previously reported that the aftercontraction becomes strong after hold-short conditioning with a contraction and muscle vibration (Ishihara et al. 2004), our current study did not differentiate such an aftercontraction from the TVR response. Thus, the changes in EMG activity of the biceps potentially included this type of aftercontraction. Nevertheless, it is certain that muscle vibration at a short length promotes intrafusal thixotropy because the aftercontraction is also because of intrafusal thixotropy (Hagbarth and Nordin 1998).
In conclusion, muscle vibration at 80 Hz at a short length followed by passive lengthening increases subsequent TVRs. When this vibration was applied during muscle contraction, it increased the TVR further, even though the muscle was fully contracted. The effects of vibration on the TVR are greater in the presence than in the absence of a conditioning contraction. Our results suggest that (1) intrafusal thixotropy is involved in the residual facilitation of muscle vibration on the TVR and (2) muscle vibration and contraction work together synergistically to develop intrafusal muscle thixotropy after hold-short conditioning.
References
Burke D, Gandevia SC (1995) The human muscle spindle and its fusimotor control. In: Ferrell W, Proske U (eds) Neural control of movement. Plenum Press, New York, pp 19–25
Burke D, Hagbarth KE, Lofstedt L, Wallin BG (1976) The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol 261:673–693
Campbell KS, Lakie M (1998) A cross-bridge mechanism can explain the thixotropic short-range elastic component of relaxed frog skeletal muscle. J Physiol 510:941–962
Eklund G, Hagbarth KE (1966) Normal variability of tonic vibration reflexes in man. Exp Neurol 16:80–92
Eklund G, Hagbarth KE, Hagglund JV, Wallin EU (1982) The ‘late’ reflex responses to muscle stretch: the ‘resonance hypothesis’ versus the ‘long-loop hypothesis’. J Physiol 326:79–90
Enoka RM, Hutton RS, Eldred E (1980) Changes in excitability of tendon tap and Hoffmann reflexes following voluntary contractions. Electroencephalogr Clin Neurophysiol 48:664–672
Gilhodes JC, Gurfinkel VS, Roll JP (1992) Role of Ia muscle spindle afferents in post-contraction and post-vibration motor effect genesis. Neurosci Lett 135:247–251
Gooey K, Bradfield O, Talbot J, Morgan DL, Proske U (2000) Effects of body orientation, load and vibration on sensing position and movement at the human elbow joint. Exp Brain Res 133:340–348
Gregory JE, Morgan DL, Proske U (1988) Aftereffects in the responses of cat muscle spindles and errors of limb position sense in man. J Neurophysiol 59:1220–1230
Gregory JE, Wise AK, Wood SA, Prochazka A, Proske U (1998) Muscle history, fusimotor activity and the human stretch reflex. J Physiol 513:927–934
Hagbarth KE, Hagglund JV, Nordin M, Wallin EU (1985) Thixotropic behaviour of human finger flexor muscles with accompanying changes in spindle and reflex responses to stretch. J Physiol 368:323–342
Hagbarth KE, Nordin M (1998) Postural after-contractions in man attributed to muscle spindle thixotropy. J Physiol 506:875–883
Hill DK (1968) Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. J Physiol 199:637–684
Hutton RS, Suzuki S (1979) Postcontraction discharge of motor neurons in spinal animals. Exp Neurol 64:567–578
Hyngstrom AS, Johnson MD, Miller JF, Heckman CJ (2007) Intrinsic electrical properties of spinal motoneurons vary with joint angle. Nat Neurosci 10:363–369
Ishihara Y, Izumizaki M, Atsumi T, Homma I (2004) Aftereffects of mechanical vibration and muscle contraction on limb position-sense. Muscle Nerve 30:486–492
Kiehn O, Eken T (1997) Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol 78:3061–3068
Morgan DL, Proske U, Gregory JE (1991) Responses of primary endings of cat muscle spindles to locally applied vibration. Exp Brain Res 87:530–536
Nordin M, Hagbarth KE (1996) Effects of preceding movements and contractions on the tonic vibration reflex of human finger extensor muscles. Acta Physiol Scand 156:435–440
Proske U, Gregory JE (1999) Vibration sensitivity of cat muscle spindles at short muscle lengths. Exp Brain Res 124:166–172
Proske U, Morgan DL, Gregory JE (1992) Muscle history dependence of responses to stretch of primary and secondary endings of cat soleus muscle spindles. J Physiol 445:81–95
Proske U, Morgan DL, Gregory JE (1993) Thixotropy in skeletal muscle and in muscle spindles: a review. Prog Neurobiol 41:705–721
Proske U, Wise AK, Gregory JE (2000) The role of muscle receptors in the detection of movements. Prog Neurobiol 60:85–96
Roll JP, Vedel JP, Ribot E (1989) Alteration of proprioceptive messages induced by tendon vibration in man: a microneurographic study. Exp Brain Res 76:213–222
Sapirstein M, Herman R, Wallace G (1937) A study of after-contraction. Am J Physiol 119:549–561
Sekihara C, Izumizaki M, Yasuda T, Nakajima T, Atsumi T, Homma I (2007) Effect of cooling on thixotropic position-sense error in human biceps muscle. Muscle Nerve 35:781–787
Steyvers M, Levin O, Verschueren SM, Swinnen SP (2003) Frequency-dependent effects of muscle tendon vibration on corticospinal excitability: a TMS study. Exp Brain Res 151:9–14
Whitehead NP, Gregory JE, Morgan DL, Proske U (2001) Passive mechanical properties of the medial gastrocnemius muscle of the cat. J Physiol 536:893–903
Yasuda T, Izumizaki M, Ishihara Y, Sekihara C, Atsumi T, Homma I (2006) Effect of quadriceps contraction on upper limb position sense errors in humans. Eur J Appl Physiol 96:511–516
Acknowledgments
We thank Dr. Uwe Proske for reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakajima, T., Izumizaki, M., Sekihara, C. et al. Combined effects of preceding muscle vibration and contraction on the tonic vibration reflex. Exp Brain Res 192, 211–219 (2009). https://doi.org/10.1007/s00221-008-1571-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-008-1571-x