Abstract
Although several features of brain motor function appear to be preserved even in chronic complete SCI, previous functional MRI (fMRI) studies have also identified significant derangements such as a strongly reduced volume of activation, a poor modulation of function and abnormal activation patterns. It might be speculated that extensive motor imagery training may serve to prevent such abnormalities. We here report on a unique patient with a complete traumatic SCI below C5 who learned to elicit electroencephalographic signals β-bursts in the midline region upon imagination of foot movements. This enabled him to use a neuroprosthesis and to “walk from thought” in a virtual environment via a brain–computer interface (BCI). We here used fMRI at 3T during imagined hand and foot movements to investigate the effects of motor imagery via persistent BCI training over 8 years on brain motor function and compared these findings to a group of five untrained healthy age-matched volunteers during executed and imagined movements. We observed robust primary sensorimotor cortex (SMC) activity in expected somatotopy in the tetraplegic patient upon movement imagination while such activation was absent in healthy untrained controls. Sensorimotor network activation with motor imagery in the patient (including SMC contralateral to and the cerebellum ipsilateral to the imagined side of movement as well as supplementary motor areas) was very similar to the pattern observed with actual movement in the controls. We interpret our findings as evidence that BCI training as a conduit of motor imagery training may assist in maintaining access to SMC in largely preserved somatopy despite complete deafferentation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Several approaches have been proposed to promote recovery of functions following spinal cord injury (SCI) (Kirshblum et al. 2007). Based on the concept of a “central pattern generator,” activation of spinal cord circuitry independent of higher centres represents one strategy (Kalb 2003). An alternative approach involves the use of brain–computer interfaces (BCI) to bypass the area of damage which causes a disconnection of transmission (Lauer et al. 1999; Friehs et al. 2004; Neuper et al. 2006; Birbaumer and Cohen 2007). Here, patients need to learn to voluntarily control a specific movement via electroencephalographic (EEG) signals (Pfurtscheller et al. 2000; Birbaumer and Cohen 2007).
Although several features of brain motor function appear to be preserved even in chronic complete SCI (Sabbah et al. 2002; Alkadhi et al. 2005; Hotz-Boendermaker et al. 2008), other functional MRI (fMRI) studies have identified significant derangements such as a strongly reduced volume of activation, a poor modulation of function and abnormal activation patterns (Cramer et al. 2005). It can be speculated that motor imagery training (Sharma et al. 2006; de Vries and Mulder 2007) may serve to prevent such abnormalities and a recent study has already confirmed such effects on brain function (Cramer et al. 2007). To provide further support for this assumption, we here report on a unique SCI patient who learned to elicit EEG β-bursts in the midline region upon imagination of foot movements. This enabled him to use a neuroprosthesis and to “walk from thought” in a virtual environment (Pfurtscheller et al. 2003, 2006; Leeb et al. 2007). We here used fMRI during imagined hand and foot movements to investigate the effects of persistent BCI training as a conduit of motor imagery training over 8 years on brain motor function and compared these findings to a group of five untrained healthy age-matched volunteers during executed and imagined movements.
Materials and methods
Patient
The tetraplegic patient studied was a 31-year-old male with a complete traumatic SCI below C5 and an incomplete lesion below C4 since April 1998 due to a spinal cord compression by bone fragments. He had started BCI training in 1999 with different types of motor imagery (Pfurtscheller et al. 2000). First, he had been asked to imagine left versus right hand movements for 28 sessions (each containing 160 trials) which led him to achieve a classification accuracy of approximately 65% (correctly classified trials). Over the next 24 sessions, the strategies were varied, e.g. they consisted of imagination of left and right hand movements versus no specific imagination (thinking “nothing”) or imagination of left foot versus right hand movement. This led to a slight classification improvement to an average of about 75% correctly classified trials. Finally, with the beginning of session 54, the strategy was changed to imagine movement of both feet versus the right hand which led to an improvement of classification accuracy to approximately 95%. In the end, thousands of foot movement imaginations during prolonged training over several months resulted in the induction of stable midcentral focused beta-oscillations with a dominant frequency of 17 Hz. A series of follow-up experiments demonstrated that the induction of these oscillations upon foot movement imagination were reproducible. Finally, the patient also learned to use the BCI for controlling a prosthetic device applied to his paralyzed left hand via functional electrical stimulation (as exemplarily shown in Fig. 1a) and to move in virtual environments upon imagination of foot movements (Pfurtscheller et al. 2003, 2005, 2006; Leeb et al. 2007).
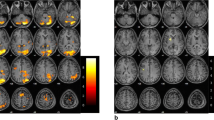
Maps of brain activation associated with imagery of foot and hand movements in the tetraplegic SCI subject after extensive BCI training. a Illustration of the possibility to use the BCI system to allow the patient grasping a glass with the paralyzed hand upon activation of a neuroprosthesis by EEG β-bursts of 17 Hz induced by foot movement imagination. These impulses are “translated” to hand/finger movements by functional electric stimulation (FES; with elbow movements accommodated by residual motor function by the patient). Brain activation maps associated with imagery of movement of the right (b) and left (c) foot and of the right (d) and left (e) hand relative to rest in the patient. Brain activation in expected somatopy in the primary sensorimotor cortex (SMC) contralateral to the side of imagined movement, supplementary and cingulate motor areas bilaterally and the cerebellum ipsilateral to the side of imagined movement becomes apparent (Z > 5.0; corrected P = 0.01; functional data overlaid on high resolution scans). These findings were confirmed by a model-free ICA-based exploratory data analysis (f–i). Brain activation elicited by active movement of the right foot (j) and dominant right hand (k) in the control group of five healthy age- and sex-matched subjects (Z > 5.0; corrected P = 0.01; functional data overlaid on mean high resolution scan). Substantial overlap in the activation patterns between the patient with covert and the controls with overt movement become apparent (compare to b and d, respectively). (All images shown in radiological convention; left side of the image is right side of the brain as indicated by “R” and “L”)
At clinical examination, the patient demonstrated residual volitional muscle activation in the left upper extremity [shoulder: active abduction and flexion up to 90° (grade 4/5); elbow: active flexion (grade 4/5), pro- and supination possible (partly trick movements); M. extensor carpi radialis (grade 1/5); all other muscles (grade 0/5)], with full or almost full passive range of motion preserved in all joints, corresponding to American Spinal Injury Association (ASIA) upper-extremity and lower-extremity motor subscores of 5/50 and 0/50, respectively, and an ASIA Impairment Scale grade of A (http://www.asia-spinalinjury.org).
To confirm the extent and location of cerebrospinal disconnection, the patient also underwent somatosensory evoked potential recordings (SEPs) and motor evoked potential recordings (MEPs). SEPs confirmed complete central conduction block whereas distal conduction was preserved. SEPs were obtained by stimulating the median nerve at the wrist and the posterior tibial nerve at the ankle with frequencies of 5 and 2 Hz, respectively (Suga et al. 1999). Recording electrodes were placed over Erb’s point, the seventh cervical vertebra, and C3’ or C4’ over the somatosensory cortex. For the lower extremities, the electrodes were placed over L1 and Cz’. Fz was used as the reference for all electrodes. The amplifier averaged at 256 for the upper and 512 for the lower extremities. Two trials were superimposed to establish reproducibility. MEPs with cortical stimulation and recording from the upper and lower extremities on either side (hypothenar and M. tibialis anterior, respectively) also were consistent with complete central conduction block. Magnetic stimuli were applied using double cone coils for stimulation of the motor cortex for the upper and lower extremities, and for elicitation of the lumbar root (L5) (Tobimatsu et al. 1998). The target muscles were the abductor pollicis brevis for the hand and the tibialis anterior for the legs. No stimulus could be applied over the seventh cervical vertebra due to safety reasons (metal implant in the cervical region).
Controls
Five 30- to 34-year-old right-handed and right-footed males free of neuropsychiatric disease acted as control group. Their neurologic exam and brain scan were normal.
Magnetic resonance imaging
Imaging was performed on a 3.0T Tim Trio system (Siemens Medical Systems, Erlangen, Germany) using a 12-element head coil. In each session, 210 functional volumes were obtained with a single shot gradient echo EPI sequence (TR = 3,000 ms, TE = 30 ms, FA = 90°, matrix size = 64 × 64, pixel size = 3.0 mm × 3.0 mm × 3.0 mm) within a scanning time of 10 min and 30 s. The first two volumes were discarded to account for T1 saturation effects. Stimuli were projected on a screen positioned at the rear of the scanner, which the subjects could comfortably see through a mirror mounted on the head coil. A high resolution image was acquired to allow functional image registration for precise localization of activations (T1-weighted 3D MPRAGE sequence; TR = 1,900 ms, TE = 2.6 ms, TI = 900 ms; 1 mm × 1 mm × 1 mm isotropic resolution). There was no evidence for structural brain damage on conventional FLAIR, T2- and T2*-weighted images in the patient and brain scans were reported as normal in the controls.
Study design
The main paradigm consisted of imagery of unilateral foot and hand movements. The patient and the healthy subjects were instructed to imagine smooth flexion and extension of the wrist or the ankle, respectively, paced by a visual cue at 1 Hz. During one run of approximately 10 min, 210 functional volumes were acquired using a simple boxplot design, where imagery of movement of either side alternated with interspersed periods of absolute rest, during which subjects fixated a flashing cross (ArBrArBr, with A and B representing five blocks of 30 s each for movement imagery of the right and left side, respectively, and r indicating rest periods). During the first run, the patient and the healthy subjects were asked to imagine foot movements in this way. The second run was devoted to imagery of hand movements in the same fashion. The third and forth run consisted of passive ankle and wrist movements of either side at 1 Hz by the experimenter (range of motion 20° and 15°, respectively) in the patient, and active ankle and wrist movements of either side at the same rate and range in the healthy subjects, again according to the block design as described above. To avoid stimulus-correlated motion, the individuals’ heads were secured with Velcro straps in a foam-cushioned holder; the knees were flexed to approximately 135° using a soft roll placed beneath the knees and the feet and forearms were fixed in a purpose-built apparatus. Participants were familiarized with the paradigm prior to entering the scanner.
FMRI data analysis
General linear model
Functional imaging analysis was carried out using FMRI Expert Analysis Tool (FEAT; version 5.63, part of FMRIB’s Software Library FSL, http://www.fmrib.ox.ac.uk/fsl). The following pre-statistical processing was applied: motion correction using MCFLIRT; non-brain removal using BET; spatial smoothing using a Gaussian kernel of 5 mm full-width half maximum; global (volumetric) multiplicative mean intensity renormalization; high pass temporal filtering (Gaussian-weighted least squares straight line fitting, with sigma 50.0 s). Time-series statistical analysis was carried out using FILM with local autocorrelation correction. Registration to high resolution and/or standard images was carried out using FLIRT. Higher-level analysis in the controls was carried out using a fixed effects model, by forcing the random effects variance to 0 in FMRIB’s Local Analysis of Mixed Effects (FLAME Beckmann et al. 2003; Woolrich et al. 2004). Z (Gaussianized T/F) statistic images were thresholded using clusters determined by Z > 5.0 and a (corrected) cluster significance threshold of P = 0.01 (Worsley et al. 1992), unless otherwise stated. Motion parameters were used as a covariate of no interest in the analyses.
Independent component analysis
Independent component analysis-based exploratory data analysis was carried out using Multivariate Exploratory Linear Decomposition into Independent Components (MELODIC) Version ln(11), an implementation for the estimation of a probabilistic independent component analysis model, in order to investigate the possible presence of unexpected artefacts or activation [alternative hypothesis testing at P < 0.05; detailed information in Beckmann and Smith (2004)].
Region of interest analysis
Functional ROIs selected from the activation clusters obtained with the contrasts of hand and foot movement in the controls and imagery of respective movements in the patient versus rest were applied to the first level analyses to compute the mean signal change at the coordinates of the activation peak using FEATQUERY, which is part of FSL.
Results
Motor imagery in the patient
The SEPs and MEPs confirmed complete central conduction block in the tetraplegic patient. Contrasts of imagery of repetitive hand (Fig. 1b, c) and foot (Fig. 1d, e) movements versus rest in the patient demonstrated significant activation of sensorimotor networks similar to the patterns defined by contrasts of active movement versus rest in the healthy control group (Fig. 1f, g—data for left sided movement not shown as analogue to right sided movement; see Table 1 for MNI coordinates of significant clusters).
Significant activation was observed in clusters in the sensorimotor cortex (SMC) contralateral to the side of movement imagined in expected somatopy, in supplementary motor and pre-motor areas (SMA and pre-SMA), and in the cerebellum ipsilateral to the side of movement imagined, with differential activation for hand and foot movements (e.g. cerebellar lobules V, VI, and vermis for foot imagery versus culmen for hand imagery). In addition, significant activation was observed in bilateral occipital areas, attributable to processing of the visual cue. SMC and cerebellar activation was strongly lateralized depending on the side of movement imagined, i.e. located in the left cerebellum and in the right SMC upon imagination of left sided limb movements and vice versa (Fig. 1b–e). Independent component analysis confirmed the results of the model-based analyses (selected components shown in Fig. 1f–i).
Although involving identical components of the sensorimotor network, compared to active movement versus rest in the healthy controls, the peak activation in the SMC cluster with motor imagery in the patient tended to be shifted towards more cranial and anterior regions within the SMC (see Table 1 for cluster-coordinates and insets in Fig. 2). Also, the cerebellar activation peak associated with imagery of hand movements was located more caudally compared to active hand movement in the control, albeit it involved the same lobule.
Region of interest analyses demonstrating the extent of signal change in the primary sensorimotor cortices (SMC) in voxels with peak activation defined by motor imagery versus rest in the patient and by active movement versus rest in the controls. Insets show the clusters of activation comprising the SMC in coronal, sagittal and axial orientation with respective coordinates for imagery of movement in the patient (upper rows; designated by the label PC for patient cluster) and for active movement in the healthy controls (lower rows; designated by the label CC for controls cluster). The MNI coordinates of the PC and CC within the SMC were then used to compute the percent signal change versus rest at each of these locations for the respective contrasts. Grey columns indicate the signal change in the PC while the black columns indicate signal changes in the CC. a shows the results for the dominant right hand and b for the right foot, respectively. The following contrasts were generated: motor imagery versus rest in the patient (Patient), active movement versus rest in controls (Controlsmov), and motor imagery versus rest in the controls (Controlsimg). Further details are provided in the text
Passive limb movements in the patient
Consistent with complete deafferentiation, contrasts between passive movement of both feet and the right hand versus rest did not elicit significant brain activation, apart from sparse activation of primary and secondary somatosensory cortices observed with passive movement of the left hand versus rest, consistent with residual sensorimotor function.
Motor imagery in healthy controls
Cluster-based 2nd level statistics of contrasts of motor imagery versus rest in the healthy subjects did not reveal significant activation within the SMC (even after lowering the threshold of the statistical analysis to >3.1; P = 0.01; also see Table 1). Similarly, ICA did not detect a component with significant SMC activation. Imagery of hand movement was associated with a greater brain response reflected by a larger number of activated areas compared to the pattern of activities seen with imagery of foot movement versus rest. Apart from activation in occipital and parietal areas, contrasts of imagery of hand movements versus rest showed activation of pre-SMA (Table 1).
Region of interest analyses
The coordinates of SMC peak activation associated with imagery of movement in the patient versus rest [insets designated as patient cluster (PC) in Fig. 2] and with active movement in the healthy control group versus rest [insets designated as control cluster (CC) in Fig. 2] were used to compute signal changes at ROIs for different contrasts, both for the dominant hands and feet (MNI coordinates in Fig. 2 indicating peak activation), in order to scrutinize in more detail for differences and similarities in SMC activation between the patient and the controls. With imagery of hand movement in the patient, the peak signal change in SMC was twice as high (2.18%; grey bar above designation “Patient”) compared to the signal change at the same ROI observed with active movement in the controls (1.05%; grey bar above designation “Controlsmov,” Fig. 2a). Signal change with imagery of hand movement in the patient at the ROI of peak activation associated with active movement in the control group (black bar above designation “Patient”; 0.70%) was similar to the magnitude of signal change observed with active hand movement in controls at the same ROI (0.81%; black bar above designation “Controlsmov”). Similar findings were noted for the foot area (Fig. 2b). Consistent with cluster-based statistics, only marginal signal change in both SMC ROIs were noted with imagery of foot or hand movement in the healthy controls (grey and black bars above designation “Controlsimg”).
Discussion
In this fMRI study, we were able to demonstrate SMC activity following extensive motor imagery training in a tetraplegic patient years after traumatic SCI while such activation was absent in healthy untrained controls. Previous studies reported on robust activations of secondary motor networks and ipsilateral cerebellum in SCI, but inconsistently found SMC to be activated (Sabbah et al. 2002; Alkadhi et al. 2005; Cramer et al. 2005). Until recently, it thus has been unclear if SMC function can be preserved years after SCI (Cramer et al. 2005). We therefore interpret our findings as evidence that BCI training as a conduit of motor imagery training may assist in maintaining access to SMC in largely preserved somatopy despite complete deafferentation.
Through extensive BCI training, our patient learned to generate EEG bursts of beta-oscillations by imagination of foot movements (Pfurtscheller et al. 2000). In EEG studies, the generation network of these beta bursts has been attributed to the foot representation area and/or SMA, but differences between beta-oscillations in able-bodied subjects and the induced beta-oscillations in tetraplegic and paraplegic patients have been noted (Muller-Putz et al. 2007). Our study demonstrates substantial overlap between the activation patterns elicited by motor imagery in the patient and actual movement execution in healthy subjects. Importantly, this preservation of higher order motor activity was confirmed by a principal component analysis, which remains unaffected by a priori assumptions concerning the experimental model or characteristics of the hemodynamic function of the BOLD response (Beckmann and Smith 2004). Also, electrophysiological testing and use of a passive movement paradigm [where activation of SMC would have to be expected in case of reciprocal stimulation (Weiller et al. 1996)], supported the concept that these activations are related to motor imagery only and cannot be explained by residual subclinical efferent or afferent motor activity.
In line with previous studies (Cramer et al. 2005), subtle deviations from normal motor control also became apparent in the SCI patient. Covert movement in the patient was associated with a cranial and anterior shift of the peak activation within SMC (and differential cerebellar activation) compared to activation patterns observed with overt movement in the healthy controls. The patient predominantly trained imagery of foot movements with BCI. It is therefore of special interest that even upon imagery of hand movements, significant activation of the SMC contralateral to and the cerebellum ipsilateral to the side of hand movement imagined could be achieved. This suggests that persistent motor imagery training per se might suffice to maintain refined access to the motor system, irrespective of the actual movement component trained. This notion concurs with the observation that motor imagery training has been shown to partially reverse abnormalities in central motor control in SCI patients (Cramer et al. 2007). Such strategies might therefore constitute a back-door to the motor system in the context of sensorimotor rehabilitation subsequent to CNS injury (Johnson-Frey 2004; Sharma et al. 2006; de Vries and Mulder 2007).
Motor imagery both in healthy controls and in chronic SCI patients have been associated with some degree of activation in pre-motor and parietal areas (Binkofski and Seitz 2004; Hotz-Boendermaker et al. 2008), which has been attributed to increased cognitive demand in the latter. The lack of activation in these areas in our patient might indicate that these mechanisms loose importance after years of intensive BCI training. There is also some controversy regarding the neural substrates of motor learning in the literature (e.g. Jackson et al. 2003; Floyer-Lea and Matthews 2005). In this context, it also needs to be noted that the signal change of SMC with covert movement in our patient was higher compared to overt movement in controls. Together, this might reflect central plastic changes due to long-term deefferentation/deafferentiation, as also suggested by the “cerebral hyperactivity” reported in spinal cord-injured patients for other areas most recently (Hotz-Boendermaker et al. 2008).
However, several limitations also have to be considered in the interpretation of our findings. The unique case presented cannot be considered representative for the general population of SCI victims, in particular because it is still unclear what proportion of patients will both be capable of reliably eliciting EEG bursts of activity upon imagery of movement and then also will endure BCI training over sufficiently long time (Wolpaw et al. 2002). Further, the lack of activation of key components of the motor system with motor imagery in the healthy controls in our study prohibits a direct comparison between the fMRI activation patterns associated with motor imagery between the patient and the controls. For a better description of the performance of our controls, we lack a motor imagery skill survey (Sharma et al. 2006; de Vries and Mulder 2007). Previous studies suggested that SMC activation might be obtained with imagery in healthy controls after training (Ehrsson et al. 2003; Hanakawa et al. 2003; Lacourse et al. 2005; de Vries and Mulder 2007). However, consistent with earlier reports (Gerardin et al. 2000; Alkadhi et al. 2005), we observed only activation of SMA and pre-SMA with motor imagery in our controls, corroborating that the subjects were engaged with the task. This also concurs with a recent high temporal resolution fMRI study using dynamic causal modeling which not only highlighted the importance of the SMA for the preparation and execution of intended movements, but also for suppressing movements that are represented in the motor system but not to be performed (Kasess et al. 2008).
References
Alkadhi H, Brugger P, Boendermaker SH, Crelier G, Curt A, Hepp-Reymond M-C, Kollias SS (2005) What disconnection tells about motor imagery: evidence from paraplegic patients. Cereb Cortex 15:131–140
Beckmann CF, Smith SM (2004) Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging 23:137–152
Beckmann CF, Jenkinson M, Smith SM (2003) General multilevel linear modeling for group analysis in FMRI. Neuroimage 20:1052
Binkofski F, Seitz RJ (2004) Modulation of the BOLD-response in early recovery from sensorimotor stroke. Neurology 63:1223–1229
Birbaumer N, Cohen LG (2007) Brain-computer interfaces: communication and restoration of movement in paralysis. J Physiol 579:621–636
Cramer SC, Lastra L, Lacourse MG, Cohen MJ (2005) Brain motor system function after chronic, complete spinal cord injury. Brain 128:2941–2950
Cramer SC, Orr EL, Cohen MJ, Lacourse MG (2007) Effects of motor imagery training after chronic, complete spinal cord injury. Exp Brain Res 177:233–242
de Vries S, Mulder T (2007) Motor imagery and stroke rehabilitation: a critical discussion. J Rehabil Med 39:5–13
Ehrsson HH, Geyer S, Naito E (2003) Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part-specific motor representations. J Neurophysiol 90:3304–3316
Floyer-Lea A, Matthews PM (2005) Distinguishable brain activation networks for short- and long-term motor skill learning. J Neurophysiol 94:512–518
Friehs GM, Zerris VA, Ojakangas CL, Fellows MR, Donoghue JP (2004) Brain-machine and brain-computer interfaces. Stroke 35:2702–2705
Gerardin E, Sirigu A, Lehericy S, Poline JB, Gaymard B, Marsault C, Agid Y, Le Bihan D (2000) Partially overlapping neural networks for real and imagined hand movements. Cereb Cortex 10:1093–1104
Hanakawa T, Immisch I, Toma K, Dimyan MA, Van Gelderen P, Hallett M (2003) Functional properties of brain areas associated with motor execution and imagery. J Neurophysiol 89:989–1002
Hotz-Boendermaker S, Funk M, Summers P, Brugger P, Hepp-Reymond M-C, Curt A, Kollias SS (2008) Preservation of motor programs in paraplegics as demonstrated by attempted and imagined foot movements. Neuroimage 39:383–394
Jackson PL, Lafleur MF, Malouin F, Richards CL, Doyon J (2003) Functional cerebral reorganization following motor sequence learning through mental practice with motor imagery. Neuroimage 20:1171–1180
Johnson-Frey SH (2004) Stimulation through simulation? Motor imagery and functional reorganization in hemiplegic stroke patients. Brain Cogn 55:328–331
Kalb RG (2003) Getting the spinal cord to think for itself. Arch Neurol 60:805–808
Kasess CH, Windischberger C, Cunnington R, Lanzenberger R, Pezawas L, Moser E (2008) The suppressive influence of SMA on M1 in motor imagery revealed by fMRI and dynamic causal modeling. Neuroimage 40:828–837
Kirshblum SC, Priebe MM, Ho CH, Scelza WM, Chiodo AE, Wuermser LA (2007) Spinal cord injury medicine. 3. Rehabilitation phase after acute spinal cord injury. Arch Phys Med Rehabil 88:S62–S70
Lacourse MG, Orr ELR, Cramer SC, Cohen MJ (2005) Brain activation during execution and motor imagery of novel and skilled sequential hand movements. Neuroimage 27:505
Lauer RT, Peckham PH, Kilgore KL (1999) EEG-based control of a hand grasp neuroprosthesis. Neuroreport 10:1767–1771
Leeb R, Friedman D, Müller-Putz G, Scherer R, Slater M, Pfurtscheller G (2007) Self-paced (asynchronous) BCI control of a wheelchair in Virtual Environments: a case study with a tetraplegic. Comput Intell Neurosci:1–8
Muller-Putz GR, Zimmermann D, Graimann B, Nestinger K, Korisek G, Pfurtscheller G (2007) Event-related beta EEG-changes during passive and attempted foot movements in paraplegic patients. Brain Res 1137:84–91
Neuper C, Muller-Putz GR, Scherer R, Pfurtscheller G (2006) Motor imagery and EEG-based control of spelling devices and neuroprostheses. Prog Brain Res 159:393–409
Pfurtscheller G, Guger C, Muller G, Krausz G, Neuper C (2000) Brain oscillations control hand orthosis in a tetraplegic. Neurosci Lett 13:211–214
Pfurtscheller G, Muller GR, Pfurtscheller J, Gerner HJ, Rupp R (2003) ‘Thought’—control of functional electrical stimulation to restore hand grasp in a patient with tetraplegia. Neurosci Lett 351:33–36
Pfurtscheller G, Müller-Putz G, Pfurtscheller J, Rupp R (2005) EEG-based asynchronous BCI controls functional electrical stimulation in a tetraplegic patient. EURASIP J Appl Signal Processing 19:3152–3155
Pfurtscheller G, Leeb R, Keinrath C, Friedman D, Neuper C, Guger C, Slater M (2006) Walking from thought. Brain Res 1071:145–152
Sabbah P, de SS, Leveque C, Gay S, Pfefer F, Nioche C, Sarrazin JL, Barouti H, Tadie M, Cordoliani YS (2002) Sensorimotor cortical activity in patients with complete spinal cord injury: a functional magnetic resonance imaging study. J Neurotrauma 19:53–60
Schmahmann JD, Doyon J, Toga AW, Petrides M, Evans AC (2000) MRI atlas of the human cerebellum. Academic, San Diego
Sharma N, Pomeroy VM, Baron J-C (2006) Motor imagery: a backdoor to the motor system after stroke? Stroke 37:1941–1952
Suga R, Tobimatsu S, Kira J, Kato M (1999) Motor and somatosensory evoked potential findings in HTLV-I associated myelopathy. J Neurol Sci 167:102–106
Tobimatsu S, Sun SJ, Fukui R, Kato M (1998) Effects of sex, height and age on motor evoked potentials with magnetic stimulation. J Neurol 245:256–261
Weiller C, Juptner M, Fellows S, Rijntjes M, Leonhardt G, Kiebel S, Muller S, Diener HC, Thilmann AF (1996) Brain representation of active and passive movements. Neuroimage 4:105
Wolpaw JR, Birbaumer N, McFarland DJ, Pfurtscheller G, Vaughan TM (2002) Brain-computer interfaces for communication and control. Clin Neurophysiol 113:767–791
Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM (2004) Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage 21:1732
Worsley KJ, Evans AC, Marrett S, Neelin P (1992) A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 12:900–918
Acknowledgments
This work has been supported by the FWF Austrian Science Fund (SR, grant number P15158), “Lorenz Böhler Gesellschaft” and “Allgemeine Unfallversicherung AUVA” (GP, G-M P). We thank the patient for his enthusiasm with the BCI training and this project and Karin Brodtrager for technical assistance.
Conflict of interest:
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Enzinger, C., Ropele, S., Fazekas, F. et al. Brain motor system function in a patient with complete spinal cord injury following extensive brain–computer interface training. Exp Brain Res 190, 215–223 (2008). https://doi.org/10.1007/s00221-008-1465-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-008-1465-y