Abstract
A recent study by our group showed that the scaling of reach trajectories to target size is independent of conscious visual awareness of that intrinsic target property (Binsted et al. in Proc Natl Acad Sci USA 104:12669–12672, 2007). The present investigation sought to extend previous work and determine whether unconscious target information represents a temporally durable or evanescent visuomotor characteristic. To accomplish that objective, we employed Di Lollo et al’s (J Exp Psychol Gen 129:481–507, 2000) object substitution masking paradigm and asked participants to complete verbal reports and reaching responses to different sized (1.5, 2.5, 3.5, 4.5, 5.5 cm) targets under masked and non-masked target conditions. To determine whether visuomotor networks retain unconscious target information, reaching trials were cued concurrent with target presentation or 1,000 or 2,000 ms after target presentation. For the perceptual trials, participants readily identified the size of non-masked trials but demonstrated only chance success identifying target size during masked trials. Interestingly, however, reaches directed to non-masked and masked targets exhibited comparable and robust scaling with target size; that is, lawful speed-accuracy relations related to movement planning and execution times were observed regardless of whether participants were aware (i.e., non-masked trials) or unaware (i.e., masked trials) of target size. What is more, the length of the visual delay period used here did not differentially influence the scaling of reach trajectories. These results indicate that a conscious visual percept is not necessary to support motor output and that unconscious visual information persists in visuomotor networks to support the kinematic parameterization of action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An introspective experience related to our ability to reach and grasp objects is that we have conscious access to the visual information supporting movement. It is, however, important to note that reaches can be elicited in the absence of conscious visual awareness. For example, lesions to the primary visual cortex (V1) preclude visual awareness in the impaired hemifield but do not universally impede visual tracking or pointing to visual stimuli in the scotoma (so-called action-blindsight: Perenin and Jeannerod 1975; Weiskrantz et al. 1974; see Danckert and Rossetti 2005 for recent review). Further, the study of an individual (DF) with bilateral lesions to the lateral occipitotemporal cortex (LOC) (James et al. 2003) provides a more subtle demonstration of the separation between conscious visual perception and visuomotor control. Specifically, DF cannot identify line forms (i.e., visual form agnosia) nor can she report the size and orientation of objects; however, she is able to tune the parameters of reaching and grasping movements to the veridical size and orientation of to-be-grasped/touched objects (Goodale et al. 1991; see also Milner and Goodale 1995). In other words, DF interacts with her visual world successfully without conscious awareness of object properties.
A framework for understanding the separation between conscious visual awareness and motor control is provided by Goodale and Milner’s perception/action model (PAM) (Goodale and Milner 1992; see Goodale et al. 2004 for recent review). The PAM asserts that projections from VI to perception-based networks residing in the inferotemporal cortex of the ventral visual pathway mediate visual judgments. As such, early (i.e., V1; blindsight) or late (LOC; visual form agnosia) lesions to the ventral visual pathway are predicted to encumber visuo-perceptual judgments. In turn, the PAM states that V1 or extrageniculate projections provide visual input to dedicated visuomotor networks residing in the posterior parietal cortex (PPC) of the dorsal visual pathway. Thus, in the face of impaired visuo-perceptual abilities, the PAM predicts that individuals with blindsight or visual agnosia can retain adequate visuomotor abilities because the structural deficits characterizing the aforementioned do not elicit a salient impact on visual inputs to the dorsal visual pathway.
As an extension to clinical populations, the double-step paradigm has shown a separation between conscious visual awareness and visuomotor control in neurologically intact individuals (Bridgeman et al. 1979; Goodale et al. 1986). In the double-step paradigm, participant’s limb position and visual gaze is directed to a home position in advance of reaching to a peripheral target. Importantly, on a limited number of trials the location of the target is unexpectedly perturbed at or near peak ocular velocity; that is, during saccadic suppression. The results of this paradigm have consistently shown that participants amend their reach trajectories online in response to the change in target location in spite of the fact that saccadic suppression disrupts conscious awareness of the target change (see also Chua and Enns 2005). Further, it has been shown that PPC lesions impair the fast corrective movements associated with the double-step paradigm (Pisella et al. 2000). Thus, evidence from clinical and non-clinical populations supports the PAM’s assertion that visuomotor processing within the dorsal visual pathway is independent of visual awareness.
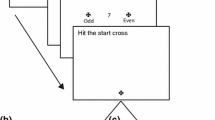
More recent work has shown that unconscious visuomotor processing includes integration of a wider range of target features than the exogenous change in target location characterizing the double-step paradigm. Indeed, semantic cues which prime the direction of a target (Cressman et al. 2007) and intrinsic object properties (Binsted et al. 2007) have also been shown to shape reaching trajectories without participant’s awareness. For example, recent work by our group (Binsted et al. 2007) required participants to make perceptual reports and complete reaches to targets using a variant of Di Lollo et al’s (2000) four-dot object-substitution masking paradigm (Di Lollo et al. 2000; see Enns and Di Lollo 2000 for review). In our group’s earlier study, an array of circles of different sizes (1.5, 2.5, 3.5, 4.5, 5.5 cm) was briefly presented (13 ms). The array included a target circle identified by four small red dots (i.e., four-dot mask) that surrounded but did not touch the target (see Fig. 1). When the array and four-dot mask disappeared simultaneously there was no masking and participants were able to report the size of the cued target (i.e., the prime condition: mean accuracy = 94%). In contrast, when the four-dot mask remained visible for a period of time (i.e., 320 ms) following offset of the circles array then participants were unable to report the size of the target (i.e., the mask condition: mean accuracy = 56%). Interestingly, when participants were instructed to complete reaching movements to the cued target, trajectory parameters of prime and mask responses elicited speed-accuracy relations corresponding to veridical target size (Fitts 1954). In other words, trajectories were specified according to the size of the target regardless of whether participants were consciously aware of physical target properties. Notably such findings are in line with neuropsychological research demonstrating that individuals with object agnosia and some individuals with action-blindsight can scale their reach and grasp trajectories to the dimensions of a to-be-touched or to-be-grasped object (e.g., Goodale et al. 1994; see also Danckert and Rossetti 2005 for review).
Display sequence in prime (a) and mask (b) conditions. Participants were instructed to maintain gaze on a fixation point during a variable foreperiod (1,000–2,000 ms) after which point an array of circles appeared for 13 ms. One circle in the array (i.e., the target circle) was surrounded by four red dots (i.e., the four-dot mask and displayed in this figure as four solid black dots) and in the example shown here corresponds to a left space target (targets also appeared equidistant to the right of fixation). In the prime condition a blank screen followed presentation of the array of circles whereas in the mask condition the four-dot mask remained visible for a further 320 ms. For all perceptual trials, participants were prompted to provide a verbal report 320 ms following offset of the circles array. For reaching trials, an auditory initiation tone was provided coincident (i.e., the D0 condition), 1,000 (D1000), or 2,000 (D2000) ms after presentation of the array of circles. Note that D1000 and D2000 trials are shown to have been cued 680 and 1,680 ms after onset of the fourth panel (i.e., the blank screen). Those times in combination with the 320 ms interval of the third panel produce respective movement delays of 1,000 and 2,000 ms. Note: due to limitations in page size-scaling the second panels of Fig. 1 a and b do not contain the three circles of each target width (i.e., the panels shown here contain 13 as opposed the 15 circles used in experimental sessions)
In the present research, we again used the four-dot masking paradigm to elucidate the timeframe that unconscious target information can be retained and used by the visuomotor system. According to the real-time component of the PAM, the dorsal visual pathway accesses metrical visual information only on a moment-to-moment basis and thus does not operate when a response is initiated after any period of visual delay (Westwood and Goodale 2003; see Goodale and Westwood 2004 for review). In support of this view, some evidence from the pictorial illusions literature shows that actions planned with direct visual input are mostly—if not entirely—refractory to the cognitive effects of illusions (e.g., Aglioti et al. 1995; Westwood et al. 2000) whereas movements performed following even the briefest of visual delays (i.e., when visual stimuli is occluded coincident with the cue to initiate a response) are influenced by the context-dependent properties of illusions (e.g., Haffenden and Goodale 1998; Hu and Goodale 2000; Westwood et al. 2000). According to the PAM, such a pattern reflects the fact that in the absence of real time visual input, a cognitive representation laid down and maintained by the ventral visual pathway is used to support motor output (Westwood and Goodale 2003). It is, however, important to note that mounting research has shown that illusory features influence actions planned with direct visual input from the reaching and grasping environment (Daprati and Gentilucci 1997; Glover and Dixon 2001; Heath et al. 2004a; see Glover 2004 or Mendoza et al. 2005 for reviews). Thus, the pictorial illusions literature does not provide systematic nor reliable evidence related to the timeframe by which unconscious target information can be stored and used to support motor output (Bruno et al. 2008).
Here we asked participants to complete perceptual reports and reaching responses under prime and mask conditions of the four-dot masking paradigm. As in our previous work (Binsted et al’s 2007), one block of reaching trials was cued concurrent with presentation of the target array (i.e., planned in real time). In addition, we included blocks of trials wherein reaches were cued 1,000 or 2,000 ms following offset of the circles array (i.e., planned offline). If access to unconscious visual information is limited by the evanescent property of the dorsal visual pathway, as predicted by the PAM, then lawful speed-accuracy relations should be restricted to situations wherein responses are cued concurrent with presentation of the target stimuli. If, however, unconscious visual information is resistant to visual delays than speed-accuracy relations should characterize performance for the 1,000 and possibly the 2,000 ms delay conditions used here.
Methods
Participants
Eleven participants from the University of Western Ontario community volunteered for this research study (age range = 20–33 years: 5 men and 6 women). Participants were right-handed and had normal or corrected-to-normal vision (contact lenses only). This research was approved by the Office of Research Ethics, University of Western Ontario, and was conducted in accord with the Declaration of Helsinki (1964).
Apparatus and procedure
We used an apparatus similar to that developed by Held and Gottlieb (1958). The apparatus consisted of a rectangular frame containing three shelves. The top shelf supported a computer monitor (Dell 1707FP, 8 ms response rate; Austin, TX, USA) that was used to project visual stimuli onto a one-way mirror (i.e. the middle shelf). The lower shelf was a solid surface (96 cm wide by 65 cm deep) and was the area where participants completed reaching movements. The distance between the top shelf and the middle shelf, and the middle shelf and the bottom shelf was constant at 34 cm. Thus, the optical geometry of this setup created a situation wherein participants perceived visual stimuli projected onto the mirror as being located on the lower surface of the apparatus. A constant optical geometry was maintained via a head/chin rest (ASL-6000: Bedford, MA, USA). All visual and auditory events were controlled via Eprime (ver 1.1: Psychology Software Tools, Pittsburgh, PA, USA). The lights in the experimental suite were darkened throughout data collection, and in combination with the one-way mirror, occluded vision of the reaching limb (see details below).
Participants were seated at the apparatus for the duration of the experiment. In advance of each trial a central fixation cross was presented for a randomized foreperiod (1,000–2,000 ms). Following this foreperiod, an array of five differently sized circles (1.5, 2.5, 3.5, 4.5, and 5.5 cm in diameter; 3 circles per width) was presented for 13 ms (Fig. 1). As in our previous work (Binsted et al. 2007), this array included one target circle identified by four small red dots arranged in an imaginary square (36 cm2) (i.e., the four-dot mask). In the prime condition, the array and the four-dot-mask were simultaneously presented for 13 ms. Importantly, the array and four-dot-mask were then simultaneously extinguished. In the mask condition, the array and four-dot mask were simultaneously presented for 13 ms; however, the four-dot-mask remained visible for an additional 320 ms (see Fig. 1 for timeline of experimental events). Target circles were always located 22.7 cm anterior to a common midline home position (i.e., a microswitch located 5 cm anterior to the front edge of the reaching surface) and 17 cm to the left (i.e., left space) and right (i.e., right space) of participant’s midline.
Perceptual task
To avoid confusion with the naming of intermediate-sized targets only the 2.5 and 4.5 cm circles were presented as targets during perceptual trials. Prior to data acquisition, participants were shown each target to provide advance knowledge of target characteristics. Participants were prompted to provide a verbal report (forced-choice binary decision) of whether the cued target was “small” (i.e., 2.5 cm) or “large” (i.e., 4.5 cm): the prompt occurred 320 ms following offset of the circles array (see panel 4 of Fig. 1). Prime and mask conditions were performed in separate and randomly ordered blocks. Within each block, small and large targets were presented randomly in left and right space on four separate occasions for a total of 32 perceptual trials. Perceptual trials were completed in advance of reaching trials. Binsted et al’s (2007) perceptual trials were performed following reaching trials, and as will be demonstrated below, that previous work in combination with the present study demonstrates that perceptual trial performance is not influenced by the ordering of reaching trials.
Reaching task
From the home position, participants completed goal-directed reaching movements (specifically a pointing response with the right index finger) to the cued target circle as quickly and accurately as possible. Reaching movements were completed in three visual conditions: 0 ms delay (D0), 1,000 ms delay (D1000) and 2,000 ms delay (D2000). In the D0 condition, participants were cued (via auditory tone) to initiate their reaching movement concurrent with onset of the circles array (see panel 2 of Fig. 1). In the D1000 and D2000 conditions, the initiation tone was provided 1,000 or 2,000 ms after onset of the target array (see panel 4 of Fig. 1). Target sizes were 1.5, 2.5, 3.5, 4.5, and 5.5 cm and produced respective index of difficulty (ID) values of 5.2, 4.5, 4.0, 3.6 and 3.3 bits [log2(2A/W): see Fitts 1954].Footnote 1 Visual conditions were completed in separate and randomly ordered trial blocks. Within each visual condition, prime and mask trials were blocked and presented randomly. In the prime and mask blocks, target size and location (i.e., right space vs. left space) were randomized and eight trials were completed to each target size by reaching space combination. Thus, for each visual condition block (i.e., D0, D1000, D2000) participants completed 160 trials resulting in 480 total reaching trials. We also note that trials in the different visual conditions, as well as presentation of prime and masked trials within each visual condition, were presented in separate trial blocks because previous work has shown that randomly interleaving different visual conditions on a trial-by-trial basis impacts the type of visual information and the motor strategies used by participants to implement their reach trajectories (Elliott and Allard 1985; Heath et al. 2006; Neely et al. 2008).
As mentioned above, the lights in the experimental suite were dimmed and in combination with the one-way mirror prevented participants from directly viewing their limb. In the place of veridical limb vision, a splint complex containing dual light emitting diodes (LEDs) affixed to the nail of the right index finger was used to provide visual feedback about limb position. The LEDs were continuously illuminated during reaching trials. Additionally, the splint complex contained an infra-red emitting diode (IRED). IRED position data were sampled at 200 Hz for 1 s following the auditory initiation tone via an OPTOTRAK 3020 (Northern Digital Inc: Waterloo, ON, Canada). Offline, IRED position data were filtered via a second-order dual-pass Butterworth filter employing a low-pass cut-off frequency of 15 Hz. Instantaneous velocities were computed via a three-point central finite difference algorithm. Movement onset was determined by an analogue signal driven by release of pressure from the home position microswitch and movement offset was defined as the first frame wherein limb velocity fell below 50 mm/s for ten consecutive frames (i.e., 50 ms).
Dependent variables and statistical analyses
For the perceptual task, the frequency and proportion of correct and incorrect responses were computed. The frequency of correct mask and prime trials was contrasted via repeated measures t statistic. In addition, signal detection values (d 1) were computed for each participant and grouped prime and mask d 1 values were separately contrasted to a null value of 0 (via single-sample t statistics). The dependent variables examined for the reaching task included: reaction time (RT: time from auditory initiation tone to movement onset), movement time (MT: time from movement onset to movement offset), peak velocity (PV: maximum resultant velocity) time after peak velocity (TAPV: time from PV to movement offset) and constant error in the mediolateral (CEML: negative value = leftward bias, positive value = rightward bias) and anteroposterior (CEAP: negative value = undershoot, positive value = overshoot) movement directions and their associated variable error (i.e., VEML and VEAP) values. All variables for the reaching task were examined via 2 (stimulus presentation: prime, mask) by 3 (visual delay: D0, D1000, D2000) by 5 (target ID: 5.2, 4.5, 4.0, 3.6 and 3.3 bits) repeated-measures ANOVA.Footnote 2 Significant main effects were decomposed via simple effects and/or power polynomials (P < 0.05) (see Pedhazur 1997). Means and between-participant standard deviations are reported in the body of the manuscript and Tables 1 and 2.
Results
Perceptual task
Perceptual judgments were more accurate in the prime as compared to the mask condition [t(10) = 6.05, P < 0.001]. More specifically, in the prime condition participants were able to accurately report the size of the target [mean proportion correct = 0.88, SD 0.12, mean d 1 = 1.66, t(10) = 6.84, P < 0.001]. In contrast, mask condition trials yielded only a chance level of performance [mean proportion correct = 0.54, SD 0.12, mean d 1 = 0.17, t(10) = 1.26, P = 0.23]. It is also worth noting that in the mask condition participants frequently reported not being consciously aware of what size target was presented to them: similar reports were not associated with the prime condition. Interestingly, this pattern of behaviour persisted throughout reaching trials as well.
Reaching task
The analysis of RT produced main effects for visual delay, F(2, 20) = 15.80, P < 0.001, and target ID, F(4, 40) = 7.08, P < 0.001. RTs for D0 trials were 271 ms (SD 32) and were slower than the respective 218 ms (SD 21) and 214 ms (SD 26) RTs characterizing D1000 and D2000 trials [significant quadratic effect: F(1, 10) = 5.23, P < 0.05]. In addition, RTs slowed in relation to increasing target ID [only linear effect significant: F(1, 10) = 14.85, P < 0.01] (Table 1). For MT, overall movement durations increased with increasing target ID, F(4, 40) = 8.22, P < 0.001 [only linear effect significant: F(1, 10) = 9.73, P < 0.02]. Notably, Fig. 2 demonstrates that MT for prime and mask conditions were comparable and did not interact with the different visual delays and target IDs used here (P’s > 0.50) (see also Table 2).Footnote 3
The results for PV showed that peak movement speed decreased with increasing target ID, F(4, 40) = 3.77, P < 0.02 [only linear effect significant, F(1, 10) = 5.02, P < 0.05]. For TAPV, it was found that the movement deceleration period increased with increasing target ID, F(4, 44) = 5.80, P < 0.02 [only linear effect significant: F(1, 10) = 5.8, P < 0.05] (Table 1).
Analysis of CEML produced main effects for stimulus presentation, F(1, 10) = 6.15, P < 0.04, and target ID, F(4, 40) = 7.37, P < 0.001). Trials performed in the mask condition showed less rightward bias (7.4 mm SD 9.1) than prime counterparts (11.5 cm SD 11.2). In addition, rightward bias decreased with increasing target ID [only linear effect significant: F(1, 10) = 12.50, P < 0.01] (Table 1). In terms of CEAP, D0 (−3.7 mm SD 7.1), D1000 (−7.9 mm SD 9.2) and D2000 (−10.1 mm SD 8.7) trials exhibited an increase in undershooting with visual delay, F(2, 28) = 8.58, P < 0.01 [only linear effect significant: F(1, 10) = 13.55, P < 0.01]. The results for VEML showed that longer visual delays resulted in a wider distribution of movement endpoints, F(2, 20) = 3.75, P < 0.05; i.e., 10.6 mm (SD 2.7), 12.1 mm (SD 4.5) and 13.5 mm (SD 4.2) characterized the respective variability of D0, D1000 and D2000 trials [only linear effect significant: F(1, 10) = 4.02, P < 0.05]. Similarly, results for VEAP showed increased endpoint variability across D0 (6.9 mm SD 1.9), D1000 (8.0 mm, SD 2.5) and D2000 (8.5 mm SD 2.1) visual conditions, F(2, 20) = 8.61, P < 0.01 [only linear effect significant: F(1, 10) = 30.64, P < 0.01]. In addition, VEAP increased with decreasing target ID, F(4, 40) = 9,12, P < 0.001 [only linear effect significant: F(1, 10) = 30.63, P < 0.001] (see Table 1).
Discussion
We sought to determine if unconscious information concerning an intrinsic target characteristic (i.e., size) could be used to support reaching performance following a visual delay. To accomplish that objective, perceptual reports and reaches to targets of different sizes were examined using a variation of Di Lollo et al’s (2000) object-substitution masking paradigm (Binsted et al. 2007). Importantly, we manipulated the time between the offset of primed and masked targets and the onset of a goal-directed reaching response such that movements were cued concurrent with presentation of the target (i.e., planned in real time) or 1,000 and 2,000 ms after removal of the target. These procedures provided us an emergent understanding of the temporal durability of the unconscious target information supporting motor output.
Four-dot masking impedes conscious awareness of intrinsic target features
Prime and mask conditions differentially influenced conscious awareness of target size. When the circles array and four-dot mask were blanked at the same time (i.e., the prime condition) participants accurately reported the size of the target circle. In contrast, when the four-dot mask remained visible after the circles array was blanked (i.e., the mask condition) participants demonstrated only a chance ability to report the size of the target circle. These results are in line with Di Lollo et al’s (2000; see also Enns and Di Lollo 2000) computational model of object substitution. According to this model, concurrent blanking of the circles array and four-dot mask allows target information to be processed on the basis of a “visible persistence” maintained at high-level visual processing areas (i.e., the ventral visual pathway). Moreover, and because each part of the display is simultaneously removed, a uniform decay between the target and the four-dot mask permits conscious access to perception-based target features. When the four-dot mask remains visible following blanking of the circles array however, reentrant information of the mask processed at a low-level visual system (V1) conflicts with the visible persistence (i.e., the original stimulus array) maintained at high-level visual processing areas (see Di Lollo et al. 2000; Weidner et al. 2006). Importantly, non-uniformity of decay associated with reentrant visual processing of the four-dot mask renders the original percept (i.e., the target array and the four-dot mask) unavailable for conscious perceptual report.
Visuomotor memory operates without conscious awareness of intrinsic target features
Before turning to the principal issue of the impact of visual delays on prime and mask reaching trials, we discuss the general influence of the 0 (D0), 1,000 (D1000) and 2,000 (D2000) ms visual delays. Overall, D1000 and D2000 conditions elicited faster reaction times than D0 counterparts; this is not a surprising finding because target circles were presented in both left and right reaching space. Thus, the presentation of target location in advance of movement cuing (i.e., D1000 and D2000 trials) provided a valid precue related to the direction (i.e., target presented in right or left space) of a to-be-initiated reaching response (Rosenbaum 1980). In contrast, the D0 condition entailed concurrent movement cuing and target presentation: a situation precluding advanced identification of movement direction. In terms of movement execution, movement time, the magnitude and timing of peak velocity as well as endpoint accuracy in the mediolateral axis did not differ across the visual delays. We did, however, observe that an increase in visual delay was accompanied by enhanced undershooting of target location (i.e., CEAP) as well as greater endpoint variability in mediolateral and anteroposterior reaching directions. That pattern of results represents a well-documented finding in the memory-guided reaching literature and is interpreted to reflect the visuomotor system’s access to reasonably accurate, albeit temporally unstable, target information (e.g., Adamovich et al. 1999; Binsted and Heath 2004; Elliott 1988; Heath 2005; Heath and Binsted 2007; Heath and Westwood 2003; Heath et al. 2004b; McIntyre et al. 1997; Rolheiser et al. 2006; Westwood et al. 2001, 2003). Importantly, our results demonstrate that the visual delays used here produced a salient impact on the effectiveness of reach endpoints.Footnote 4
We next turn to the principal issue of whether visual awareness influenced the parameterization of limb trajectories across the different delay conditions. Table 1 and Fig. 2 show that D0, D1000 and D2000 prime and mask trials elicited reaction time, movement time, and time after peak velocity values that increased with increasing target ID. In addition, peak velocity, constant error (mediolateral direction only), and endpoint variability (anteroposterior direction only) decreased with increasing target ID.Footnote 5 Taken together, the performance and kinematic results demonstrate lawful speed-accuracy trade-offs related to target width and the need to devote longer planning times and slower movements to “hit” the centre of a target (e.g., Elliott et al. 1999; Fitts 1954; Fitts and Peterson 1964; Heath et al. 1998; Langolf et al. 1976; Woodworth 1899; see Plamondon and Alimi 1997 for overview). These results, in conjunction with the results for the perceptual trials, demonstrate that reach trajectories scaled in relation to target size regardless of explicit visual awareness.
The D0 condition used here directly corresponds to that used in an early study by our group (Binsted et al. 2007). Importantly, both studies demonstrate that visual awareness of target size is not required to support a response initiated in time with the presentation of a target. Of course, that D0 reaches scaled in relation to target size during prime and mask trials is congruent with the PAM’s assertion that actions planned in real time are mediated by the visuomotor networks of the dorsal visual pathway: a pathway thought to processes metrical visual information on a moment-to-moment basis without top-down conscious awareness (Goodale and Milner 1992; Westwood and Goodale 2003). Recall that support for this view is garnered by some studies showing that visual input from the movement environment at the time of response planning renders actions refractory to the context-dependent properties of pictorial illusions (e.g., Aglioti et al. 1995; Haffenden and Goodale 1998; Westwood and Goodale 2003; Westwood et al. 2000). In turn, pictorial illusions have been shown to reliably “trick” actions following a period of brief visual delay and this result has been taken as evidence that the dorsal visual pathway has no appreciable visuomotor memory. As such, the PAM states that responses initiated following a visual delay are supported by consciously derived and temporally durable visual information laid down and maintained by the perceptual networks of the ventral visual pathway (Hu and Goodale 2000; Westwood and Goodale 2003; Westwood et al. 2001). Support for this position is also drawn from observations of patient DF (see “Introduction”) and her inability to scale grip aperture to target size when a period of visual delay (2,000 ms) is introduced between target viewing and movement onset (Goodale et al. 1994). Thus, a logical prediction derived from the PAM is that the absence of visual awareness (i.e., the mask trials) would preclude reliable speed-accuracy relations from being observed in the D1000 and D2000 reaching conditions used here. That prediction, however, was not borne out as D1000 and D2000 trials (across prime and masks conditions) elicited speed-accuracy relations comparable to D0 counterparts. Put another way, the present results indicate that motor output following a period of visual delay is not reliant on an obligatory visual percept maintained by visuo-perceptual networks.
One issue to be addressed is why the present findings depart from the theoretical predictions of the PAM. As an exemplar to this issue, and as mentioned just above, DF demonstrates an inability to appropriately scale her reach/grasp trajectories when a statically previewed (i.e., for up to 5,000 ms) target object is removed from her visual field prior to response cuing (e.g., Goodale et al. 1994): a result thought to reflect the fact that dorsal visuomotor networks operate only when real time visual information is available to the performer (see Westwood and Goodale 2003). In the present investigation however, we examined reaching performance in neurologically intact individuals and visual stimuli were exogenously presented (i.e., 13 ms presentation). We believe that the study population in combination with the rapid stimulus presentation technique used here resulted in mediation of reach trajectories via extrageniculate connections to dorsal visuomotor networks and permitted such networks to maintain a temporally durable and enriched (Schindler et al. 2004) target representation (see Michael and Buron 2005). Indeed, such an assertion is supported by the fact that participants were able to scale reach trajectories without conscious awareness of target size. Moreover, it is worth noting that our assertion is not completely at odds with the action-blindsight literature. Although it is typical in current blindsight literature to present visual stimuli concurrent with a response imperative (i.e., the D0 condition used here; see Danckert et al. 2003 for example), Weiskrantz et al’s (1974) classic study of patient DB introduced a period of delay between initial target viewing and movement cuing. In their study, DB was prompted—via verbal command—to point at the guessed location of a target when the experimenter perceived that the target was extinguished. Although, the exact period of delay associated with Weiskrantz et al’s (1974) cuing technique is unclear, it is clear that in spite of DB’s inability to perceive the location/presence of visual stimuli, he demonstrated preserved visuomotor function following at least some period of delay.Footnote 6
A final issue to be addressed is the nature of the information used to support reaching performance following a period of visual delay. In terms of D0 reaches, it is clear that visual-to-motor transformations occurred at movement initiation: after all, the target was not presented to participants until response cuing. As such, in the D0 condition unconsciously derived information related to target size was immediately transformed into appropriate motor coordinates. In terms of D1000 and D2000 reaches however, it is possible that participants developed a movement plan following target presentation and held that information in memory for subsequent response execution (i.e., offline control). In other words, the visual representation of target size (whether conscious or unconscious) was used to precompute the kinematic parameters of a movement prior to response cuing. Alternatively, it is possible that a sensory (specifically visual) representation of the target was held in visuomotor memory and used to specify a motor plan at the time of response cuing. We believe that the extant literature favours the latter hypothesis and present three lines of evidence supporting that position. First, the classic work of Henry and Rogers (1960) and Klapp (1975) demonstrate that movement planning times increase with movement complexity and the spatial demands of a task. Such findings argue that the internal structure of a motor plan is instantiated at the time of response cuing and not before. Second, many studies involving pictorial illusions and non-illusory geometric structure (i.e., spatial landmarks) report that responses become increasingly sensitive to context-dependent visual features following a period of visual delay (e.g., Bridgeman et al. 2000; Gentilucci et al. 1996; Hu et al. 1999; Hu and Goodale 2000; Krigolson and Heath 2004; Krigolson et al. 2007; Obhi and Goodale 2005; Lemay et al. 2004; Velay and Beaubaton 1986). Presumably non-target features become increasingly salient following a visual delay because memory-based actions are supported by context-dependent visual information maintained by perception-based networks in the ventral visual pathway (see Goodale et al. 2004 for review). Third, Heath and Westwood (2003) used a video-based aiming task that prevented participants from pre-computing the trajectory of memory-guided aiming movements. More specifically, participants moved a computer mouse to manipulate the location of a cursor under conditions wherein the mapping between the mouse and cursor was altered from trial-to-trial. The results of Heath and Westwood showed that participants were able to achieve memory-based target locations regardless of the inability to pre-compute a movement trajectory. Taken as a whole, the results describe above provide support for the view that sensory-based target information is maintained in memory and used to construct a movement plan at response cuing.
Conclusions
The present results combined with other work (Binsted et al. 2007; Chua and Enns 2005; Cressman et al. 2007; Goodale et al. 1986) provide a picture of the visuomotor system as being largely unreliant on conscious visual information related to a movement goal. Such a finding is in line with the PAM’s assertion of independent cortical visual pathways supporting conscious visual perception and unconscious visual regulation of action. Notably, however, the fact that unconscious target size information was available to support motor output for up to 2,000 ms of delay counters the PAM’s view that visuomotor networks maintain movement-dependent visual information only on a moment-to-moment basis. Rather, convergent evidence provides a view that the visuomotor networks process a spatially enriched (Schindler et al. 2004) and temporally durable (i.e., 2,000 ms or longer) representation of the movement environment.
Notes
The speed at which movements are completed is defined by a lawful speed-accuracy relation (i.e., MT = log2(2A/W): where MT is movement time, A is movement amplitude and W is target width (see Fitts 1954). The present investigation used radial amplitude between the movement start position and the target location (i.e., 28.1 cm) to compute index of difficulty.
We randomly presented targets left and right of fixation so that participants did not point to a single location for the duration of the experiment. We, however, did not include reaching space as a factor in our ANOVA in order to simplify our statistical model. Although reaches in right space were faster, F(1, 10) = 44.92, P < 0.001, and demonstrated reduced rightward bias, F(1, 10) = 10.94, P < 0.001, than left space counterparts, the present results parallel those of an earlier study by our group (Binsted et al. 2007) in that reaching space did not differentially influence prime and mask trials (i.e., visual stimulus by reaching space interaction: P’s > 0.35). For examination of issues related to asymmetries in left and right space see Neely et al. (2005) or Barthelemy and Boulinguez (2002).
As a matter of course we do not report null effects across all variables; however, we felt it important to document that movement times were not differentially influenced by the prime and mask conditions.
The present study did not include a condition in which targets were continuously visible to participants. It is, however, interesting to note that the average slope for MT observed in the present study (average = 13 ms) is steeper than the slope associated with a previous study by our group employing similar ID’s in the context of a visually guided reaching task (average = 5 ms: see Binsted and Heath 2005). Such memory-based size-scaling is in line with work showing robust amplitude-scaling of visually and memory-guided reach trajectories (e.g., Heath et al. 2004a, b; Heath 2005).
Mask trials demonstrated reduced rightward aiming bias relative to prime counterparts. This was a somewhat surprising finding, however, it may be that persistence of the four-dot mask during mask trials served as a spatial landmark (Krigolson et al. 2007) facilitating ocular gaze anchoring (Neggers and Bekkering 2001) thus reducing visuomotor uncertainty of target location. In addition, the fact that endpoint error and stability did not demonstrate a consistent effect of target ID in the mediolateral and anteroposterior reaching directions is consistent with work showing that speed-accuracy reach parameters differentially influence the effective coding of target distance and direction.
Personal communication with Larry Weiskrantz (March 8, 2008) indicated that trials were cued via verbal command when the experimenter perceived that the target light was extinguished. Hence, we believe that the time required for the experimenter to perceive removal of the target light, and the time required for the experimenter to produce the verbal imperative in combination with the time required for DB to identify and respond to the imperative induced a delay akin to the 1,000 ms delay used here.
References
Adamovich SV, Berkinblit MB, Fookson O, Poizner H (1999) Pointing in 3D space to remembered targets II. Effects of movement speed toward kinesthetically defined targets. Exp Brain Res 125:200–210
Aglioti S, DeSouza JFX, Goodale M (1995) Size-contrast illusions deceive the eye but not the hand. Curr Biol 5:679–685
Barthelemy S, Boulinguez P (2002) Manual asymmetries in the directional coding of reaching: further evidence for hemispatial effects and right hemisphere dominance for movement planning. Exp Brain Res 147:305–312
Binsted G, Heath M (2004) Can the motor system use a stored representation to control movement? Behav Brain Sci 27:25–27
Binsted G, Brownell K, Vorontsova Z, Heath M, Saucier D (2007) Visuomotor system uses target information unavailable to conscious awareness. Proc Natl Acad Sci USA 104:12669–12672
Bridgeman B, Lewis S, Heit G, Nagle M (1979) Relation between cognitive and motor-oriented systems of visual position perception. J Exp Psychol Hum Percept Perform 5:692–700
Bridgeman B, Gemmer A, Forsman T, Huemer V (2000) Processing spatial information in the sensorimotor branch of the visual system. Vision Res 40:3539–3552
Bruno N, Bernardis P, Gentilucci M (2008) Visually guided pointing, the Müller–Lyer illusion, and the functional interpretation of the dorsal-ventral split: conclusions from 33 independent studies. Neurosci Biobehav Rev 32(3):423–437
Chua R, Enns JT (2005) What the hand can’t tell the eye: illusion of space constancy during accurate pointing. Exp Brain Res 162:109–114
Cressman EK, Franks IM, Enns JT, Chua R (2007) On-line control of pointing is modified by unseen visual shapes. Conscious Cogn 16:265–275
Danckert J, Rossetti Y (2005) Blindsight in action: what can the different sub-types of blindsight tell us about the control of visually guided actions? Neurosci Biobehav Rev 29:1035–1046
Danckert J, Revol P, Pisella L, Krolak-Salmon P, Vighetto A, Goodale MA, Rossetti Y (2003) Measuring unconscious actions in action-blindsight: exploring the kinematics of pointing movements to targets in the blind field of two patients with cortical hemianopia. Neuropsychologia 41:1068–1081
Daprati E, Gentilucci M (1997) Grasping an illusion. Neuropsychologia 35:1577–1582
Di Lollo V, Enns JT, Rensink R. (2000) Competition for consciousness among visual events: the psychophysics of reentrant visual processes. J Exp Psychol Gen 129:481–507
Elliott D (1988) The influence of visual target and limb information on manual aiming. Can J Psychol 42:57–68
Elliott D, Allard F (1985) The utilization of visual feedback information during rapid pointing movements. Q J Exp Psychol 37:407–425
Elliott D, Binsted G, Heath M (1999) The control of goal-directed limb movements: correcting errors in the trajectory. Hum Mov Sci 18:121–136
Enns JT, Di Lollo V (2000) What’s new in visual masking? Trends Cogn Sci 4:345–352
Fitts PM (1954) The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 47:381–391
Fitts PM, Peterson JR (1964) Information capacity of discrete motor responses. J Exp Psychol 67:103–112
Gentilucci M, Chieffi S, Daprati E, Saetti MC, Toni I (1996) Visual illusion and action. Neuropsychologia 34:369–376
Goodale MA, Milner AD (1992) Separate visual pathways for perception and action. Trends Neurosci 15:20–25
Goodale MA, Westwood DA (2004) An evolving view of duplex vision: separate but interacting cortical pathways for perception and action. Curr Opin Neurobiol 14:203–211
Goodale MA, Pelisson D, Prablanc C (1986) Large adjustments in visually guided reaching do not depend on vision of the hand or perception of target displacement. Nature 320:748–750
Goodale MA, Milner AD, Jakobson LS, Carey DP (1991) A neurological dissociation between perceiving objects and grasping them. Nature 349:154–156
Goodale MA, Jakobson LS, Keillor JM (1994) Differences in the visual control of pantomimed and natural grasping movements. Neuropsychologia 32:1159–1178
Goodale MA, Westwood DA, Milner AD (2004) Two distinct modes of control for object-directed action. Prog Brain Res 144:31–144
Glover S (2004) Separate visual representations in the planning and control of action. Behav Brain Sci 27:3–78
Glover S, Dixon P (2001) Motor adaptation to an optical illusion. Exp Brain Res 137:254–258
Haffenden AM, Goodale MA (1998) The effect of pictorial illusion on prehension and perception. J Cogn Neurosci 10:122–136
Heath M (2005) Role of limb and target vision in the online control of memory-guided reaches. Motor Control 9:281–311
Heath M, Westwood DA (2003) Can a visual representation support the online control of memory-dependent reaching? Evidence from a variable spatial mapping paradigm. Motor Control 7:346–361
Heath M, Binsted G (2007) Visuomotor memory for target location in near and far reaching space. J Mot Behav 39:169–177
Heath M, Hodges NJ, Chua R, Elliott D (1998) On-line control of rapid aiming movements: unexpected target perturbations and movement kinematics. Can J Exp Psychol 52:163–173
Heath M, Rival C, Binsted G (2004a) Can the motor system resolve a premovement bias in grip aperture? Online analysis of grasping the Müller–Lyer illusion. Exp Brain Res 158:378–384
Heath M, Westwood DA, Binsted G (2004b) The control of memory-guided reaching movements in peripersonal space. Motor Control 8:76–106
Heath M, Rival C, Neely K (2006) Visual feedback schedules influence visuomotor resistance to the Müller–Lyer figures. Exp Brain Res 168:348–356
Henry FM, Rogers DE (1960) Increased response latency for complicated movements and a “memory drum” theory of neuromotor reaction. Res Q Exerc Sport 31:448–458
Held R, Gottlieb N (1958) Technique for studying adaptation to disarranged hand-eye coordination. Percept Mot Skills 8:83–86
Hu Y, Goodale MA (2000) Grasping after a delay shifts size-scaling from absolute to relative metrics. J Cogn Neurosci 12:856–868
Hu Y, Eagleson R, Goodale MA (1999) The effects of delay on the kinematics of grasping. Exp Brain Res 126:109–116
James TW, Culham J, Humphrey GK, Milner A, Goodale MA (2003) Ventral occipital lesions impair object recognition but not object-directed grasping: an fMRI study. Brain 126:2463–2475
Klapp ST (1975) Feedback versus motor programming in the control of aimed movements. J Exp Psychol Hum Percept Perform 104:161–169
Krigolson O, Heath M (2004) Background visual cues and memory-guided reaching. Hum Mov Sci 23:861–877
Krigolson O, Clark N, Heath M, Binsted G (2007) The proximity of visual landmarks impacts reaching performance. Spat Vis 20:317–336
Langolf GD, Chaffin DB, Foulke JA (1976) An investigation of Fitts’ law using a wide range of movement amplitudes. J Mot Behav 8:113–128
Lemay M, Bertram CP, Stelmach GE (2004) Pointing to an allocentric and egocentric remembered target. Motor Control 8:16–32
McIntyre J, Stratta F, Lacquaniti F (1997) Viewer-centered frame of reference for pointing to memorized targets in three-dimensional space. J Neurophysiol 78:1601–1618
Mendoza J, Hansen S, Glazebrook CM, Keetch KM, Elliott D (2005) Visual illusions affect both movement planning and on-line control: A multiple cue position on bias and goal-directed action. Hum Mov Sci 24:760–773
Michael G, Buron V (2005) The human pulvinar and stimulus-driven attentional control. Behav Neurosci 119:1353–1367
Milner AD, Goodale MA (1995) The visual brain in action. Oxford University Press, Oxford
Neely K, Binsted G, Heath M (2005) Manual asymmetries in bimanual reaching: the influence of spatial compatibility and visuospatial attention. Brain Cogn 57:102–105
Neely K, Tessmer A, Binsted G, Heath M (2008) Goal-directed reaching: movement strategies influence the weighting of allocentric and egocentric visual cues. Exp Brain Res 86:375–84
Neggers SF, Bekkering H (2001) Gaze anchoring to a pointing target is present during the entire pointing movement and is driven by a non-visual signal. J Neurophysiol 86:961–970
Obhi SS, Goodale MA (2005) The effects of landmarks on the performance of delayed and real-time pointing movements. Exp Brain Res 167:335–344
Pedhazur EA (1997) Mutliple regression in behavioural research, 3rd edn. Harcourt Brace, Orlando
Perenin MT, Jeannerod M (1975) Residual vision in cortically blind hemifields. Neuropsychologia 13:1–7
Pisella L, Grea H, Tilikete C, Vighetto A, Desmurget M, Rode G, Boisson D, Rossetti Y (2000) An ‘automatic pilot’ for the hand in human posterior parietal cortex: toward reinterpreting optic ataxia. Nat Neurosci 3:729–736
Plamondon R, Alimi AM (1997) Speed/accuracy trade-offs in target directed movements. Behav Brain Sci 20:279–349
Rolheiser TM, Binsted G, Brownell KJ (2006) Visuomotor representation decay: influence on motor systems. Exp Brain Res 173:698–707
Rosenbaum DA (1980) Human movement initiation: specification of arm, direction and extent. J Exp Psychol Gen 109:444–474
Schindler I, Rice NJ, McIntosh RD, Rossetti Y, Vighetto A, Milner AD (2004) Automatic avoidance of obstacles is a dorsal stream function: evidence from optic ataxia. Nat Neurosci 7:779–784
Velay JL, Beaubaton D (1986) Influence of visual context on pointing movement accuracy. Cah Psychol Cogn 6:447–456
Weidner R, Shah NJ, Fink GR (2006) The neural basis of perceptual hypothesis generation and testing. J Cogn Neurosci 18:258–266
Weiskrantz L, Warrington EK, Sanders MD, Marshall J (1974) Visual capacity in the hemianopic field following a restricted occipital ablation. Brain 97:709–728
Westwood DA, Goodale MA (2003) Perceptual illusion and the real-time control of action. Spat Vis 16:243–254
Westwood DA, Heath M, Roy EA (2000) The effect of a pictorial illusion on closed-loop and open-loop prehension. Exp Brain Res 134:456–463
Westwood DA, Heath M, Roy EA (2001) The accuracy of reaching movements in brief delay conditions. Can J Exp Psychol 55:304–10
Westwood DA, Heath M, Roy EA (2003) No evidence for accurate visuomotor memory: systematic and variable error in memory-guided reaching. J Mot Behav 35:127–133
Woodworth RS (1899) The accuracy of voluntary movement. Psychol Rev 3:1–114
Acknowledgments
Natural Sciences and Engineering Research Council of Canada Discovery Grants (MH, GB) and a University of Western Ontario Major Academic Development Fund (MH) supported this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heath, M., Neely, K.A., Yakimishyn, J. et al. Visuomotor memory is independent of conscious awareness of target features. Exp Brain Res 188, 517–527 (2008). https://doi.org/10.1007/s00221-008-1385-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-008-1385-x