Abstract
Paired associative stimulation (PAS) can increase motor cortical excitability, possibly by long-term potentiation (LTP)-like mechanisms. As the capability of the cortex for plasticity decreases with age, we were interested here in testing interindividual variability and age-dependency of the PAS effect. Motor-evoked potentials (MEPs) were recorded from the resting right abductor pollicis brevis muscle before and for 30 min after PAS in 27 healthy subjects (22–71 years of age). PAS consisted of 225 pairs (rate, 0.25 Hz) of right median nerve stimulation followed at an interval equaling the individual N20-latency of the median nerve somatosensory-evoked cortical potential plus 2 ms by transcranial magnetic stimulation of the hand area of left primary motor cortex (PASN20+2). The PASN20+2-induced changes in MEP amplitude (ratio post PAS/pre PAS) were highly variable (1.00 ± 0.07, range 0.36–1.68). Fourteen subjects showed the expected LTP-like MEP increase (responders) while 13 subjects showed a long-term depression (LTD)-like MEP decrease (non-responders). Responders had a significantly lower resting motor threshold (RMT) and minimum stimulus intensity to elicit MEPs of 1 mV (MEP1 mV) than non-responders. RMT and MEP1 mV correlated significantly negatively with the PASN20+2 effect. The absolute PASN20+2 effect size irrespective of its direction decreased with age (r = −0.57, P = 0.002), i.e., LTP-like and LTD-like plasticity were large in young subjects but substantially smaller in elderly subjects. In conclusion, measures of motor cortical excitability (RMT, MEP1 mV) and age determine direction and magnitude of PAS effects in individual subjects.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paired associative stimulation (PAS) is a now widely used protocol to non-invasively induce lasting changes in the excitability of the human motor cortex. First described in 2000 (Stefan et al. 2000), it employs repetitive pairing of a peripheral electrical stimulus (most often applied to the median nerve), which precedes a single pulse of transcranial magnetic stimulation (TMS) applied to the hand area of the contralateral primary motor cortex (M1) by a distinct interstimulus interval. Shaped after models of associative plasticity in animal neocortical slices (Markram et al. 1997), PAS induces a rapidly evolving (<30 min), long-lasting (>60 min), yet reversible, and input-specific increase in corticomotor excitability when the interval between the two associative stimuli is timed to generate near-synchronous events in the motor cortex (Stefan et al. 2000; Wolters et al. 2003; Ziemann et al. 2004; Morgante et al. 2006; Weise et al. 2006; Meunier et al. 2007; Muller et al. 2007). The site of this action has been located at a cortical level (Stefan et al. 2002; Wolters et al. 2003), but recently it was shown that spinal mechanisms might also be involved (Meunier et al. 2007). The facilitatory effect of PAS on corticomotor excitability could be blocked by administration of an N-methyl-d-aspartate (NMDA) receptor antagonist (Stefan et al. 2002). Given these properties, it was proposed (Stefan et al. 2002) that PAS relies on mechanisms similar to long-term potentiation (LTP) as studied at a cellular level (Bliss and Collingridge 1993; Cooke and Bliss 2006). A possible neural substrate for the effects induced by PAS may be LTP of horizontal cortico-cortical connections within M1 (Rioult-Pedotti et al. 1998; Rioult-Pedotti et al. 2000).
Several PAS studies from different groups are now reported in the literature (Wolters et al. 2003; Stefan et al. 2004; Ziemann et al. 2004; Stinear and Hornby 2005; Fratello et al. 2006; Morgante et al. 2006; Quartarone et al. 2006; Rosenkranz and Rothwell 2006; Ueki et al. 2006; Meunier et al. 2007; Muller et al. 2007; Sale et al. 2007). It emerges from these studies that PAS-induced changes in corticomotor excitability seem to be quite variable across subjects. Screening data from the work of Stefan and colleagues (2004) suggested that only ∼75% of naïve subjects show an LTP-like increase in motor-evoked potential (MEP) amplitude after PAS. Similar results were reported for effects induced by a PAS protocol on lower limb muscles (Fig. 3a in Stinear and Hornby 2005). In addition, Fratello and colleagues (2006) showed low intraindividual reproducibility of PAS effects across sessions with some subjects showing a PAS-induced decrease rather than increase in MEP amplitude in single sessions (Fig. 2 in Fratello et al. 2006). Although several studies have now reported high levels of interindividual variability (Stefan et al. 2000; Quartarone et al. 2003; Stinear and Hornby 2005; Bagnato et al. 2006), individual data were most often not provided.
There is considerable evidence for age-dependency of other forms of plastic changes within human motor cortex (Sawaki et al. 2003; Ward et al. 2007). Practice-dependent plasticity declines as a function of age with subjects older than ∼50 years experiencing only minor gains (Sawaki et al. 2003). Moreover, this age-related decline in motor performance may be due to a reduced ability to modulate activity appropriately (i.e., task-specifically) in motor networks (Ward et al. 2007). Furthermore, normal aging is associated with a relative decrease in the excitability of inhibitory circuits within M1 (Peinemann et al. 2001), which may have a role in gating stimulation-induced motor cortical plasticity (Ziemann et al. 1998).
In the present study, we specifically addressed interindividual variability and age-dependency of PAS-induced effects on MEP amplitude in a small hand muscle. Given previous evidence for an age-dependency of other forms of motor cortical plasticity (Sawaki et al. 2003; Ward et al. 2007), we hypothesized that PAS-induced effects on MEP amplitude decline with age.
Methods
Subjects
Twenty-seven healthy subjects aged 22–71 years, participated in this study (16 females; mean age, 38.2 ± 3.1 years; median, 31 years). All subjects were consistent right-handers according to the Edinburgh Handedness Inventory (Oldfield 1971). No subject had a history of neurological disease or was on CNS-active drugs at the time of the experiments. All subjects completed the adult safety screen questionnaire (Keel et al. 2001) and written informed consent was obtained prior to participation. The experiments conformed to the Declaration of Helsinki and were approved by the ethics committee of Johann Wolfgang Goethe University Hospital, Frankfurt am Main, Germany.
Electromyography
Subjects were seated comfortably in a reclining chair with their eyes open. Motor-evoked potentials (MEPs) were recorded from the right abductor pollicis brevis (APB) muscle by surface electromyography (EMG), using 1-cm Ag–AgCl cup electrodes in a belly-tendon montage. The EMG raw signal was amplified and band-pass filtered (20 Hz to 2 kHz; Counterpoint Mk2 electromyograph; Dantec, Skovlunde, Denmark), digitized at an A/D rate of 5 kHz (CED Micro 1401; Cambridge Electronic Design, Cambridge, UK) and stored in a laboratory computer for online visual display and later offline-analysis using customized data collection and conditional averaging software (Spike 2 for Windows, Version 3.05, CED).
Transcranial magnetic stimulation
Transcranial magnetic stimulation (TMS) was delivered through a figure-of-eight coil (diameter of each wing, 70 mm) connected to a Magstim 200 magnetic stimulator (Magstim Co., Carmarthenshire, Wales, UK) with a monophasic current waveform. The coil was held tangential to the scalp with the handle pointing backwards and 45° away from the midline to activate the corticospinal system preferentially transsynaptically via horizontal cortico-cortical connections (Di Lazzaro et al. 2004). The optimal coil position over the hand area of the left primary motor cortex (M1) for eliciting MEPs in the right APB (‘hot spot’) was determined as the site where TMS at a slightly suprathreshold intensity consistently produced the largest MEPs. This site was marked with a soft-tipped pen in order to assure a constant placement of the coil throughout the session. Resting motor threshold (RMT) was defined to the nearest 1% of maximum stimulator output (MSO) as the lowest stimulus intensity which elicited MEPs > 50 μV in at least five out of ten consecutive trials.
Paired associative stimulation
Paired associative stimulation (PAS) was applied according to a protocol previously used by our group inducing LTP-like increases in MEP amplitude (Ziemann et al. 2004; McDonnell et al. 2007; Muller et al. 2007). It consisted of 225 pairs of electrical stimulation of the right median nerve at the level of the wrist and a single TMS pulse over the hot spot of the APB motor representation of the left M1 (see above) at a rate of 0.25 Hz. Electrical stimulation was applied through a bipolar electrode (cathode proximal), using constant current square wave pulses (duration, 1 ms) at an intensity of three times the perceptual threshold. The intensity of TMS was adjusted to produce MEPs of, on average, 1 mV peak-to-peak amplitude (MEP1 mV) in the resting APB when given without the preceding median nerve stimulus. The interstimulus interval (ISI) between median nerve and M1 stimulation equaled the individual N20-latency of the median nerve somatosensory-evoked cortical potential plus 2 ms (PASN20+2). In order to maintain a standardized level of attention during the PAS intervention, subjects were instructed to stay alert, voluntarily relax the right APB and count the number of median nerve stimuli.
Time line of experiments
The time points of MEP measurements are shown in Fig. 1 (B0, B1–B6). At time point B0, the TMS intensity was adjusted to MEP1 mV. After 15 min of PASN20+2 (see above) MEPs were recorded at time points B1–B6 using exactly the same stimulus intensity as at baseline. At each time point (B0, B1–B6), 20 MEPs were obtained in the resting right APB muscle at a rate of 0.1 Hz with a random intertrial interval variation of 25% to limit anticipation of the next trial. Complete voluntary relaxation of the APB muscle was monitored audio-visually with high-gain EMG (50 μV/division). Trials contaminated with voluntary EMG activity were discarded from the analysis. MEP measurements at time points B1–B6 were conducted every 5 min; thus, measurements at time points B1–B6 covered a period of 30 min after PASN20+2.
Time line of experiments. B0 and B1–B6 denote time points of motor-evoked potential (MEP) measurements recorded in the resting right abductor pollicis brevis muscle. At time point B0, transcranial magnetic stimulation (TMS) intensity was adjusted to produce MEPs of, on average, 1 mV peak-to-peak amplitude (MEP1 mV). Paired associative stimulation (PAS) was applied at an interstimulus interval of the individual N20-latency of the median nerve somatosensory-evoked cortical potential plus 2 ms (PASN20+2). Measurements of MEP amplitudes at time points B1–B6, using the same TMS intensity as at time point B0, covered a period of 30 min after the end of PASN20+2. RMT, resting motor threshold
Data analysis and statistics
For each subject and time point, the single-trial peak-to-peak MEP amplitudes were averaged and normalized to the MEP amplitude measured at baseline (time point B0). PAS-induced plasticity was assessed by the grand average of normalized MEP amplitudes measured at time points B1–B6. Values > 1.0 indicate responders to PASN20+2, whereas values ≤ 1.0 indicate non-responders to PASN20+2.
To test for the effects of PASN20+2 we conducted a repeated measures analysis of variance (rmANOVA) with the within-subjects factor TIME (B0, B1, B2, B3, B4, B5, B6). Sphericity was tested by Mauchly’s test.
To quantify the effect size of PASN20+2-induced changes in MEP amplitudes irrespective of their direction (absolute PASN20+2 effect) we calculated |1 − ΣB i /6B0| with i = 1,...,6, that is the absolute value of 1 minus the grand average of MEPs at time points B1–B6 normalized to the baseline at time point B0.
In order to evaluate the age-dependency of PASN20+2-induced motor cortical plasticity, a linear regression analysis was computed with the absolute PASN20+2 effect as the dependent and age as the independent variable. To evaluate the dependency of the PASN20+2 effect on motor cortical excitability, the same analysis was performed with the PASN20+2 effect as the dependent variable and RMT and MEP1 mV, respectively, as the independent variables.
In all tests, the level of statistical significance was set to P < 0.05. All data are expressed as the mean ± 1 standard error of the mean (SEM).
Results
Interindividual variability
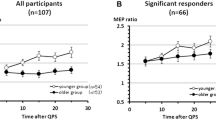
RMANOVA did not show a significant main effect of TIME (F 6,156 = 0.47, P = 0.83). This result was due to substantial interindividual variability of the PASN20+2-induced changes in MEP amplitude (1.00 ± 0.07, range 0.36–1.68). Fourteen of the 27 subjects (52%) showed the expected increase in MEP amplitude after PASN20+2 (responders), while 13 subjects (48%) showed a decrease (non-responders; see Fig. 2a).
Interindividual variability of paired associative stimulation (PASN20+2) effects. a PASN20+2 effects were assessed by the grand average of motor evoked potential (MEP) amplitudes at time points B1 to B6 normalized to the baseline at time point B0. Values > 1.0 indicate responders to PASN20+2, whereas values ≤ 1.0 indicate non-responders to PASN20+2. PASN20+2 induced motor cortical plasticity showed substantial interindividual variability (PASN20+2 effect 1.00 ± 0.07, range 0.36–1.68) with 14 of 27 subjects (52%) being responders (1.26 ± 0.06), and the remaining 13 subjects (48%) being non-responders to PASN20+2 (0.73 ± 0.06). The PASN20+2 effects from two subjects with the same age (25 years) were virtually identical and, therefore, their data points map to the same spot. Note the decreasing magnitude of PASN20+2 induced MEP changes with age. b Resting motor threshold (RMT) was measured at baseline and expressed as percentage of maximum stimulator output (% MSO). RMT correlated negatively with the PASN20+2 effect (r = −0.53, P = 0.005). c MEP1 mV denotes the transcranial magnetic stimulation intensity at baseline adjusted to produce MEPs of, on average, 1 mV peak-to-peak amplitude in the resting right abductor pollicis brevis muscle (in % MSO). MEP1 mV correlated negatively with the PASN20+2 effect (r = −0.48, P = 0.01)
Responders (PASN20+2 effect, 1.26 ± 0.06) had a significantly lower RMT (39.3 ± 1.3% MSO) than non-responders (PASN20+2 effect, 0.73 ± 0.06; RMT, 47.6 ± 2.1% MSO; unpaired two-tailed Student’s t test; T = 3.45, P = 0.002). RMT correlated negatively with the PASN20+2 effect, i.e., a low RMT was associated with a strong LTP-like increase in MEP amplitude, whereas a high RMT was associated with a long-term depression (LTD)-like decrease in MEP amplitude (r = −0.53, P = 0.005; Fig. 2b). Likewise, the stimulus intensity needed to elicit MEP1 mV was significantly lower in responders (48.4 ± 2.2% MSO) than in non-responders (59.2 ± 2.7% MSO; unpaired two-tailed Student’s t test; T = 3.10, P = 0.005), while these intensities produced MEPs of comparable size in responders (0.93 ± 0.06 mV) and non-responders (1.06 ± 0.1 mV) at baseline (time point B0; unpaired two-tailed Student’s t test; T = 1.12, P > 0.2). MEP1 mV correlated negatively with the PASN20+2 effect, i.e., a low MEP1 mV was associated with an LTP-like increase in MEP amplitude, whereas a high MEP1 mV was associated with an LTD-like decrease in MEP amplitude (r = −0.48, P = 0.01; Fig. 2c).
Age-dependency
The magnitude of the absolute PASN20+2 effect, that is the effect size of PASN20+2-induced changes in MEP amplitude irrespective of their direction, decreased linearly as a function of age (r = −0.57, P = 0.002; Fig. 3). This indicates that PASN20+2 induced large-size LTP- and LTD-like changes in MEP amplitude in young subjects whereas these effects were substantially decreased in elderly subjects. This result was confirmed by dividing our study sample into two groups (young vs. elderly subjects). The cut-off between groups was either set to the median of age of our study sample (31 years) or to a biologically more meaningful age of 50 years, which according to previous work revealed an age-dependent group effect on other forms of motor cortical plasticity (Sawaki et al. 2003; Floel et al. 2005). The young groups showed a significantly larger absolute PASN20+2 effect than the elderly groups for both cut-off ages (Table 1). Importantly, neither RMT nor MEP1 mV were correlated with age (RMT, r = 0.19, P > 0.3; MEP1 mV, r = 0.11, P > 0.5; see also Table 1), and therefore cannot explain the age-related decline in PASN20+2 induced motor cortical plasticity.
Age-dependency of paired associative stimulation (PASN20+2 ) effects. The absolute PASN20+2 effect was calculated as |1 – ΣB i /6B0| with i = 1,...,6, that is the absolute value of 1 minus the grand average of MEPs at time points B1 to B6 normalized to the baseline at time point B0. The magnitude of the absolute PASN20+2 effect decreased as a function of age (r = −0.57, P = 0.002)
Discussion
The results of our study show that there is substantial interindividual variability of the after-effects induced by paired associative stimulation (PASN20+2 protocol) (Ziemann et al. 2004; McDonnell et al. 2007; Muller et al. 2007). Fourteen of the 27 subjects (52%) showed the expected increase in MEP amplitude after PASN20+2 (responders) while thirteen subjects (48%) showed a decrease (non-responders). Responders had a lower resting motor threshold (RMT) and transcranial magnetic stimulation intensity needed to elicit motor-evoked potentials (MEPs) of 1 mV amplitude (MEP1 mV) than non-responders. Both RMT and MEP1 mV were correlated negatively with the PASN20+2 effect on MEP amplitude. Moreover, findings revealed that the effect size of motor cortical plasticity induced by PASN20+2 decreased linearly with age: while the effect size was large in young subjects, PASN20+2 failed to induce motor cortical plasticity in elderly subjects.
Interindividual variability of PAS effects
Several studies reported considerable interindividual variability to PAS protocols both for upper and lower limb muscles (Stefan et al. 2000; Stinear and Hornby 2005; Bagnato et al. 2006; Fratello et al. 2006). Our findings suggest that only about two-thirds (9/14) of young healthy subjects [mean age, 25.4 ± 0.8 (SEM) years; see Table 1; Fig. 2a] respond with an LTP-like increase in MEP amplitude to PASN20+2. The rate of responders to PASN20+2 in our study is in line with prior work by Stefan and colleagues (2004), where 76% of naïve young healthy subjects [mean age, 24.6 ± 3.8 (SD) years] showed an increase in MEP amplitude after PAS in a screening session. Comparable results also came from a study by Fratello and colleagues (2006) where a rate of ∼78% of naïve young subjects [mean age, 26.1 ± 0.6 (SEM) years] was reported as responders to PAS (cf. Fig. 2, first session in Fratello et al. 2006). Also, previous work from our group reported the occurrence of LTD-like MEP depression in single subjects after the PASN20+2 protocol (cf. Fig. 2, placebo condition in McDonnell et al. 2007; cf. Fig. 4 in Muller et al. 2007). Furthermore, it emerges that a number of protocol parameters may influence PAS-induced changes in MEP amplitude; among them the frequency, intensity, number and interstimulus interval of the two associative stimuli (Wolters et al. 2003), attention (Stefan et al. 2004), the activation level of the target muscle (Kujirai et al. 2006) and the time of day (Sale et al. 2007).
A high interindividual variability of MEP changes induced by other repetitive TMS protocols (rTMS) was also noted previously: most young subjects respond by an MEP decrease to low-frequency (1 Hz) rTMS and an increase to high-frequency (>5 Hz) rTMS, but a smaller subgroup (∼25% of the subjects) showed the opposite effects, i.e., an MEP increase to low-frequency rTMS and a decrease to high-frequency rTMS (Maeda et al. 2000; Gangitano et al. 2002). Although those authors already speculated on individual anatomical differences in the orientation of sulci and gyri or differences in the orientation of motor cortical interneurons, the reason for the observed substantial interindividual variation in stimulation-induced motor cortical plasticity remained unclear.
There is strong evidence that the neuronal mechanisms underlying PAS involve those of synaptic plasticity (Bliss and Collingridge 1993; Cooke and Bliss 2006). PAS-induced effects on corticomotor excitability evolve rapidly (<30 min), are long-lasting (>60 min), yet reversible, input-specific (Stefan et al. 2000; Wolters et al. 2003; Ziemann et al. 2004; Morgante et al. 2006; Weise et al. 2006; Meunier et al. 2007; Muller et al. 2007) and can be blocked by administration of an NMDA receptor antagonist (Stefan et al. 2002; Wolters et al. 2003). Accordingly, one possible explanation for the observed substantial interindividual variability to PAS may be constituted by genetic polymorphisms of neural signals involved in synaptic plasticity. For example, a single-nucleotide missense polymorphism in the gene coding for the brain-derived neurotrophic factor (BDNF), one of the key neural signals orchestrating synaptic plasticity, is associated with modified experience-dependent motor cortical plasticity (Kleim et al. 2006). It is intriguing to assume that polymorphisms of key neural signals for synaptic plasticity are also associated with the broad variation of PAS-induced motor cortical plasticity among subjects observed in this and previous studies. This idea gains support from our findings that responders to PASN20+2 showed a more excitable motor cortex (lower values for RMT and MEP1 mV) than non-responders, and that these measures correlated negatively with the PASN20+2 effect (Fig. 2b–c). RMT and MEP1 mV may thus reflect part of a genetically determined endophenotype (Wassermann 2002), which is involved in determining magnitude and direction of stimulation-induced motor cortical plasticity in a given individual.
In contrast, there was no difference between the sensory perception threshold of electrical stimulation of the median nerve between responders (0.80 ± 0.07 mA) and non-responders (0.92 ± 0.07 mA; P > 0.2), and sensory perception threshold did not correlate with the PASN20+2 effect (r = −0.30, P = 0.13). These findings point to a network-specific conjunction between motor cortical excitability measures and PAS effects. RMT is thought to reflect excitability of cortico-cortical axons projecting onto corticospinal neurons (Ziemann et al. 1996), and this can be directly linked to the notion that the same cortico-cortical connections serve as the neural substrate of LTP (Rioult-Pedotti et al. 1998, 2000) and PAS-induced LTP-like plasticity (Ziemann et al. 2004) in motor cortex.
Recently, it was shown that musicians regulate motor cortical excitability and plasticity with a higher gain than non-musicians (Rosenkranz et al. 2007). Musicians showed steeper recruitment curves for excitatory corticospinal and intracortical inhibitory projections as compared to non-musicians and their range of PAS-induced changes of MEP amplitudes and corticospinal projection recruitment was wider. As these neurophysiological characteristics partly depended on the age at which instrumental playing was commenced and on practice intensity, the authors hypothesized that the motor cortical excitability measures may reflect neural adaptations to long-term musical practice such as an increase in the number and modifiability of synapses. Thus, it is well possible that responders as opposed to non-responders to PAS show different anatomical and physiological characteristics, partly as a consequence of endophenotypic traits, and partly reflecting experience-/training-induced neural plasticity. These traits likely allow responders to recruit corticospinal projections and mechanisms of synaptic plasticity more efficiently than non-responders. Musicians then constitute a special and long-term practice-trained group of strong responders.
Fratello and colleagues (2006) reported high intraindividual test–retest variability of PAS effects, with some subjects even showing a decrease in MEP amplitude in one and an increase in another PAS session (Fig. 2 in Fratello et al. 2006). Therefore, an alternative explanation to account for the interindividual variability of PAS effects may be homeostatic metaplasticity. This is currently thought to constitute a fundamental stabilizing mechanism to enable both selective modification and maintenance of synaptic strength in a physiological range. The PAS effect on motor cortical excitability critically depends on the recent history of neuronal activity in M1. The LTP-like effect of PASN20+2 could be suppressed or, in some instances, even switched to an LTD-like effect, if it was preconditioned by another PASN20+2 protocol (Muller et al. 2007). However, homeostatic metaplasticity is unlikely to explain the present findings, as only one PAS protocol was performed per subject under standardized conditions, largely excluding systematic variation of motor activity prior to PAS.
Age-dependency of PAS effects
We found that the magnitude of PASN20+2-induced motor cortical plasticity substantially decreases with age: while the effect size of PASN20+2-induced motor cortical plasticity was large in young subjects, PASN20+2 failed to induce motor cortical plasticity in elderly subjects (Fig. 3; Table 1). There is considerable evidence for age-dependency of other forms of plastic changes within human motor cortex (Sawaki et al. 2003; Ward et al. 2007). Practice-dependent plasticity declines as a function of age with subjects older than ∼50 years experiencing only minor gains (Sawaki et al. 2003). Moreover, this age-related decline in motor performance may be due to a reduced ability to modulate activity appropriately (i.e., task-specifically) in motor networks (Ward et al. 2007). Our findings are in line with these reports of an age-related decline in the capacity of the human motor cortex to undergo plastic change. It is important to note that, consistent with previous findings (Wassermann 2002; Pitcher et al. 2003), there was no effect of age on RMT, and therefore variation of motor cortical excitability per se cannot explain the age-related decline in PAS induced plasticity.
In contrast, other studies showed a preserved LTP-like response to PAS in elderly subjects (Bagnato et al. 2006; Morgante et al. 2006; Ueki et al. 2006). The reasons for this discrepancy are not entirely clear. Differences in the PAS protocols with respect to the frequency, number and interstimulus interval of the two associative stimuli may contribute, but further experiments are needed to clarify this.
Several mechanisms may account for the age-related decline of PAS-induced motor cortical plasticity. It is well known that both morphological and biochemical substrates deteriorate with age (Agnati et al. 1990; Hedden and Gabrieli 2004). Neurotransmitters, which play an important role in supporting synaptic plasticity, in particular acetylcholine, dopamine and norepinephrine, show declining concentrations in the brain in the course of normal aging. Specifically, dopamine, which constitutes one of the major heterosynaptic modulators of synaptic plasticity (Jay 2003), has been shown to critically influence encoding of a new motor memory (Floel et al. 2005). In subjects older than ∼50 years of age, substantially diminished motor memory encoding was found, but upregulation of dopaminergic activity restored the encoding ability in elderly subjects to levels similar to those seen in healthy young subjects (Floel et al. 2005). Likewise, acetylcholine and norepinephrine facilitate practice-dependent plasticity in the human motor cortex (Butefisch et al. 2002; Sawaki et al. 2002; Meintzschel and Ziemann 2006). As the mechanisms of practice-dependent synaptic plasticity and PAS-induced motor cortical plasticity interact closely (Ziemann et al. 2004; Stefan et al. 2006), it is well conceivable that degeneration of cholinergic, adrenergic and/or dopaminergic neuronal circuits play an important role in the age-related decline of PAS-induced motor cortical plasticity. Furthermore, neurotrophic factors involved in synaptic plasticity such as BDNF and its receptor tyrosine kinase B, which decrease with age in the human cortex (Romanczyk et al. 2002; Webster et al. 2006), may contribute to the age-related decline in PAS-induced plasticity. Finally, synaptic connectivity decreases as a function of age in the human M1 (Adams 1987), and the present and recent work suggests that the magnitude of motor cortical plasticity depends on the number and modifiability of synapses in the motor cortex (Rosenkranz et al. 2007).
Normal aging is associated with a relative decrease in the excitability of inhibitory circuits within M1 (Peinemann et al. 2001). Short-interval intracortical inhibition in M1 correlated negatively with age, whereas intracortical facilitation was preserved in the elderly (Peinemann et al. 2001). At the spinal level, an age-related reduction in both inhibitory and facilitatory control mechanisms of motor neuron pool recruitment has been suggested in the elderly (Morita et al. 1995; Earles et al. 2001; Kido et al. 2004). Moreover, in elderly subjects, descending corticospinal volleys have a reduced capacity to recruit or synchronize spinal motor neurons appropriately (Pitcher et al. 2003; Oliviero et al. 2006). The site of action of PAS has been located to a cortical level (Stefan et al. 2002; Wolters et al. 2003), but spinal mechanisms may contribute as well to PAS-induced changes in corticomotor excitability (Meunier et al. 2007). These cortical and spinal age-related functional adaptations in motor control may also significantly contribute to the reduced PAS-induced plasticity in elderly subjects.
The age-related decline in PASN20+2-induced motor cortical plasticity closely compares to the age-related decline in use-dependent plasticity induced by repetitive thumb movement training (Sawaki et al. 2003). Thus, the question arises as to the relationship between stimulation- and practice-induced plasticity. Both plasticity protocols are thought to rely on mechanisms of synaptic plasticity on a cellular level (Butefisch et al. 2000; Stefan et al. 2002), but recently it has been reported that they may target different sets of circuits in the motor cortex (Rosenkranz and Rothwell 2006). Our results show that age and measures of motor cortical excitability (RMT, MEP1 mV) contribute to predicting the magnitude and direction of PASN20+2-induced changes in corticomotor excitability in individual subjects. Yet, how the capacity of the human motor cortex to undergo PAS-induced plastic changes links to use-dependent plasticity remains to be established.
References
Adams I (1987) Plasticity of the synaptic contact zone following loss of synapses in the cerebral cortex of aging humans. Brain Res 424:343–351
Agnati LF, Zoli M, Grimaldi R, Fuxe K, Toffano G, Zini I (1990) Cellular and synaptic alterations in the aging brain. Aging (Milano) 2:5–25
Bagnato S, Agostino R, Modugno N, Quartarone A, Berardelli A (2006) Plasticity of the motor cortex in Parkinson’s disease patients on and off therapy. Mov Disord 21:639–645
Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361:31–39
Butefisch CM, Davis BC, Sawaki L, Waldvogel D, Classen J, Kopylev L, Cohen LG (2002) Modulation of use-dependent plasticity by d-amphetamine. Ann Neurol 51:59–68
Butefisch CM, Davis BC, Wise SP, Sawaki L, Kopylev L, Classen J, Cohen LG (2000) Mechanisms of use-dependent plasticity in the human motor cortex. Proc Natl Acad Sci USA 97:3661–3665
Cooke SF, Bliss TV (2006) Plasticity in the human central nervous system. Brain 129:1659–1673
Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC (2004) The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol 115:255–266
Earles D, Vardaxis V, Koceja D (2001) Regulation of motor output between young and elderly subjects. Clin Neurophysiol 112:1273–1279
Floel A, Breitenstein C, Hummel F, Celnik P, Gingert C, Sawaki L, Knecht S, Cohen LG (2005) Dopaminergic influences on formation of a motor memory. Ann Neurol 58:121–130
Fratello F, Veniero D, Curcio G, Ferrara M, Marzano C, Moroni F, Pellicciari MC, Bertini M, Rossini PM, De Gennaro L (2006) Modulation of corticospinal excitability by paired associative stimulation: reproducibility of effects and intraindividual reliability. Clin Neurophysiol 117:2667–2674
Gangitano M, Valero-Cabre A, Tormos JM, Mottaghy FM, Romero JR, Pascual-Leone A (2002) Modulation of input–output curves by low and high frequency repetitive transcranial magnetic stimulation of the motor cortex. Clin Neurophysiol 113:1249–1257
Hedden T, Gabrieli JD (2004) Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci 5:87–96
Jay TM (2003) Dopamine: a potential substrate for synaptic plasticity and memory mechanisms. Prog Neurobiol 69:375–390
Keel JC, Smith MJ, Wassermann EM (2001) A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol 112:720
Kido A, Tanaka N, Stein RB (2004) Spinal excitation and inhibition decrease as humans age. Can J Physiol Pharmacol 82:238–248
Kleim JA, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC (2006) BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci 9:735–737
Kujirai K, Kujirai T, Sinkjaer T, Rothwell JC (2006) Associative plasticity in human motor cortex during voluntary muscle contraction. J Neurophysiol 96:1337–1346
Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A (2000) Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res 133:425–430
Markram H, Lubke J, Frotscher M, Sakmann B (1997) Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275:213–215
McDonnell MN, Orekhov Y, Ziemann U (2007) Suppression of LTP-like plasticity in human motor cortex by the GABA(B) receptor agonist baclofen. Exp Brain Res 180:181–186
Meintzschel F, Ziemann U (2006) Modification of practice-dependent plasticity in human motor cortex by neuromodulators. Cereb Cortex 16:1106–1115
Meunier SO, Russmann H, Simonetta-Moreau M, Hallett M (2007) Changes in spinal excitability after PAS (paired associative stimulation). J Neurophysiol (in press)
Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R (2006) Motor cortex plasticity in Parkinson’s disease and levodopa-induced dyskinesias. Brain 129:1059–1069
Morita H, Shindo M, Yanagawa S, Yoshida T, Momoi H, Yanagisawa N (1995) Progressive decrease in heteronymous monosynaptic Ia facilitation with human ageing. Exp Brain Res 104:167–170
Muller JF, Orekhov Y, Liu Y, Ziemann U (2007) Homeostatic plasticity in human motor cortex demonstrated by two consecutive sessions of paired associative stimulation. Eur J Neurosci 25:3461–3468
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Oliviero A, Profice P, Tonali PA, Pilato F, Saturno E, Dileone M, Ranieri F, Di Lazzaro V (2006) Effects of aging on motor cortex excitability. Neurosci Res 55:74–77
Peinemann A, Lehner C, Conrad B, Siebner HR (2001) Age-related decrease in paired-pulse intracortical inhibition in the human primary motor cortex. Neurosci Lett 313:33–36
Pitcher JB, Ogston KM, Miles TS (2003) Age and sex differences in human motor cortex input–output characteristics. J Physiol 546:605–613
Quartarone A, Bagnato S, Rizzo V, Siebner HR, Dattola V, Scalfari A, Morgante F, Battaglia F, Romano M, Girlanda P (2003) Abnormal associative plasticity of the human motor cortex in writer’s cramp. Brain 126:2586–2596
Quartarone A, Rizzo V, Bagnato S, Morgante F, Sant’angelo A, Girlanda P, Roman Siebner H (2006) Rapid-rate paired associative stimulation of the median nerve and motor cortex can produce long-lasting changes in motor cortical excitability in humans. J Physiol 575:657–670
Rioult-Pedotti MS, Friedman D, Donoghue JP (2000) Learning-induced LTP in neocortex. Science 290:533–536
Rioult-Pedotti MS, Friedman D, Hess G, Donoghue JP (1998) Strengthening of horizontal cortical connections following skill learning. Nat Neurosci 1:230–234
Romanczyk TB, Weickert CS, Webster MJ, Herman MM, Akil M, Kleinman JE (2002) Alterations in trkB mRNA in the human prefrontal cortex throughout the lifespan. Eur J Neurosci 15:269–280
Rosenkranz K, Rothwell JC (2006) Differences between the effects of three plasticity inducing protocols on the organization of the human motor cortex. Eur J Neurosci 23:822–829
Rosenkranz K, Williamon A, Rothwell JC (2007) Motorcortical excitability and synaptic plasticity is enhanced in professional musicians. J Neurosci 27:5200–5206
Sale MV, Ridding MC, Nordstrom MA (2007) Factors influencing the magnitude and reproducibility of corticomotor excitability changes induced by paired associative stimulation. Exp Brain Res 181(4):615–626
Sawaki L, Boroojerdi B, Kaelin-Lang A, Burstein AH, Butefisch CM, Kopylev L, Davis B, Cohen LG (2002) Cholinergic influences on use-dependent plasticity. J Neurophysiol 87:166–171
Sawaki L, Yaseen Z, Kopylev L, Cohen LG (2003) Age-dependent changes in the ability to encode a novel elementary motor memory. Ann Neurol 53:521–524
Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J (2002) Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol 543:699–708
Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J (2000) Induction of plasticity in the human motor cortex by paired associative stimulation. Brain 123 (Pt 3):572–584
Stefan K, Wycislo M, Classen J (2004) Modulation of associative human motor cortical plasticity by attention. J Neurophysiol 92:66–72
Stefan K, Wycislo M, Gentner R, Schramm A, Naumann M, Reiners K, Classen J (2006) Temporary occlusion of associative motor cortical plasticity by prior dynamic motor training. Cereb Cortex 16:376–385
Stinear JW, Hornby TG (2005) Stimulation-induced changes in lower limb corticomotor excitability during treadmill walking in humans. J Physiol 567:701–711
Tecchio F, Zappasodi F, Pasqualetti P, Gennaro L, Pellicciari M, Ercolani M, Squitti R, Rossini P (2008) Age dependence of primary motor cortex plasticity induced by paired associative stimulation. Clin Neurophysiol 119(3):675–682. doi:10.1016/j.clinph.2007.10.023
Ueki Y, Mima T, Kotb MA, Sawada H, Saiki H, Ikeda A, Begum T, Reza F, Nagamine T, Fukuyama H (2006) Altered plasticity of the human motor cortex in Parkinson’s disease. Ann Neurol 59:60–71
Ward NS, Swayne OB, Newton JM (2007) Age-dependent changes in the neural correlates of force modulation: an fMRI study. Neurobiol Aging (in press)
Wassermann EM (2002) Variation in the response to transcranial magnetic brain stimulation in the general population. Clin Neurophysiol 113:1165–1171
Webster MJ, Herman MM, Kleinman JE, Shannon Weickert C (2006) BDNF and trkB mRNA expression in the hippocampus and temporal cortex during the human lifespan. Gene Expr Patterns 6:941–951
Weise D, Schramm A, Stefan K, Wolters A, Reiners K, Naumann M, Classen J (2006) The two sides of associative plasticity in writer’s cramp. Brain 129:2709–2721
Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J (2003) A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol 89:2339–2345
Ziemann U, Corwell B, Cohen LG (1998) Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci 18:1115–1123
Ziemann U, Ilic TV, Pauli C, Meintzschel F, Ruge D (2004) Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci 24:1666–1672
Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W (1996) Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol 40:367–378
Acknowledgments
We thank B. Bliem for constructive comments on the manuscript. This work was supported by grant ZI 542/4-1 from the German Research Foundation (DFG). A similar study has been published during the review process on this manuscript (Tecchio et al. (2008) Clin Neurophysiol Jan 4 [Epub ahead of print]).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Müller-Dahlhaus, J.F.M., Orekhov, Y., Liu, Y. et al. Interindividual variability and age-dependency of motor cortical plasticity induced by paired associative stimulation. Exp Brain Res 187, 467–475 (2008). https://doi.org/10.1007/s00221-008-1319-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-008-1319-7