Abstract
In the present experiment we used a version of the attention network test (ANT) similar to that of Callejas et al. (Exp Brain Res 167:27–37, 2005) to assess the Posner’s attention networks (alerting, orienting and conflict), and their interactions. We observed shorter reaction times with alerting tone than with no alerting tone trials (the alerting effect); with cued than with uncued trials (the orienting effect); and with congruent than with incongruent trials (the conflict effect). These results replicate previous findings with the ANT. We also manipulated cue–target interval at five stimulus onset asynchrony (SOA) values (100, 300, 500, 800, and 1,200 ms) to trace the alerting network influence over the orienting network. The SOA manipulation showed that cuing effects peaked at 300 ms SOA irrespective of whether an alerting tone was present or not, and the alerting tone improved the cuing effect equally for 100–500 SOAs, but it did not at the longest 800–1,200 ms SOAs. These results suggest that alerting improves rather than accelerates orienting effects, a result that agrees with data from neuropsychological rehabilitation of parietal patients with spatial bias.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The attention network test (ANT; Fan et al. 2002) is becoming a useful and broadly used instrument to measure the functioning of the three attention networks proposed by Posner and his colleagues: the alerting, the orienting and the executive networks (Posner and Petersen 1990). The test provides individual scores of the three networks, and allows researchers to compare performance of normal individuals with that of persons suffering from different pathologies (for a review, see Posner and Rothbart 2007). However, few studies have been conducted to look at the interactions between the networks, even when it has been assumed that the networks form part of a cognitive system, the attention system, which might work in a rather interactive way (Fuentes et al. 1999; Posner and Raichle 1994; see Fuentes 2004, for a review). Although the interaction between the executive and the other networks seems to be well established, the interaction between alerting and orienting is rather controversial. Fernandez-Duque and Posner (1997) did not find any interaction between alerting and orienting, claiming that these two networks are independent. The lack of significant correlations between the alerting and the orienting scores in the original ANT study by Fan et al. (2002) also suggests that both networks are completely independent. Finally, alerting and orienting activate different anatomical brain areas and these networks are modulated by different neurotransmitters (Fernandez-Duque and Posner 2001). The alerting network uses norepinephrine coming from the locus coeruleus (Marrocco and Davidson 1998), whereas orienting is a cholinergic network arising in the basal forebrain (Davidson and Marrocco 2000).
The aforementioned independence of alerting and orienting contrasts with data from neuropsychology. It is well documented that visual neglect is associated with a deficit in orienting attention to the contralesional side, with larger prevalence of unilateral neglect for right than left hemisphere lesions. In some patients, those lesions affecting the right orienting network might also affect sustained (tonic) alert, which depends on the integrity of right parietal and frontal areas (Heilman and van den Abell 1979; Pardo et al. 1991). Several studies have shown that phasic alerting, a transient alert state that depends on ascending thalamic projections to the right parietal lobe which are intact in these patients, can be used to ameliorate sustained alert and either right-biased (Robertson et al. 1998, 1995; see Robertson 1999, for a review) or left-biased (Dove et al. 2007) attention deficits in right-hemisphere neglect patients. Thus, the use of alerting stimuli for improving attention orienting performance in neglect patients calls for an interaction between the two networks.
One reason why some studies did not find correlation between the networks, whereas neuropsychological studies did, might be due to the way phasic alerting is implemented in the attentional tasks. Whereas the ANT uses asterisks for both alerting and orienting, studies with neglect patients used tones for alerting. This might have fostered any modulation of orienting by alerting to emerge. Callejas et al. (2005; see also Callejas et al. 2004) found an interaction between the two networks. The authors found larger cuing effects when an auditory signal served as a warning signal supposed to tap the alerting network, than when the auditory signal was not present. The effect was found just when the asynchrony between the visual cue and the target, SOA, was short (100 ms) but not long (500 ms). The authors accounted for this modulation of the cuing effect by the auditory signal as evidence of alerting accelerating rather than enhancing the effect of the visual cue. Note that the accelerating account predicts that orienting by visual cues will reach its maximal effectiveness (the asymptote) earlier with the alerting tone, than without any warning signal. A second prediction is that once the asymptote is reached in baseline conditions (i.e., without alerting tone), the alerting tone will not produce any further improvement in orienting. On the other hand, the enhancing hypothesis predicts that the time course of orienting effects will be similar for conditions with and without alerting tone, but greater orienting effects are expected with the alerting tone in all cue–target SOA values where alerting is still modulating the effect of orienting. Given that Callejas et al. (2005) based their conclusions on data from only two SOA levels, with significant effects in only one of them (100 ms SOA), their conclusion of accelerating rather than enhancing effects might have been rather premature. We think an inspection to the time course of the effect is necessary to fully understand the nature of the relationships between the alerting and the orienting networks.
In the present study, we looked at the time course of alerting modulation of orienting by manipulating cue–target SOAs at different values. We used the modified version of the ANT in which a tone served as a warning signal and cues were not informative respect to the target location (see Callejas et al. 2005). These changes from the original ANT are supposed to tap the phasic alerting network and the exogenous orienting network in an orthogonal manipulation, and therefore will allow us to assess interactive effects between the two networks.
Methods
Participants
Twenty four undergraduates form the University of Murcia participated in the experiment in return for course credit. None of the participants had prior experience with any version of the ANT, and all had normal or corrected-to-normal vision.
Stimuli and apparatus
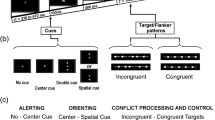
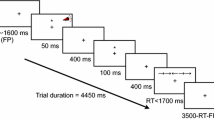
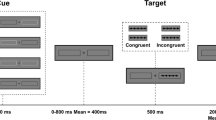
Stimuli were displayed on a 17″ CTR monitor, which was set to a screen resolution of 640 × 480 pixels. Responses were collected through the computer keyboard, and headphones were used to present the alerting tones. The basic display, which was visible throughout the experiment, consisted of a black fixation cross between two rows of five rectangular boxes vertically arranged (see Fig. 1).Footnote 1 Boxes were 40 pixels wide and 30 pixels high, with the discontinuous line that formed each box being one pixel thick. There were two pixels between every two boxes in the row, so that each five-box row was 208 pixels wide. The fixation cross was 18 pixels wide and 18 pixels high, with the line being 4 pixels wide. The distance between the fixation cross and the central box of each row was 60 pixels from centre to centre. For the visual cue, the line that formed the central box of the row increased from one to four pixels wide. In each trial, five black arrows were presented inside each of the five boxes in the row. The arrow presented in the central box was the target, whereas the arrows presented in the other four boxes were the flankers. Arrows were 36 pixels in length, with the arrowhead being from 2 to 20 pixels in height and the tail being 4 pixels high. The alerting tone was a 50 ms beep of 2,000 Hz.
Procedure
Participants were tested individually in a sound-attenuated room. The experiment consisted of two blocks of 160 trials. In each block, all possible combinations of the alerting condition (alerting tone, no alerting tone), cuing condition (cued, uncued), cue–target SOA (100, 300, 500, 800, 1,200 ms), target location (above, below), target direction (right, left), and flanker type (congruent, incongruent) were presented once. Target location and target direction were not considered as experimental factors. Thus, there were eight trials per experimental condition in the whole experiment. We did not include no cue trials in this experiment to avoid an excessive number of experimental conditions, which might have made the task too long.
The sequence of events in each trial is illustrated in Fig. 1. The basic configuration described above was presented for a variable duration between 1,200 and 2,600 ms, being the precise duration determined randomly, with the constraint that the entire range was homogeneously represented within each block of trials. The alerting tone was presented in half of the trials for 50 ms (tone condition), with an equivalent empty audio file being run in the other half of the trials (no tone condition). The orienting visual cue appeared 350 ms after the tone and was presented for 50 ms in the central box of either the upper or the lower box row. Finally, with visual cue–target SOAs of 100, 300, 500, 800, or 1,200 ms, the target and the flankers were presented either in the same row as the cue (50% of trials, the cued condition) or in the other row (50% of trials, the uncued condition). They were presented until the participant indicated whether the target arrow pointed to the right or to the left by pressing key Z or M, respectively. The target arrows pointed to the right in half of the trials and to the left in the other half. Flanker arrows could point to the same direction of the target (the congruent condition) in half of the trials, or to the opposite direction of the target (the incongruent condition) in the other half. Participants were instructed to respond as fast and accurate as possible. Ten practice trials were presented before the experimental trials.
Results
Table 1 shows the mean reaction times (RTs) and the percentage of errors per experimental condition. Accuracy was very high in this experiment with some conditions reaching 100% of correct responses. Therefore, data analyses were carried out only on RTs. In each condition, RTs above or below 2 SD from the mean were not included in the statistical analyses (3.36% of the data).
Correct RTs were submitted to a repeated measures analysis of variance (ANOVA), with alerting (tone, no tone), cuing (cued, uncued), cue–target SOA (100, 300, 500, 800, and 1,200 ms), and congruency (congruent, incongruent) as the within-subjects factors. The results showed significant main effects for all factors, F(1, 23) = 32.01 for alerting; F(1, 23) = 69.58 for cuing; F(1, 23) = 466.57 for congruency; and F(4, 92) = 4.81 for SOA (for all P < 0.001). Responses were faster for alerting tone trials than for no tone trials (alerting effect = 13.45 ms), for cued compared with uncued locations (cuing effect = 51.42 ms), and for congruent compared with incongruent flankers (congruency effect = 81.34 ms). The shortest 100 ms SOA produced longer RTs than the remaining SOAs among which there were not significant differences (an additional ANOVA without the 100 ms SOA did not show statistical significant results, F < 1).
Interactions between the networks were found here. The alerting × cuing and the cuing × congruency interactions were significant, [F(1, 23) = 23.94, P < 0.001, and F(1, 23) = 20.3, P < 0.001, respectively. That is, the alerting tone condition produced a larger cuing effect than the no alerting tone condition. This interaction between the alerting and the orienting networks replicates previous findings (Callejas et al. 2004, 2005). Also, cuing the target location reduced conflict produced by incongruent trials. That is, the cue allowed participants to orient their attention to the exact location of the forthcoming target, favouring the filtering out of distracting flankers. The cuing effect was also reduced with SOA, F(4, 92) = 4.08, P < 0.001, as is expected when non-informative cues are used for orienting attention. An inspection to Fig. 2 shows that orienting reached its asymptotic level around 300 ms SOA, irrespective of whether or not an alerting tone was presented previous to the visual cue.
However, the most relevant results in the present experiment was the significant three-way alerting × cuing × SOA interaction, F(4, 92) = 3.00, P = 0.02. Figure 2 reveals that cuing effects were larger with tone trials than with no tone trials, but that difference occurred only for 100, 300 and 500 ms SOAs. To confirm these different patterns of results as a function of SOA, we re-analyzed the three-way interaction including just the SOA values where the alerting × cuing interaction is apparent (100, 300 and 500 ms SOA), and where it is not (800 and 1,200 ms SOA). The first analysis confirmed that the alerting tone improved the cuing effect equally for the three short SOAs, that is, we observed a significant alerting × cuing interaction, F(1, 23) = 29.58, P < 0.001, that did not interact with SOA, F(2, 46) < 1. The second analysis with the two longest SOAs did not show any significant interaction, neither the alerting × cuing, nor the alerting × cuing × SOA interactions, for both F < 1. These results point out that the modulation of alerting over orienting vanishes away with cue–target intervals longer than 500 ms, at least under the parametric conditions of this experiment.
No other effects reached statistical significance.
Discussion
The present study adds to recent evidence that proves an interactive relationship between the attention networks. Overall, by using a similar version of the ANT to that of Callejas et al. (2004, 2005), we have showed an interactive pattern among the attention networks, although the interaction between alerting and conflict was not supported by statistically significant results.
Concerning the interaction between cuing and conflict, previous studies have shown that the orienting and the executive networks relate to each other in rather complex ways. For instance, Posner et al. (1987) found that when participants are told to perform the visual cuing task (primary task) concurrently with a task that involved the executive network (e.g., counting backward by threes from a fixed three-digit number), the validity effect was affected by the secondary task. Posner (1988) suggested a hierarchical relation between the networks so that the executive network would command the orienting network functioning. However, Fuentes et al. (1999) argued that the kind of relationship between the two networks may depend on the attention demands, or priorities, of tasks used to tap each network. For instance, in a task that combined inhibition of return, supposed to tap the orienting network, and attention-dependent semantic processing, supposed to tap the executive network, Fuentes et al. (1999) found that semantic priming (both facilitatory and inhibitory) vanished when targets were presented at cued (inhibited) locations. Thus, in Posner et al. (1987) the executive task might have demanded more attention than the orienting task, and therefore it affected the functioning of the visual task. However, in Fuentes et al. (1999) the orienting task might have had priority over the executive task, being the latter affected by the former. These two studies show that the two networks might interfere with each other. In contrast, Vivas and Fuentes (2001) found that inhibitory mechanisms related to orienting and executive networks might co-ordinate to accomplish an important function: to favour the organism to explore new objects or locations (see Fuentes 2004, for a review of these studies). In the present experiment we found that the visual cue helped attention to focus on the target location by filtering out distracting flankers, an operation that previous studies have attributed to the pulvinar nucleus of the thalamus (LaBerge and Buchsbaum 1990). Taken together, all these studies suggest that the way the orienting and executive networks interact depends on the main goals imposed by the tasks.
We also observed an interaction between alerting and cuing effects. This finding replicates that of Callejas et al. (2005), although as we will discuss later on we did not reach the same conclusion as to the nature of such interaction. However, it does not agree with the lack of correlation between alerting and orienting scores found in previous studies with the original ANT (e.g., Fan et al. 2002). One major difference between the present and Fan’s task is that we used an auditory signal for alerting whereas Fan et al. used visual signals (asterisks). Research has shown that auditory stimuli acting as accessory signals reduce RTs to visual targets more than visual stimuli do to auditory targets (Posner 1978). This asymmetry in the facilitatory effects of warning signals from different modalities suggests that auditory stimuli are more able to activate the alerting mechanism automatically than visual stimuli.
However, the main aim of the present study was to assess the nature of the relationships between the alerting and the orienting networks, by looking at the time course of alerting effects over orienting attention. We obtained two main findings: (1) the cuing effects lasted for a prolonged period of time peaking at 300 ms; (2) but the beneficial effect of alerting over orienting disappeared with SOAs longer than 500 ms. These results clearly contrast with those of Callejas et al. (2005) in that these authors did not observe any alerting effect over orienting at 500 ms SOA. A major difference between the two studies is that we did not include no cue trials in our experiment, which might have altered both the strength and the temporal window of alerting effects over orienting. The use of visual cues in all conditions in the present study might have increased the general alerting level throughout the experiment. This higher sustained alertness level, which is mediated by a cortical network involving right frontal and parietal lobes, might have increased the beneficial effect of the alerting tone over spatial orienting, probably due to extra activation in the superior parietal lobe, the common site for both the alerting and the orienting networks. Therefore we predict larger and longer lasting effects of the alerting tone over orienting when no cue trials are omitted than when they are included in the ANT protocol.Footnote 2 Thus, the decision of not including no cue trials in the present experiment had a dramatic effect on the alerting and conflict interaction, which is mainly observed with this kind of trials,Footnote 3 but it was beneficial for better understanding the nature of the relationships between the alerting and the orienting networks.
Finally, cuing effects improved with the alerting tone and followed a similar time course to that without the alerting tone. These results do not meet the predictions of the accelerating account proposed by Callejas et al. (2004, 2005). According to that account, the orienting asymptotic level should be observed earlier with the alerting than without the alerting tone, and once that level is reached, alerting should not produce any further benefit. However, the present results showed that orienting effects peaked at 300 ms with and without the alerting tone, and the benefit of the alerting tone was of similar magnitude for all cue–target intervals where alerting had an influence over orienting. Thus, the present results favour the enhancing hypothesis that predicts that alerting will affect attention orientation by improving the effect of the visual cue on target responses.
These results are also in line with data from neuropsychological rehabilitation of right parietal patients with visual orienting deficits. Robertson (1999) suggests that lesions to the parietal lobe might be compensated by thalamic projections from the midbrain arousal system to the parietal lobe, which are spared in these patients. The midbrain arousal system is activated by warning signals that produce a transient (phasic) alert state in the patient, similar to the alerting tone used here. The result of such rehabilitation technique is that patients’ alertness ameliorates the spatial bias, and reorienting attention to the contralateral side is clearly improved (Robertson et al. 1995).
The results of the present research not only help to understand the nature of the relationships between the alerting and the orienting networks, but also draw attention to the relevance of looking at the interactions between the attention networks in clinical settings. This might help to better characterize attention dysfunction in pathology, and reorient rehabilitation programs for remediation.
Notes
We used boxes containing the rows to have a version of the ANT more appropriate to test patients with attention pathology. The boxes help the patients to localize the target display.
The comparison between the results of common conditions in Callejas et al. (2005, Experiment 2, the only experiment that manipulated SOA) and the present study (only 100 and 500 ms SOAs are considered) showed that the improvement of cuing effects by the alerting tone were 8 versus 27 ms, respectively.
As Callejas et al. (2005, Experiment 1) acknowledge, the alerting × congruency interaction was clearer when the statistical analysis was conducted only with no cue trials. In fact when all trials were included, the interaction was marginally significant.
References
Callejas A, Lupiáñez J, Tudela P (2004) The three attentional networks: on its independence and interactions. Brain Cogn 54:225–227
Callejas A, Lupiáñez J, Funes MJ, Tudela P (2005) Modulations among the alerting, orienting and executive control networks Exp Brain Res 167:27–37
Davidson MC, Marrocco RT (2000) Local infusion of scopolamine into intraparietal cortex slows covert orienting in rhesus monkeys. J Neurophysiol 83:1536–1549
Dove ME, Eskes G, Klein RM, Shore DI (2007) A left attentional bias in chronic neglect: a case study using temporal order judgments. Neurocase 13:37–49
Fan J, McCandliss BD, Sommer T, Raz A, Posner MI (2002) Testing the efficiency and independence of attentional networks. J Cogn Neurosci 14:340–347
Fernandez-Duque D, Posner MI (1997) Relating the mechanisms of orienting and alerting. Neuropsychologia 35:477–486
Fernandez-Duque D, Posner MI (2001) Brain imaging of attentional networks in normal and pathological states. J Clin Exp Neuropsychol 23:74–93
Fuentes LJ (2004) Inhibitory processing in the attentional networks. In: Posner MI (ed) Cognitive neuroscience of attention. Guilford, New York
Fuentes LJ, Vivas AB, Humphreys GW (1999) Inhibitory mechanisms of attentional networks: spatial and semantic inhibitory processing. J Exp Psychol Hum 25:1114–1126
Heilman K, Van Den Abell T (1979) Right hemispheric dominance for mediating cerebral activation. Neuropsychologia 17:315–321
LaBerge D, Buchsbaum MS (1990) Positron emission tomographic measurements of pulvinar activity during an attention task. J Neurosci 10:613–619
Marrocco RT, Davidson MC (1998) Neurochemistry of attention. In: Parasuraman R (ed) The attentive brain. MIT Press, Cambridge
Pardo JV, Fox PT, Raichle ME (1991) Localization of a human system for sustained attention by positron emission tomography. Nature 349:61–64
Posner MI (1978) Chronometric exploration of mind. Oxford University Press, New York
Posner MI (1988) Structures and functions of selective attention. In: Boll T, Bryant BK (eds) Clinical neuropsychology and brain functions: research, measurement, and practice. APA, Washington DC
Posner MI, Petersen SE (1990) The attention system of the human brain. Ann Rev Neurosci 13:25–42
Posner MI, Raichle ME (1994) Images of mind. Scientific American Library, New York
Posner MI, Rothbart MK (2007) Research on attention networks as a model for the integration of psychological science. Ann Rev Psychol 58:1–23
Posner MI, Inhoff A, Friedrich RJ, Cohen A (1987) Isolating attentional systems: a cognitive-anatomical analysis. Psychobiology 15:107–121
Robertson IH (1999) Cognitive rehabilitation: attention and neglect. Trends Cogn Sci 3:385–393
Robertson IH, Tegnér R, Tham K, Lo A, Nimmo-Smith I (1995) Sustained attention training for unilateral neglect: theoretical and rehabilitation implications. J Clin Exp Neuropsychol 17:416–430
Robertson IH, Mattingley JB, Rorden C, Driver J (1998) Phasic alerting of neglect patients overcomes their spatial deficit in visual awareness. Nature 395:169–172
Vivas AB, Fuentes LJ (2001) Stroop interference is affected in inhibition of return. Psychon Bull Rev 8:315–323
Acknowledgments
This research was supported by the Spanish Ministerio de Educación y Ciencia (grant SEJ2005–01223/PSIC) and by the Fundación Séneca (grant 03066/PHCS/05).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fuentes, L.J., Campoy, G. The time course of alerting effect over orienting in the attention network test. Exp Brain Res 185, 667–672 (2008). https://doi.org/10.1007/s00221-007-1193-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-007-1193-8