Abstract
The aim of this study was to investigate, in healthy subjects, the modulation of amplitude and phase precision of the auditory steady-state response (ASSR) to 40 Hz stimulation in two resting conditions varying in the level of arousal. Previously, ASSR measures have shown to be affected by the level of arousal, but the findings are somewhat controversial. Generally, ASSR is diminished in sleep but it may be increased in drowsiness. Besides, ASSR reduction has been observed in schizophrenia. However, schizophrenic patients are known to have a disturbance of arousal level, what makes it pertinent to know the effects of fluctuations in arousal on passive response to gamma-range stimulation. In nine healthy volunteers trains of 40 Hz click stimuli were applied during two conditions: in the “high arousal” condition subjects were sitting upright silently reading a book of interest; in the “low arousal” condition subjects were sitting in a reclined position with eyes closed and the lights turned off. The 64-channel EEG data was wavelet transformed and the amplitude and phase precision of the wavelet transformed evoked potential were decomposed by the recently proposed multi-subject non-negative multi-way factorization (NMWF) (Morup et al. in J Neurosci Methods 161:361–368, 2007). The estimates of these measures were subjected to statistical analysis. The amplitude and phase precision of the ASSR were significantly larger during the low arousal state compared to the high arousal condition. The modulation of ASSR amplitude and phase precision by differences in the arousal level during recording warrants caution when investigating oscillatory brain activity and interpreting the findings of reduced ASSR in schizophrenia. It also emphasizes the necessity of standardized recording procedures and monitoring the level of arousal during ASSR testing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
The auditory steady-state response (ASSR) is observed when stimuli are presented periodically resulting in electroencephalographic (EEG) entrainment (Picton et al. 2003a). The frequency of the ASSR is close to the frequency of stimulation and the greatest magnitude is observed when stimuli are presented at 40 Hz (Galambos et al. 1981). The source of ASSR has been localized in the primary auditory cortex, supratemporal gyrus, and brainstem with additional activity arising from the cerebellum (Makela and Hari 1987; Hari et al. 1989; Pantev et al. 1996; Pastor et al. 2006).
Predominately, ASSR is used for testing hearing sensitivity or as a marker of the state of consciousness during anesthesia (Picton et al. 2003a, b). ASSRs have been reported to be diminished during sleep when compared to wakefulness (Linden et al. 1985; Jerger et al. 1986; Cohen et al. 1991). Thus arousal, defined as a non-specific activation of cerebral cortex in relation to sleep–wake states (Oken et al. 2006), probably affects the capacity of sensory-neural circuits to synchronize at gamma frequencies, but the details of these effects are not well known.
The 40 Hz ASSR has also been used as an index of the ability for gamma band frequency generation in local cortical networks in schizophrenia, showing a decrease in 40 Hz ASSR power and phase precision (Kwon et al. 1999; Brenner et al. 2003; Hong et al. 2004; Light et al. 2006). This was considered as a reflection of limitations in the intrinsic binding capacity of pyramidal cell networks (Kwon et al. 1999; Light et al. 2006). Notably, schizophrenic patients frequently deviate from healthy subjects in two directions, being either hyper- or hypo-aroused, i.e. arousal is an important factor in schizophrenic functioning (Venables 1984; Bernstein 1987; Cannon et al. 1988). Considering this aspect of the illness, it is pertinent to know how the fluctuations in arousal level affect the generation of the 40 Hz ASSR.
Consequently, the present study in healthy volunteers aimed at the investigation of the difference between the auditory 40 Hz ASSR in two experimental conditions differing in the level of arousal. Considering the abovementioned findings, it was initially conjectured that 40 Hz activity would be larger in the high arousal condition compared to the low arousal condition.
Methods
Subjects
Eleven healthy subjects (6 females) were included into the study. Written informed consent was obtained, as approved by the Ethics Committee, and the subjects were paid for the participation. Due to technical reasons, data of 2 subjects could not be used. The final sample consisted of 9 subjects (4 females). The mean age of the sample was 23.1 years (standard deviation, SD 1.6).
Stimulation
A stimulus was a 1-s train of clicks consisting of 40 identical clicks of 1.5 ms duration, 1–10,000 Hz white noise bursts at peak SPL of 60 dB, delivered through Sennheiser HD 565 Ovation© headphones. Seventy-two presentations of the 40 Hz train were interspersed with trains of other frequencies (8, 10, 12, 20, 30, 46 and 60 Hz, not reported here) with an inter-train interval of 1 s. Two runs lasting 22 min were recorded in each of two conditions used. Conditions were defined as follows: “high arousal” condition when the subject was sitting upright and reading a self-selected book (the attention to reading was not controlled); “low arousal” condition when the subject was sitting very reclined with closed eyes and the lights turned down. In general, the instruction given to the subjects was to let their thoughts wander and not pay attention to the stimulation. The order of stimulation conditions was not counterbalanced across subjects.
EEG recordings
Electroencephalographic data was recorded with 64-scalp electrodes (BioSemi Active electrodes system) arranged according to the International 10–10 system. Digitally linked earlobes electrodes were used as reference. The grounding electrodes were placed centrally, close to POz. Data was recorded continuously at 2048 Hz/channel and band passed at 0.1–760 Hz by a LabView© application (ActivView©) on a Windows© based PC.
Data analysis
Off-line processing was performed in ERPWAVELAB and EEGLAB for MatLab© (Delorme and Makeig 2004; Morup 2007). Wavelet transformation (WT; complex Morlet wavelet from MatLab© Wavelet Toolbox; frequencies represented from 4 to 80 Hz, 2 Hz intervals between each frequency) was performed. This yields both the wavelet transformed evoked potential measure (avWT, corresponding to WT amplitude measure) and phase synchronization index (inter-trial phase coherence, ITPC), that is best conceptualized as phase precision or synchronization of the evoked oscillations from trial to trial ranging from 0 (random phase) to 1 (nearly identical phase) (Morup et al. 2006). These measures describe evoked activity dealing with different domains of the signal—amplitude and phase—that allows a more detailed description of the response. Prior to WT, 15% of the epochs with the largest variability were rejected automatically. The avWT and the ITPC were decomposed through non-negative multi-way factorization (NMWF) (Morup et al. 2006). The application of NMWF creates time–frequency plots of the avWT and ITPC while indicating how this precision varies with experimental manipulation. In other words, the multi-subject NMWF analysis of the 3-way array of channel × time − frequency × subject—condition gives the subject-specific strength to the activity that is most common across subjects, conditions and runs, i.e. creates a subjects-weighted collapse and makes it possible to quantify (by giving the single estimation of the measure of interest) how the measure of interest varies with experimental manipulation for all the subjects in all conditions (Morup et al. 2006, 2007). Prior to NMWF analysis, random avWT and ITPC activity, estimated by calculating the mean of an artificially generated random avWT and ITPC samples, was extracted (Morup et al. 2006). The window for mathematical decomposition of avWT and ITPC was set as 20–60 Hz and −50 to +1,100 ms.
Grand averaged evoked potentials of ASSR for both conditions were created (individual averages based on 122 epochs, referenced to digitally linked earlobes, cut into epochs (−500 to +1500 ms), filtered band pass 10–50 Hz).
Baseline EEG beta power was measured at 7 electrodes (F3, F4, Fz, C3, C4, Cz, Pz), through the fast Fourier transformation of the 1,000 ms inter-trial interval of each trial. The averaged power in the beta range (12–32 Hz) was divided by the total power in the 1–32 Hz range then multiplied by 100 to get a percentage estimate for each electrode and the electrode percentages were then averaged (Cardenas et al. 1997).
The results of the NMWF decompositions were normally distributed (as indicated by Shapiro-Wilk test) and were further tested in repeated measures analysis of variance (r.m. ANOVA) (SPSS© v. 9.1) for effects of “condition”, “run” and “condition * run” interaction. Baseline power measures were tested by Student’s t test.
Results
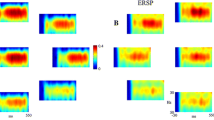
The ASSRs were detected for all subjects in both conditions. Grand averaged steady-state evoked potentials for low and high arousal conditions are presented in Fig. 1a.
a Grand averaged evoked potential for 40 Hz ASSR in low arousal (LA) and high arousal (HA) conditions. Headplots of avWT collapsed across subjects for the ASSR b and noise component e. Time-frequency plots as a weighted collapse across subjects and electrodes for the ASSR c and noise f components in low arousal (LA) and high arousal (HA) conditions. Means and standard deviations of NMWF scores of avWT for the ASSR d and noise g components in both experimental conditions
NMWF scores of avWT
The NMWF decomposition of avWT resulted in the observation of two distinct components: a component at the frequency of stimulation (Fig. 1b) and a noise component (Fig. 1e). Time–frequency plots as a weighted collapse (relative weighting strength is given by the subject scores) across subjects and electrodes for the ASSR and noise component in both conditions are presented in Fig. 1c and f. The r.m. ANOVA demonstrated that avWT of the focal 40 Hz ASSR component is significantly larger in the low arousal condition (F 1,8 = 7.463, P = 0.026; Fig. 1d). Whereas avWT of the noise component was significantly larger in the alert reading condition (F 1,8 = 30.488, P = 0.001; Fig. 1g).
NMWF scores of ITPC
The NMWF decomposition of ITPC resulted in the detection of one component (Fig. 2a). Time–frequency plots of ITPC as a weighted collapse across subjects and electrodes for 40 Hz in both conditions are presented in Fig. 2b. The r.m. ANOVA demonstrated a significant effect of condition on the ITPC: (F 1,8 = 15.391, P = 0.004), but no effect of run and no interaction effect. NMWF scores of the ITPC were higher in the low arousal condition (Fig. 2c).
Baseline beta power
The percentage of beta power was significantly lower in the low arousal condition (22.11% (SD 3.59) vs. 19.45% (SD 4.62); t = −2,833; df = 17, P = 0.017).
Discussion
The main finding of the present study is opposite to the study hypothesis: an increase of amplitude and phase precision of the auditory 40 Hz ASSR during the low arousal condition compared to the high arousal condition.
Most of the previous ASSR studies investigating the effect of arousal level evaluated ASSR in sleep (Linden et al. 1985; Jerger et al. 1986; Cohen et al. 1991). Generally, ASSR amplitude is diminished during sleep. The finding of higher avWT in the low arousal condition is in line with the report by Pockett and Tan (2002), who showed an increase of power of the ASSR during low arousal state (Pockett and Tan 2002). The experimental conditions used in their study were very similar to ones used in this paper. In the present study, all the subjects stated that they entered a relaxed resting state and felt sleepy during the low arousal condition. The lower arousal level in the low arousal condition was confirmed by the lower percentage of beta power (Regan 1989; Cardenas et al. 1997). Pockett and Tan (2002) also reported that about 50% of subjects do not show ASSRs when awake but during drowsiness (low arousal condition). That was not the case in our study as ASSRs were detected in all subjects and in both experimental conditions. Picton et al. (2003) suggested that the power of the ASSR could occasionally be increased during drowsiness due to activity in the postauricular muscles recorded from a mastoid reference (Picton et al. 2003b). Presently, the increased amplitude of ASSR was not an effect of muscular noise. Apart from detection of the focal ASSR component, the noise component was also extracted by the decomposition (Fig. 1). The avWT of ASSR was higher in the low arousal condition, whereas the noise component was much more prominent in the high arousal condition. The topography of both focal ASSR component and noise component (Fig. 1) shows that postauricular muscles had no impact on the measured signal.
The finding of higher phase precision measured as ITPC in the low arousal condition is novel. Previously, only in one study investigating effects of sleep on ASSR exploration of phase coherence (measured similarly to the ITPC, but with fast Fourier transformed data) was employed (Jerger et al. 1986). In the report, the amplitude of the response was significantly diminished in sleep, whereas the phase coherence measure showed no differences across conditions (Jerger et al. 1986). In the ITPC analysis, the noise component did not emerge as, due to the nature of ITPC measure, the amplitude information is removed leaving only phase information and all epochs are given the same weight. Thus, a noisy epoch with high amplitude activity only influences the measure by a possible phase degree shift divided by the number of epochs (n = 122). The ITPC increase indicates that gamma-range phase synchronization becomes more organized in the low arousal condition.
The discrepancy between the results of sleep and drowsiness studies probably arises from the difference in sensory processing in sleep and drowsiness. It has been shown that in wakefulness the pattern of thalamic neuronal firing is regular, while inhibitory processes predominate in the cortex (Gottesmann 1999). Slow wave sleep is marked by burst neuronal firing in thalamic neurons and disinhibition in the cortex (Gottesmann 1999). Recently, increased ASSR was found in Alzheimer patients and this was interpreted as the lack of inhibition in cortical auditory processing (Osipova et al. 2006). Several studies have shown that power and phase precision of 40 Hz ASSR is diminished in schizophrenic patients when compared to healthy controls (Kwon et al. 1999; Brenner et al. 2003; Light et al. 2006). This could also point to the changes in inhibition perhaps mediated by dysregulated NMDA-modulated GABAergic activity as this is suggested to be basic to the deficient binding capacity of pyramidal cell networks involved in gamma rhythm generation (Kwon et al. 1999; Light et al. 2006). We speculate that the inhibitory balance between the thalamic and cortical processes is changed during drowsiness and differs from that in sleep, modulating sensory processing and gamma rhythm generation.
It is important to mention, that attention has been shown to cause increase of ASSR. There are several papers dealing with the effects of attention on ASSR (Linden et al. 1987; Ross et al. 2004). The general concept of these studies was either to ignore stimulation or to actively attend and respond to it, thus making subjects to pay direct attention to the stimulation. Linden et al. (1987) failed to show any effect of attention on ASSRs, whereas a recent study by Ross et al. (2004) reported some modulation of focal attention on ASSR during a specific time moment. We assume that attention modulation in our study is sparse due to the study design and the instructions given to the subjects, i.e., to let their thoughts wander and not pay attention to the stimulation.
Our results show that gamma activity elicited under passive conditions is sensitive to the level of arousal. This observation promotes supplementary assessment of arousal in ASSR studies. In the future, it is pertinent to investigate how arousal affects transient gamma activity that plays an important role not only in sensory processes, but also in higher cognitive processes like attention, memory and categorization. Additional studies are necessary to investigate whether ASSR is modulated by the level of arousal in schizophrenia.
References
Bernstein AS (1987) Orienting response research in schizophrenia: where we have come and where we might go. Schizophr Bull 13:623–641
Brenner CA, Sporns O, Lysaker PH, O’Donnell BF (2003) EEG synchronization to modulated auditory tones in schizophrenia, schizoaffective disorder, and schizotypal personality disorder. Am J Psychiatry 160:2238–2240
Cannon TD, Fuhrmann M, Mednick SA, Machon RA, Parnas J, Schulsinger F (1988) Third ventricle enlargement and reduced electrodermal responsiveness. Psychophysiology 25:153–156
Cardenas VA, Gill P, Fein G (1997) Human P50 suppression is not affected by variations in wakeful alertness. Biol Psychiatry 41:891–901
Cohen LT, Rickards FW, Clark GM (1991) A comparison of steady-state evoked potentials to modulated tones in awake and sleeping humans. J Acoust Soc Am 90:2467–2479
Delorme A, Makeig S (2004) EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods 134:9–21
Galambos R, Makeig S, Talmachoff PJ (1981) A 40-Hz auditory potential recorded from the human scalp. Proc Natl Acad Sci USA 78:2643–2647
Gottesmann C (1999) Neurophysiological support of consciousness during waking and sleep. Prog Neurobiol 59:469–508
Hari R, Hamalainen M, Joutsiniemi SL (1989) Neuromagnetic steady-state responses to auditory stimuli. J Acoust Soc Am 86:1033–1039
Hong LE, Summerfelt A, McMahon R, Adami H, Francis G, Elliott A, Buchanan RW, Thaker GK (2004) Evoked gamma band synchronization and the liability for schizophrenia. Schizophr Res 70:293–302
Jerger J, Chmiel R, Frost JD Jr, Coker N (1986) Effect of sleep on the auditory steady state evoked potential. Ear Hear 7:240–245
Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW (1999) Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry 56:1001–1005
Light GA, Hsu JL, Hsieh MH, Meyer-Gomes K, Sprock J, Swerdlow NR, Braff DL (2006) Gamma band oscillations reveal neural network cortical coherence dysfunction in schizophrenia patients. Biol Psychiatry 60:1231–1240
Linden RD, Campbell KB, Hamel G, Picton TW (1985) Human auditory steady state evoked potentials during sleep. Ear Hear 6:167–174
Linden RD, Picton TW, Hamel G, Campbell KB (1987) Human auditory steady-state evoked potentials during selective attention. Electroencephalogr Clin Neurophysiol 66:145–159
Makela JP, Hari R (1987) Evidence for cortical origin of the 40 Hz auditory evoked response in man. Electroencephalogr Clin Neurophysiol 66:539–546
Morup M, Hansen LK, Herrmann CS, Parnas J, Arnfred SM (2006) Parallel factor analysis as an exploratory tool for wavelet transformed event-related EEG. Neuroimage 29:938–947
Morup M, Hansen LK, Arnfred SM (2007) ERPWAVELAB A toolbox for multi-channel analysis of time-frequency transformed event related potentials. J Neurosci Methods 161:361–368
Oken BS, Salinsky MC, Elsas SM (2006) Vigilance, alertness, or sustained attention: physiological basis and measurement. Clin Neurophysiol 117:1885–1901
Osipova D, Pekkonen E, Ahveninen J (2006) Enhanced magnetic auditory steady-state response in early Alzheimer’s disease. Clin Neurophysiol 117:1990–1995
Pantev C, Roberts LE, Elbert T, Ross B, Wienbruch C (1996) Tonotopic organization of the sources of human auditory steady-state responses. Hear Res 101:62–74
Pastor MA, Thut G, Pascual-Leone A (2006) Modulation of steady-state auditory evoked potentials by cerebellar rTMS. Exp Brain Res 175:702–709
Picton TW, John MS, Dimitrijevic A, Purcell D (2003a) Human auditory steady-state responses. Int J Audiol 42:177–219
Picton TW, John MS, Purcell DW, Plourde G (2003b) Human auditory steady-state responses: the effects of recording technique and state of arousal. Anesth Analg 97:1396–1402
Pockett S, Tan SM (2002) The auditory steady-state response is not a suitable monitor of anesthesia. Anesth Analg 95:1318–1323, table of contents
Regan D (1989) Human brain electrophysiology: evoked potentials and evoked magnetic fields in science and medicine. Elsevier, New York
Ross B, Picton TW, Herdman AT, Pantev C (2004) The effect of attention on the auditory steady-state response. Neurol Clin Neurophysiol 2004:22
Venables P (1984) Arousal: an examination of its status as a concept. In: Stern JA (ed) Psychophysiological perspectives—festschrift for Beatrice and John Lacey. Van Nostrand Reinhold, New York, pp 134–142
Acknowledgments
The study was financially supported by the Lundbeck Foundation, the Gangsted Foundation, the Novo Nordic Foundation, the Danish Research Council and Cirius. We thank Sv. Christoffersen and Ch. Tarrild for stimulation apparatus and software development and Dr. A. Alaburda for his comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Griskova, I., Morup, M., Parnas, J. et al. The amplitude and phase precision of 40 Hz auditory steady-state response depend on the level of arousal. Exp Brain Res 183, 133–138 (2007). https://doi.org/10.1007/s00221-007-1111-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-007-1111-0