Abstract
It has been hypothesized that impaired task-switching underlies some of the behavioural deficits in schizophrenia. However, task-switching involves many cognitive operations. In this study our goal was to isolate the effects on latency and accuracy that can be attributed to specific task-switch processes, by studying the inter-trial effects in blocks of randomly mixed prosaccades and antisaccades. By varying the preparatory interval between an instructional cue and the target, we assessed the costs of both (1) an active reconfiguration process that was triggered by the cue, and (2) passive carry-over effects persisting from the prior trial. We tested 15 schizophrenic subjects and 14 matched controls. A very short preparatory interval increased error rates and saccadic latencies in both groups, but more so in schizophrenia, suggesting difficulty in rapidly activating saccadic goals. However, the contrast between repeated and switched trials showed that the costs of task switching in schizophrenia were not significantly different from the controls, at either short or long preparatory intervals, for both antisaccades and prosaccades. These results confirm prior observations that passive carry-over effects are normal in schizophrenia, and show that active reconfiguration is also normal in this disorder. Thus problems with executive control in schizophrenia may not affect specific task-switching operations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Executive functions are cognitive control operations that allow flexible rather than reflexive responses to events. They are thought to rely on prefrontal cortex (Stuss et al. 1995). In schizophrenia, deficient executive function predicts poor functional outcome (Green et al. 2000) and is reflected in behavior that is perseverative and stereotyped, stimulus-bound rather than guided by context.
Task switching, the process of shifting from one task or one aspect of a task to another in the face of changing environmental contingencies, is considered to be an executive function. Task switching, however, has many dimensions. For one, the nature of the switch can involve different cognitive processes in the linkage between stimuli and responses. This is evident in the range of tasks used to assess task switching. In some studies, one must switch between attending to one stimulus and another—i.e. when presented with a letter and a number, such as G7, responding to the letter versus the number (Sohn and Anderson 2001). In others the switch is between one aspect of the stimulus and another—i.e. with Stroop stimuli, responding to the ink colour versus the letter form (Wylie and Allport 2000). One can also switch the modality of the response—i.e. manual versus eye movement. Yet other studies use the same stimuli and the same response modality but change stimulus-response mappings—that is, the task-set rules that state which response to make to a stimulus (i.e., given the same stimulus, press button one versus button two [Shaffer 1965; Meiran 2000)]. Whether the effects of switching are equivalent at all levels of processing between the stimulus and the response remains to be determined.
Yet another aspect of the multi-dimensional nature of task-switching is revealed when studies manipulate task parameters, particularly those involving timing. These suggest that there are probably multiple cognitive processes involved in task switching. One hypothesized process is an active reconfiguration that switches the system from one task to another. At least part of this reconfiguration can be done in advance of a target, if prompted by an instructional cue that precedes the target by a sufficient interval. This active preparatory switching has been labeled “advance reconfiguration” (Rogers and Monsell 1995; Monsell et al. 2000). Another hypothesized group of processes is “passive” inhibitory influences left over and decaying relatively slowly from the previous trial. An example is “task-set inertia”, which proposes that activation of one task-set also generates an inhibition of the competing task-set that persists into following trials (Allport et al. 1994).
Recent studies have manipulated timing parameters in task switch experiments to isolate the effects of active reconfiguration and passive inhibition on performance. First, if active reconfiguration can be triggered by an instructional cue in advance of the target, then its effects on performance will vary as a function of the interval between the cue and the target. If there is no advance warning, because the cue occurs simultaneous with the target, the time costs of reconfiguration will be included in the latency of the response to the target. With sufficiently long time, of more than 600 ms (Allport et al. 1994; Rogers and Monsell 1995; Weber 1995), reconfiguration may be completed in advance of the target, so that its effects are no longer reflected in latency measures. Second, varying the interval between the prior response and the current trial’s target can reveal inhibitory effects from the prior task. Because these decay with time, inhibitory effects are strongest when the next response occurs shortly after the prior response. By varying either the cue-to-target or prior-response-to-target interval while holding the other interval constant, a study produced evidence for both active reconfiguration and passive inhibitory effects (Meiran 2000).
These newly revealed complexities in task-switching prompt re-consideration of the status of this function in conditions such as schizophrenia. Based on observations of perseverative behavior (Sandson and Albert 1984; Crider 1997) and performance on standard neuropsychological instruments such as the Wisconsin Card Sort Test (Braff et al. 1991; Perry and Braff 1998), subjects with schizophrenia are often presumed to have task-switching deficits. However, tasks such as the Wisconsin Card Sort Test require multiple cognitive processes, and failure is as likely to reflect defects in sustained attention, concept formation, or working memory, as impaired to task switching (Cohen and Servan-Schreiber 1992; Sullivan et al. 1993; Gold et al. 1997; Smith et al. 1998). Moreover, the presence of a task-switching deficit has not been well established empirically, with reports of both defective (Elliott et al. 1995; Smith et al. 1998) and normal (Cools et al. 2000) task-switching.
Two studies have used more recently developed protocols in attempts to more specifically isolate task-switching functions in schizophrenia. One examined the switching of stimulus-response mappings between visual stimuli and manual keypresses (Meiran et al. 2000b) and found that the proportional increase of latency induced by switching was similar in schizophrenic and control subjects. The conclusion was that the slower and less accurate performance of schizophrenic subjects reflected “poor memory for task context information”, not dysfunctional task-switching. The second report (Manoach et al. 2002) studied ocular motor stimulus-response re-mappings, as subjects switched between prosaccades (gaze shifts towards a suddenly appearing target) and antisaccades (gaze shifts away from the target that require inhibition of prosaccades and generation of the novel behavior of looking away). While the data reproduced the well-known difficulty of schizophrenic subjects with antisaccade execution (Reuter and Kathmann 2004; Hutton and Ettinger 2006), the effects of task-switching on latency and accuracy did not differ between schizophrenic and control subjects, again suggesting that schizophrenic subjects did not have a task-switch deficit.
Because this last study (Manoach et al. 2002) used long cue-to-target intervals of 1,850–2,150 ms, it is likely that any cue-triggered active reconfiguration of the saccadic system had been completed before the stimulus appeared. Therefore the latency and accuracy costs incurred by task-switching in this study likely reflected passive carry-over effects from the prior trial. While the study established that these passive carry-over effects are of similar magnitude in schizophrenic and control subjects, it left open the possibility that schizophrenic subjects may have abnormal active (advance) reconfiguration processes. To address this possibility, the present study contrasted performance with a short cue-to-target interval against that with a long cue-to-target interval. Our hypothesis was that, if schizophrenic subjects have a defect in active reconfiguration, the latency and error rates of switched trials will be elevated when these are performed with short cue-to-target intervals that provide little time for advance preparation.
Methods
Subjects
We recruited 17 schizophrenic outpatients. Diagnoses were confirmed with the Structured Clinical Interviews for DSM-IV, and all subjects had been maintained on stable dose of antipsychotic medication for a minimum of 6 weeks. We recruited 16 healthy subjects from the community using poster advertisements. Potential control subjects were interviewed using the screening portion of the Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition (First et al. 1997) to rule out any history of psychiatric illness and substance abuse or dependence within the preceding 6 months. A brief medical history was taken to exclude subjects with any independent condition that might affect brain function. Two schizophrenic subjects and two control subjects did not complete the entire protocol, leaving us with final sample sizes of 15 schizophrenic and 14 control subjects. These two groups were matched for age, gender, handedness as assessed with the modified Edinburgh handedness inventory (White and Ashton 1976), and parental socioeconomic status as determined by the Hollingshead index (Hollingshead 1965) (Table 1). Control subjects had significantly more years of education and higher verbal intelligence quotient estimates, based on a test of single word reading, the American National Adult Reading Test (Blair and Spreen 1989). The committees on clinical investigations at the Beth Israel Deaconess Medical Center and the Massachusetts Department of Mental Health approved the study and all subjects gave written informed consent after the study was thoroughly explained.
Apparatus and eye movement protocol
We recorded eye movements with a magnetic search coil technique. A scleral coil was placed in the subject’s left eye, though the subject was permitted to view the stimulus binocularly. Images were programmed on a Power Macintosh 9600/233 in C++ using the Vision Shell platform (http://www.kagi.com/visionshell). These were projected onto a screen 81 cm from the subject. Participants’ heads were secured in a chin rest. Eye position was digitized at 500 samples/s. Before the test session the system was calibrated for each subject by having the subject successively fixate on nine targets in a grid spanning 50°, averaging 12 data points for each of the nine locations, and submitting the data to a regression analysis to obtain the best linear fit. Eye velocity was derived from eye position by a five-point central difference algorithm (Bahill and McDonald 1983).
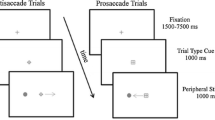
We presented blocks of 48 trials, with prosaccade trials and antisaccade trials in a randomized order. The duration of the cue-to-target interval varied between blocks. Half of the blocks contained a 200 ms delay between the cue and the target presentation, while the other half contained a 2,000 ms cue-to-target interval. Eight blocks were presented in a counterbalanced order, half of the subjects starting with four 200 ms cue-to-target interval blocks and half with four 2,000 ms cue-to-target interval blocks. The short and long delay blocks were administered on separate days to avoid fatigue. Practice blocks of 20 trials were provided before the experiment began on each day. In addition to a base payment for participation, monetary rewards of $0.025 per correct saccade were given to mitigate against potential motivational deficits, which are often found in schizophrenia.
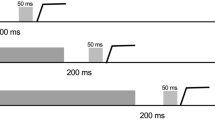
Each trial began with a white fixation ring of 1° diameter and luminance of 20 cd/M2, at the center of a dark background (Fig. 1). The fixation ring was flanked by two dots of 0.7° diameter placed 20° right and left of center. In the blocks with 200 ms cue-to-target interval, the fixation ring was replaced after 3,500 ms by an instructional cue. This was a yellow “O” to prompt prosaccades and blue “X” to prompt antisaccades. The cue lasted 200 ms and was immediately followed by the appearance of a target. Targets were a ring similar to the fixation ring, placed around one of the peripheral dots at 20° eccentricity, with the side of appearance (left vs. right) randomly determined. In the blocks with 2,000 ms cue-to-target interval, the initial fixation ring lasted 1,700 ms, followed by the 200 ms instructional cue, followed by a return of the central fixation ring, which then remained for another 1,800 ms until the target appeared. In this manner, while cue-to-target interval varied between 200 and 2,000 ms, the duration of the interval between the end of the prior trial and the onset of the target was kept constant at 3,700 ms in the two blocks. On prosaccade trials subjects were instructed to look at the target as rapidly and accurately as possible; on antisaccade trials they were to look at the location in the direction opposite to the target, again as rapidly and accurately as possible. The white ring moved back to the central fixation point after the subject’s eye reached within 3° of the desired end position, or after 3 s had elapsed.
Trial design. Top row shows cartoons of the screen displays during fixation, presentation of cues for either prosaccades or antisaccades, and presentation of the target at either left or right of center. Center row shows the time line for trials with short cue-to-target and long cue-to-target intervals. Bottom row shows data traces from typical correct prosaccade (left) and antisaccade (right) trials, showing horizontal target position (grey) and eye position (black), where a positive (upward) value indicates a position to the right of center fixation (0), plotted against time. Latency calculations are made from the start of the target jump to the start of the saccade
Data analysis
The first saccade of each block was eliminated from analysis, since it would not have the inter-trial effects of interest to us. Saccades were detected as eye movements with velocities exceeding 47°/s, and the beginning of the saccade marked as the point at which velocity exceeded 31°/s. The variables measured were directional accuracy and latency. A directional error was an initial eye movement vector with a horizontal component in the direction opposite to the intended saccadic goal. Latency was the time interval between target onset and the beginning of the saccade. We eliminated saccades with latencies less than 130 ms (about 1.9% of all trials), as these have a high likelihood of being contaminated by anticipatory (non-visually guided) responses (Kalesnykas and Hallett 1987), and those with latencies greater than 800 ms (about 0.1% of all trials) as being excessively delayed. The analysis of accuracy only included trials that had been preceded by correct responses. The latency analysis was conducted only on correct responses that were also preceded by correct responses, as these trials were felt to most accurately reflect the inter-trial effects we wished to assess.
We labeled trials according to their inter-trial context. That is, those trials preceded by the opposite task (i.e. an antisaccade after a prosaccade) were labeled “switched”, while those preceded by the same task were labeled “repeated”. We used ANOVA with repeated measures and subjects nested within group as a random effect, to analyze results for both directional accuracy and latency. The factors were subject group (schizophrenia vs. control), cue-to-target interval (200 vs. 2,000 ms), saccade type (prosaccade vs. antisaccade), and inter-trial context (repeated vs. switched). Pair-wise comparisons were made using linear contrasts.
As a more specific analysis of switch costs, we also calculated for each subject the difference between mean error rates and mean latencies for switched versus repeated trials. These differences were the error and latency switch costs. These too were subject to ANOVA with repeated measures, with factors of subject group, cue-to-target interval and saccade type.
Results
Error rate
The main effects in ANOVA showed that error rates were greater for the short than for the long cue-to-target interval (F (1,27) = 46.4, P < 0.0001), for antisaccades than for prosaccades (F (1,27) = 104.9, P < 0.0001), and for switched than for repeated saccadic trials (F (1,27) = 9.34, P < 0.0026) (Fig. 2). There was a trend for an interaction between saccade type and cue-to-target interval (F (1,27) = 3.40, P = 0.067), due to the error rates of antisaccades increasing more than that of prosaccades when the cue-to-target interval was shortened. The interaction between cue-to-target interval and inter-trial context did not reach significance (F (1,27) = 2.46, P = 0.118).
a Error rate data. Left shows data from 200 ms cue-to-target interval, and right from the 2,000 ms cue-to-target interval. Error bars show one standard error. Lines join symbols for switched and repeated trials, and their slopes index the task-switch cost. b Latency data, plotted in similar fashion. Note the paradoxical reduction in task-switch cost for antisaccades at 2,000 ms cue-to-target interval
Did schizophrenic subjects differ from controls? There was a main effect of subject group, with schizophrenic subjects making more errors than control subjects (F (1,27) = 19.35, P < 0.0002). There was also an interaction between subject group and saccade type (F (1,27) = 17.13, P < 0.0001): while schizophrenic subjects made more errors than control subjects on both prosaccades and antisaccades, this increase in error was greater for antisaccades. There was also a trend to an interaction between subject group and cue-to-target interval (F (1,27) = 3.04, P < 0.08): shortening the cue-target interval lead to a greater increase in overall error rate in schizophrenic subjects than it did in control subjects. However, the key finding was that there was no significant interaction involving both subject group and inter-trial context. This indicates that schizophrenic subjects did not differ from controls in the pattern of errors for switched versus repeated task trials.
The ANOVA on error switch costs did show a significant main effect of cue-to-target interval (F (1,27) = 10.44, P < 0.0018), with more errors induced by switching at the shorter cue-to-target interval. There was no significant main effect of subject group or any significant interactions, including those involving subject group. Hence the effect of switching on error rate is similar in schizophrenic and control subjects. Figure 3 shows the error switch costs. This difference between switch costs at 2,000 and 200 ms may index the effects of active reconfiguration (Meiran 2000), and is no different between schizophrenic and control subjects (Table 2).
Switch costs (the difference between switched and repeated data). a Error rate switch costs. Error bars show one standard error. b Latency switch costs. Note that switch costs are higher at 200 ms cue-to-target interval than 2,000 ns cue-to-target interval. The slope of the lines connecting 200 ms and 2,000 ms cue-to-target interval data index the costs of active reconfiguration that can be performed in advance of the target when triggered by an explicit cue. Switch costs in schizophrenic subjects are comparable to control subjects, and the costs of active reconfiguration are not greater in schizophrenia
Latency
The main effects of the ANOVA showed that latencies were longer for short than for long cue-to-target interval (F (1,27) = 254.8, P < 0.001), for antisaccades than for prosaccades (F (1,27) = 516.99, P < 0.001), and for switched than for repeated trials (F (1,27) = 20.14, P < 0.001) (Fig. 2). There was an interaction between saccade type and cue-to-target interval (F (1,27) = 5.12, P < 0.024), with greater reduction in latency for prosaccades than antisaccades when cue-to-target interval was lengthened. There was a significant interaction between cue-to-target interval and inter-trial context (F (1,27) = 27.12, P < 0.001), due to increased switch effects at the shorter cue-to-target interval, and between saccade type and inter-trial context (F (1,27) = 22.62, P < 0.001), due to smaller switch effects for antisaccades. This interaction reflected in part the fact that antisaccades at long cue-to-target interval had a paradoxical switch benefit (i.e., switched antisaccades had faster response latencies than repeated ones), reproducing an observation made in prior studies by our group (Cherkasova et al. 2002; Manoach et al. 2002) and since then by others (Hunt and Klein 2002; Fecteau et al. 2004; Reuter 2006b).
Analysis for differences between the subject groups showed an interaction between subject group and saccade type (F (1,27) = 81.05, P < 0.001): as with error rate, while schizophrenic subjects had longer latencies than control subjects on both prosaccades and antisaccades, this increase in latency was greater for antisaccades. There was an interaction between subject group and cue-to-target interval (F (1,27) = 9.94, P < 0.002), with schizophrenic subjects taking longer to make saccades at the shorter cue-to-target interval. There was no significant interaction between inter-trial context and subject group, and no significant interaction between cue-to-target interval, inter-trial context and group.
The ANOVA on latency switch costs showed, as expected, a significant main effect of cue-to-target interval (F (1,27) = 20.12, P < 0.001), with greater latency switch costs for the shorter cue-to-target interval. There was also a significant main effect for saccade type (F (1,27) = 14.78, P < 0.001), which was due to lower switch costs for antisaccades than prosaccades. This was particularly evident at long cue-to-target interval, where the data reproduced our finding of a paradoxical negative switch cost for antisaccades, in both schizophrenic and control subjects. There was no significant main effect of subject group or any significant interactions, including those involving subject group. Hence the latency switch effects in schizophrenic subjects are no different than those in the control subjects. Figure 3 shows the latency switch costs. Again as with error rate, the difference between switch costs at 2,000 and 200 ms, an index of active reconfiguration, is no different between schizophrenic and control subjects (Table 2).
Discussion
The main finding of this study is that schizophrenic subjects did not differ from controls in any task-switch cost (the difference between switched and repeated trials), for either prosaccades or antisaccades. First, using a long cue-to-target interval of 2,000 ms that allows ample time for completion of advance configuration processes (Rogers and Monsell 1995; Meiran et al. 2000a) we reproduced previous findings of intact “residual task-switch costs” (Meiran et al. 2000b; Manoach et al. 2002)—that is, the differences in latency and accuracy that remain even after enough time has been allowed for active switching processes to be completed. Residual costs are thought to reflect primarily the effects of passive carry-over of inhibition from a previous trial that slowly dissipate with time (Meiran 1996). Our results suggest that, after an interval of 3,700 ms following the prior response, the effects of inhibitory sets in prior trials are similar in magnitude in schizophrenic and control subjects. Second, with a short cue-to-target interval of 200 ms we also demonstrate comparable switch costs in schizophrenic and control subjects. Moreover, the difference between trials with short versus long cue-to-target intervals, which serves as an index of advance reconfiguration, did not differ between the two subject groups. These results do not support either of the two hypotheses suggested by concepts of abnormal task-switching in schizophrenia: first, that schizophrenia subjects would have abnormal residual task switch costs, costs which index the passive carry-over effects from the prior trial, and second, that they would have abnormal costs related to cue-triggered advance reconfiguration, an active switching process.
The chief effects of reduced preparatory time between the instructional cue and the target was a general increase in error rate, more so for antisaccades than prosaccades, and an increase in saccadic latencies. These findings can be taken to reflect less effective task preparation at shorter cue-to-target intervals, regardless of whether trials were repeated or switched. Examination of specific switch costs showed that both latency and error costs were increased at the short cue-to-target interval (although, since the interaction between cue-to-target interval and inter-trial context was not significant for error rate in the primary analysis, this cannot be taken as definitive proof that shortening cue-target interval increases error rate). When passive inhibitory carry-over influences from prior trial are kept constant by controlling the prior-response-to-target interval, as done in our study, the increase in switch costs with reduced preparation time is considered an index of the active reconfiguration of task-sets that can be done in advance of the stimulus (Meiran 1996). In our saccadic paradigm, these reconfiguration costs were similar for both antisaccades and prosaccades, being around 2–7% for error rate and 20–30 ms for latency in our control subjects (Table 2). These values are similar in magnitude to those in several other manual task-switch paradigms (Meiran 1996; Meiran et al. 2000a).
The present findings are also consistent with a number of prior observations in schizophrenia. First, schizophrenic subjects made more errors and had longer response latencies in general when compared to controls, as others have also found in manual task-switching paradigms (Meiran et al. 2000b). Second, they had marked difficulty with antisaccades, with greater errors and longer latencies (Fukushima et al. 1990a; Sereno and Holzman 1995; Hutton and Kennard 1998). We found an interaction between cue-to-target interval and subject group, with schizophrenic subjects showing an exaggeration of the normal tendency to more difficulty at shorter cue-to-target intervals, with either prosaccades or antisaccades. This interaction between subject group and cue-to-target interval was not found in another study using many cue-to-target intervals (Meiran et al. 2000b), although it could be consistent with their hypothesis that schizophrenic subjects are less efficient at retrieving the task-context information needed for each trial.
These data add to other results showing that, with newer paradigms that isolate specific aspects of task-switching, schizophrenic subjects appear to have normal switching behaviour. Our prior study (Manoach et al. 2002) reported on both residual task-switch costs and also mixed-list costs, which are the costs of keeping two tasks in readiness as opposed to one (Los 1996). Mixed-list costs can be indexed by comparing the performance on repeated trials in a block that has two potential tasks versus performance on a block where subjects only have one task. We found that both residual task-switch and mixed-list costs were normal in schizophrenia. Residual task-switch costs were also reported to be normal in schizophrenia on a manual task that required switching between stimulus-response maps, using cue-to-target intervals of 1,250–1,750 ms (Turken et al. 2003). These data clarify and confirm the impression from the pioneering study of Meiran et al. (2000b). That study used a variety of cue-to-target intervals in a similar manual stimulus-response re-mapping task-switch paradigm and concluded that the increased switch effects in schizophrenia were likely related to general elevations in reaction time rather than specific task-switch deficits. This study and the two prior reports (Manoach et al. 2002; Turken et al. 2003) extend those results by showing that switch costs are not just relatively but absolutely normal in schizophrenia.
There are also a few schizophrenia studies of switching with other paradigms. One study examined switching between the dimension of the stimulus to be attended (colour vs. form) and had predictable sequencing without a cue-to-target interval (Cools et al. 2000). Since prior reports have shown that advance reconfiguration is triggered by an explicit cue and that predictable set sequences are not a substitute for such a cue (Sohn and Anderson 2001; Tornay and Milan 2001; Barton et al. 2006), the costs in this study likely included effects of active reconfiguration. This study also concluded that switching effects in schizophrenia were normal. Another study using cue-to-target intervals of 1,200 ms had subjects switch between comparing the size and comparing the shape of two stimuli: while it found failures on set maintenance, it did not find deficits in set switching (Kieffaber et al. 2006). These two studies thus extend the data showing normal task switching in schizophrenia from switches involving stimulus-response maps (or task sets) to switches involving stimulus dimensions.
These studies showing normal task-switching in schizophrenia all compare the latency and error rates on switched trials to baseline characteristics of repeated trials. As such they isolate switch costs by studying inter-trial effects, the effect of the preceding trial on the next. In contrast, studies asserting switching deficits in schizophrenia have used more complicated paradigms in which cognitive factors other than task-switching may have contributed to defective performance. We have already discussed the complexity of the Wisconsin Card Sort Test, and indeed, a recent meta-analysis of this body of data has concluded that schizophrenic errors on this test likely reflect deficits in processes other than task-switching (Li 2004). [In support, our prior study found no relationship between perseverative errors on the Wisconsin Card Sort Test and indices of residual task-switch costs in schizophrenia (Manoach et al. 2002)]. One study suggested switching deficits in moving between semantic clusters or phonetic clusters in a verbal fluency test, by showing a decrease in the number of switches made (Robert et al. 1998), but this may reflect failure to generate clusters rather than a switching defect. Another study had subjects match a target stimulus for colour while viewing a string of following stimuli, then switch to matching its form once a successful match was made (Smith et al. 1998). Deficits on such a task could reflect failures in working memory for the initial target, in maintaining task-context information over time (Meiran et al. 2000b), or in performance monitoring (Turken et al. 2003), among other factors.
As the bulk of the data on inter-trial effects in schizophrenia comes from studies of task switches that involve stimulus-response re-mappings, it is possible that studies of other types of task-switching may yet reveal switching impairments in this disorder. However, these studies of stimulus-response remapping would at least argue against impairment of a general “supramodal” switching ability. Furthermore, there are at least two reports of normal schizophrenic performance in switching stimulus dimensions (Cools et al. 2000; Kieffaber et al. 2006).
The normal inter-trial effects of switching in schizophrenia must cast doubt upon assertions that perseverative behavior in this disorder reflects inflexibility in specific task-switching operations. Others have suggested that “a failure to mobilize cognitive resources in situations requiring controlled information processing” (Li 2004) or “poor memory for task context information” (Meiran et al. 2000b) may underlie some apparent difficulties in switching, and hence generate perseverative behavior. We have also provided some evidence of abnormal inter-trial effects related not to switching, but to recent execution of an antisaccade trial (Barton et al. 2005). The antisaccade is a relatively unpracticed task that requires inhibition of the alternative prosaccade task set. If this inhibition carries over into the following trial, it would explain not only the prolonged latencies of switched prosaccades, but also the paradoxical reduction in switch costs for antisaccades, which we and others reported before in healthy controls and schizophrenic subjects (Cherkasova et al. 2002; Manoach et al. 2002; Fecteau et al. 2004; Manoach et al. 2004) and which we reproduced in the current study. This is because antisaccade latencies would also be prolonged by an antisaccade in the prior trial, compared to having a prosaccade in the prior trial. We found that schizophrenic subjects showed this antisaccadic effect on both prosaccade and antisaccade latencies in not only the upcoming trial but also the trial after that, while controls did not (Barton et al. 2005). Furthermore, directional errors were more likely to be in the direction of a prior antisaccade in schizophrenic but not control subjects. We argued that these showed abnormally persistent effects on the saccadic response system from prior antisaccades, rather than abnormal task-switching, and that this could account for some types of perseverative behavior (Barton et al. 2006).
These excessively persistent inhibitory effects from antisaccades correctly performed in preceding trials are also at odds with the suggestion that weak inhibition is the cause of abnormal antisaccade performance in schizophrenia. Rather, this excessive inhibition may be a compensatory adaptation to overcome their difficulty in initiating antisaccades as manifest by their high error rate. Therefore poor antisaccade execution may reflect not so much weak inhibition (Clementz 1998; Levy et al. 1998) but deficient and inconsistent goal activation (Nieuwenhuis et al. 2004; Reuter and Kathmann 2004), a concept that has received support from both behavioural (Reuter et al. 2006b) and event-related potential studies of antisaccades (Klein et al. 2000; Reuter et al. 2006a), and which is consistent with findings of deficient set-maintenance in other paradigms (Kieffaber et al. 2006). Dynamic abnormalities in goal activation may also account for our finding that schizophrenic subjects show greater deterioration in error rates and latencies when cue-to-target intervals are shortened. This suggests a failure to rapidly establish the saccadic goal, a failure which is partly ameliorated by allowing a longer preparatory interval.
In summary, our study adds to a growing body of literature on inter-trial effects that show that the cognitive operations in task-switching are normal in schizophrenia. These data suggest that the origins of perseverative behavior in clinical observations and traditional neuropsychological tests may stem from difficulties in cognitive processes other than task-switching. However, given the complexities of task-switching, further studies of other types and other aspects of task-switching are warranted.
References
Allport A, Styles E, Hsieh S (1994) Shifting intentional set: exploring the dynamic control of tasks. In: Umiltà C, Moscovitch M (eds) Attention and performance XV. Erlbaum, Hillsdale, pp 421–452
Bahill T, McDonald J (1983) Frequency limitations and optimal step size for the two-point central difference derivative algorithm with applications to human eye movement data. IEEE Trans Biomed Eng 30:191–194
Barton J, Cherkasova M, Lindgren K, Goff D, Manoach D (2005) What is perseverated in schizophrenia? Evidence of abnormal response plasticity in the saccadic system. J Abnorm Psychol 114:75–84
Barton J, Greenzang C, Hefter R, Edelman J, Manoach D (2006) Switching, plasticity, and prediction in a saccadic task-switch paradigm. Exp Brain Res 168:76–87
Blair J, Spreen O (1989) Predicting premorbid IQ: a revision of the national adult reading test. Clin Neuropsychol 3:129–136
Braff DL, Heaton R, Kuck J, Cullum M, Moranville J, Grant I, Zisook S (1991) The generalized pattern of neuropsychological deficits in outpatients with chronic schizophrenia with heterogeneous Wisconsin Card Sorting Test results. Arch Gen Psychiatry 48:891–898
Cherkasova M, Manoach D, Intriligator J, Barton J (2002) Antisaccades and task-shifting: interactions in controlled processing. Exp Brain Res 144:528–537
Clementz B (1998) Psychophysiological measures of (dis)inhibition as liability indicators for schizophrenia. Psychophysiology 35:648–668
Cohen J, Servan-Schreiber D (1992) Context, cortex and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol Rev 99:45–77
Cools R, Brouwer W, deJong R, Sloof C (2000) Flexibility, inhibition and planning: frontal dysfunction in schizophrenia. Brain Cogn 43:108–112
Crider A (1997) Perseveration in schizophrenia. Schizophr Bull 23:63–74
Elliott R, McKenna P, Robbins T, Sahakian B (1995) Neuropsychological evidence for frontostriatal dysfunction in schizophrenia. Psychol Med 25:619–630
Fecteau J, Au C, Armstrong I, Munoz D (2004) Sensory biases produce alternation advantage found in sequential saccadic eye movement tasks. Exp Brain Res 159:84–91
First MB, Spitzer RL, Gibbon M, Williams JBW (1997) Structured Clinical Interview for DSM-IV Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-I/P W/PSY SCREEN). Biometrics Research, New York State Psychiatric Institute, New York
Fukushima J, Morita N, Fukushima K, Chiba T, Tanaka S, Yamashita I (1990a) Voluntary control of saccadic eye movements in patients with schizophrenic and affective disorders. J Psychiat Res J Psychiat Res 24:9–24
Gold J, Carpenter C, Randolph C et al. (1997) Auditory working memory and Wisconsin Card Sorting Test performance in schizophrenia. Arch Gen Psychiatry 54:159–165
Green M, Kern R, Braff D, Mintz J (2000) Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull 26:119–136
Hollingshead A (1965) Two factor index of social position. Yale University Press, New Haven
Hunt A, Klein R (2002) Eliminating the costs of task set reconfiguration. Mem Cogn 30:529–539
Hutton S, Ettinger U (2006) The antisaccade task as a research tool in psychopathology: a critical review. Psychophysiology 43:302–313
Hutton S, Kennard C (1998) Oculomotor abnormalities in schizophrenia. A critical review. Neurology 50:604–609
Kalesnykas R, Hallett P (1987) The differentiation of visually guided and anticipatory saccades in gap and overlap paradigms. Exp Brain Res 68:115–121
Kieffaber P, Kappenman E, Bodkins M, Shekhar A, O’Donnell B, Hetrick W (2006) Switch and maintenance of task set in schizophrenia. Schizophr Res 84:345–358
Klein C, Heinks T, Andresen B, Berg P, Moritz S (2000) Impaired modulation of the saccadic contingent negative variation preceding antisaccades in schizophrenia. Biol Psychiatry 47:978–990
Levy D, Mendell N, LaVancher C et al (1998) Disinhibition in antisaccade performance in schizophrenia. In: Lenzenweger M, Dworkin R (eds) Origins and development of Schizophrenia. American Psychological Association, Washington, pp 185–210
Li C-SR (2004) Do schizophrenia patients make more perseverative than non-perseverative errors on the Wisconsin Card Sorting test? A meta-analytic study. Psychiatry Res 129:179–190
Los S (1996) On the origin of mixing costs: exploring information processing in pure and mixed blocks of trials. Acta Psychol 94:145–188
Manoach DS, Lindgren KA, Cherkasova MV, Goff DC, Halpern EF, Intriligator J, Barton JJS (2002) Schizophrenic subjects show deficient inhibition but intact task-switching on saccadic tasks. Biol Psychiatry 51:816–825
Manoach D, Lindgren K, Barton J (2004) Deficient saccadic inhibition in Asperger’s disorder and the social-emotional processing disorder. J Neurol Neurosurg Psychiatr 75:1719–1726
Meiran N (1996) Reconfiguration of processing mode prior to task performance. J Exp Psychol Learn Mem Cogn 22:1423–1442
Meiran N (2000) Modeling cognitive control in task switching. Psychol Res 63:234–249
Meiran N, Chorev Z, Sapir A (2000a) Component processes in task switching. Cognit Psychol 41:211–253
Meiran N, Levine J, Henik A (2000b) Task set switching in schizophrenia. Neuropsychology 14:471–482
Monsell S, Yeung N, Azuma R (2000) Reconfiguration of task-set: is it easier to switch to the weaker task? Psychol Res 63:250–264
Nieuwenhuis S, Broerse A, Nielen M, de Jong R (2004) A goal activation approach to the study of executive function: an application to antisaccade tasks. Brain Cogn 56:198–214
Perry W, Braff D (1998) A multimethod approach to assessing perseverations in schizophrenia patients. Schizophr Res 33:69–77
Reuter B, Kathmann N (2004) Using saccade tasks as a tool to analyze executive dysfunctions in schizophrenia. Acta Psychol (Amst) 115:255–269
Reuter B, Herzog E, Endrass T, Kathmann N (2006a) Brain potentials indicate poor preparation for action in schizophrenia. Psychophysiology 43:604–611
Reuter B, Herzog E, Kathmann N (2006b) Antisaccade performance of schizophrenia patients: evidence of reduced task-set activation and impaired error detection. J Psychiatr Res 40:122–130
Robert P, Lafont V, Medecin I, Berthet L, Thauby S, Baudu C, Darcourt G (1998) Clustering and switching strategies in verbal fluency tasks: comparison between schizophrenics and healthy adults. J Int Neuropsychol Soc 4:539–546
Rogers, Monsell (1995) Costs of a predictable switch between simple cognitive tasks. J Exp Psychol Gen 124:207–231
Sandson J, Albert M (1984) Varieties of perseveration. Neuropsychologia 22:715–732
Sereno A, Holzman P (1995) Antisaccades and smooth pursuit eye movements in schizophrenia. Biol Psychiatry 37:394–401
Shaffer L (1965) Choice reaction with variable S-R mapping. J Exp Psychol 70:284–288
Smith G, Large M, Kavanagh D et al (1998) Further evidence for a deficit in switching attention in schizophrenia. J Abnorm Psychol 107:390–398
Sohn M-H, Anderson J (2001) Task preparation and task repetition: two-component model of task-switching. J Exp Psychol Gen 130:764–778
Stuss D, Shallice T, Alexander M, Picton T (1995) A multidisciplinary approach to anterior attentional functions. Ann NY Acad Sci 769:191–211
Sullivan EV, Mathalon DH, Zipursky RB, Kersteen-Tucker Z, Knight RT, Pfefferbaum A (1993) Factors of the Wisconsin Card Sorting Test as measures of frontal-lobe function in schizophrenia and in chronic alcoholism. Psychiatry Res 46:175–199
Tornay FJ, Milan EG (2001) A more complete task-set reconfiguration in random than in predictable task switch. Q J Exp Psychol A 54:785–803
Turken A, Vuilleumier P, Mathalon D, Swick D, Ford J (2003) Are impairments of action monitoring and executive control true dissociative dysfunctions in patients with schizophrenia? Am J Psychiatry 160:1881–1883
Weber H (1995) Presaccadic processes in the generation of pro and anti saccades in human subjects—a reaction time study. Perception 24:1265–1280
White K, Ashton R (1976) Handedness assessment inventory. Neuropsychologia 14:261–264
Wylie G, Allport A (2000) Task switching and the measurement of “switch costs”. Psychol Res 63:212–233
Acknowledgments
JB was supported by a Canada Research Chair, a Michael Smith Foundation for Health Research Senior Scholarship, and CIHR grant MOP-81270. DSM was supported by NIMH 1R01 MH67720 and the MIND Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Greenzang, C., Manoach, D.S., Goff, D.C. et al. Task-switching in schizophrenia: active switching costs and passive carry-over effects in an antisaccade paradigm. Exp Brain Res 181, 493–502 (2007). https://doi.org/10.1007/s00221-007-0946-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-007-0946-8