Abstract
The control of eye movements depends in part on subcortical motor centres. Gaze is often directed towards salient visual stimuli of our environment with no conscious voluntary commands. To further understand to what extent preprogrammed eye movements can be triggered subcortically, we carried out a study in normal volunteers to examine the effects of a startling auditory stimulus (SAS) on externally guided saccades. A peripheral visual cue was presented in the horizontal plane at a site distant 15° from the fixation point, and subjects were instructed to make a saccade to it. SAS was presented together with the peripheral visual cue in 20% of trials. To force rapid visual fixation at the end of the saccade, targets were loaded with a second cue, a small arrow pointing towards the right or the left (or a neutral sign), not distinguishable with peripheral vision. Subjects were requested to perform a flexion/extension wrist movement, according to the direction of the arrow (or not to move if the second cue was the neutral sign). SAS presented together with the visual target caused a significant shortening of the latency of saccadic movements. The wrist movements performed as a response to the second cue had similar reaction times regardless of whether the trial contained a SAS or not. Our results show that voluntary saccades to peripheral targets are speeded up by activation of the startle circuit, and that this effect does not cause a significant disturbance in the execution of simple in-target cues. These results suggest that subcortical structures play a main role in preparation of externally guided saccades.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A startling auditory stimulus (SAS) is known to induce a response in several muscles, known as the startle reaction (Landis and Hunt 1939; Wilkins et al. 1986; Brown et al. 1991a; Chokroverty et al. 1992; Kofler et al. 2001a, b). Reflex blinking is, by far, the most conspicuous involuntary movement produced by SAS-induced muscle contraction (Yeomans et al. 2002; Blumenthal et al. 2005; Flaten et al. 2005). Other movements may be elicited by SAS depending on factors such as body posture (Brown et al. 1991b), preparation for execution of a motor task (Valls-Solé et al. 1999), or other activities (Nieuwenhuijzen et al. 2000).
The oculomotor system has not been a frequent subject for research in the area of the startle. One of the reasons could be the interference of eyelid movements with the recording of eyeball movements (Gehricke et al. 2002; Rambold et al. 2005). During spontaneous, voluntary and reflex blinking or forced eyelid closure, the eyeball retracts 1 or 2 mm, due to co-contraction of all extraocular muscles (Evinger et al. 1984; Collewijn et al. 1985; Bour et al. 2000). Similar retraction can be expected from a blink-induced SAS. However, the effects of a SAS on saccade preparation have not been explored so far. In limb muscles, delivering a SAS during preparation for execution of an intended movement shortens dramatically its onset latency (Valls-Solé et al. 1999; Carlsen et al. 2003, 2004). The nucleus reticularis pontis caudalis (nRPC) plays a crucial role for the generation of the startle response (Leintner et al. 1980; Davis et al. 1982; Brown et al. 1991a). The premotor burst neurons for horizontal saccades in man were found to lie in the medial part of the nRPC (Horn et al. 1995). The superior colliculus (SC) has well-known connections with the nRPC (Isa and Naito 1995; Zhao and Davis 2004). Therefore, we considered the possibility that SAS may have also an effect on saccades. Our first objective was to examine the effects of SAS on eye movements during gaze fixation and preparation for the execution of externally guided saccades. Our second objective was to examine the accuracy of saccades made with and without accompanying SAS by means of evaluating the reaction time and correctness of the responses to a secondary instruction built into the saccade target.
Subjects
The experiments were conducted in 18 healthy subjects (seven females and 11 males, aged 20–54 years). They all gave their informed consent for the experiments that were approved by the local Ethics Committee. All subjects were reportedly right-handed and used their right eye as the preferred one for focussing their vision. All had normal or corrected-to-normal vision and were free from any neurological deficits that could affect vision, eye, or hand movements.
Experimental setup and recording
Subjects sat on a chair adjusted to a comfortable height, with their chin resting on a head movement restrainer attached to a table. Room lights were dimmed. Vertical and horizontal eye movements were monitored with surface electrodes for electro-oculography (EOG) attached at all four poles of the right orbit (Heide et al. 1999). The active electrode for vertical movements was attached to the lower orbital rim, while the active electrode for horizontal movements was placed over the external orbital rim. We also recorded the EMG activity from the sternocleidomastoid muscles (SCM) with surface electrodes attached to the skin overlying the bulk of the muscles. The subject’s forearm and hand were firmly attached to two joined metallic platforms in such a way that the wrist had only one degree of freedom for flexo-extension movements. A potentiometer, built in the hinge joining forearm and hand platforms, was used to monitor the wrist joint position signal. All bioelectric signals were recorded with, and temporarily stored on, a conventional electromyograph (MYSTRO5Plus; Oxford Medical Instruments, Surrey, UK). The bandpass frequency filters for eye and hand movements were 0.1–20 Hz, whereas the EMG of the SCM was recorded with a bandpass of 50–1,000 Hz. Eye movement towards the active electrode and wrist flexion movement were represented as negative-going deviations of the baseline. A circle of 2 cm diameter served as the fixation point for the subject’s right eye.
Visual cue
A computer monitor, installed within the subject’s right visual field, was used to trigger the visual stimulus used as target. This was a 5 cm2 white square, shown on a blank screen. The target, generated when one of the experimenters pressed a computer key, was always presented on the same spot, at an angular distance of 15° to the right of the gaze fixation point.
The target contained an in-target imperative signal (IS) intended for the subjects to make a secondary task after they focussed their gaze on the target and perceived the IS. This consisted of a 1 cm long arrowhead pointing to the left (left filled triangle) or to the right (right filled triangle), or a square (filled square). These signals appeared at random with a rate of 40% for each arrow head and 20% for the square and required the subject to make a ballistic right hand movement to the left (flexion) or to the right (extension) depending on whether the arrow pointed to the left or to the right, or not to move if the symbol was a square. Symbols were made to appear together with the target.
Startling auditory stimulus
Startling auditory stimulus consisted in a loud sound generated by discharging the coil of a magnetic stimulator over a metallic platform. The sound produced in this way has an intensity of 130 dB SPL, when measured at a distance of 1 m from the source with a Brüel and Kjaer Impulse Precision Sound Level Meter type 2204 and is effective in inducing a startle reaction (Valls-Solé et al. 1999). Subjects were given a demonstration of the SAS before beginning the recording session in order to avoid unsuitable reactions due to a surprise effect.
Instructions and general procedure
All experimental sessions began with explaining the tests to the subjects making sure that they understood them correctly and with demonstrating the SAS. After recording electrodes were put on, subjects were instructed to stay relaxed and follow the instructions. We started by recording five calibration trials in which subjects were requested to perform a fast eye movement along the 15° distance from the gaze fixation point to the target. Subjects were allowed to practice a sufficient number of trials to become accustomed to the task. Data collection began when subjects felt confident with their performance, usually after 4–6 trials. Subjects were instructed to look towards the gaze fixation point, and a verbal forewarning was issued for them to be prepared to make a saccadic movement at the perception of the target on their right peripheral visual field. In all instances, the initial gaze fixation point remained present. Subjects were also instructed to be prepared to react by performing a rapid wrist flexion or extension movement when they perceived the in-target symbol. We warned our subjects of the possibility that a SAS could be present in some of the trials, and encouraged them to be prepared to react to the visual cue regardless of the interference of the acoustic stimuli. No other interventions were made in control trials. In test trials, interspersed randomly, the presentation of the target was accompanied by the presentation of SAS. This was made possible by sending an external trigger signal from the Mystro5Plus electromyograph to the magnetic stimulator at the same time when the target was shown on the screen. In order to maintain the subject’s attention, SAS was applied on its own in three trials interspersed among control and test trials. In these SAS-alone trials no verbal forewarning or visual cue were presented.
Trials in which subjects began an eye movement during the 1 s period before target presentation were excluded. We also excluded trials in which subjects made a precipitated hand movement (before the end of the saccade) or moved the hand in the wrong direction. In these cases, trials were counted as errors and repeated on-line until we ended up with a total of 50 saccade trials per subject. These included 40 control trials and ten test trials for each condition. The in-target IS requiring wrist flexion or extension was presented in 20 trials each, while the ten remaining trials contained the IS requiring not to react (catch trials). In the ten test trials, the instructions were flexion in four, extension in four and not to move in two.
Data analysis
We measured the signals generated by the EOG and the wrist movement transducer. In SAS-alone trials, we calculated EOG vertical and horizontal onset latencies. In saccade trials, we measured for each subject the following variables, separately for control and test trials: saccade onset, determined as the latency of the first deviation of the baseline in the EOG recording for horizontal eye movements as previously described (Gribble et al. 2002); saccade end, determined as the time difference in ms between the presentation of the target and the end of the horizontal saccade in the EOG; saccade duration, determined as the time difference in ms between saccade end and saccade onset; saccade amplitude, determined as the amplitude of the linear displacement in the EOG, expressed in degrees after initial calibration of the eye movement (mean displacement in the calibration trials = 15°); hand movement reaction time, determined as the time difference between saccade end and the first consistent deviation from the baseline of the hand movement transducer signal. SCM–EMG was measured in test trials. The number of trials in which subjects made an error in the direction of hand movement (HM) (excluded from the main analysis) was expressed in percentage of the total number of trials. We included in the same determination the trials in which movements were made when the subject did not have to move.
We calculated the mean and SD for all parameters for each subject, grouped for type of trial. Statistical comparisons were done with one-factor (control versus test) repeated measures ANOVA, the dependent variables being saccade onset, saccade end, saccade amplitude, and hand movement reaction time.
Results
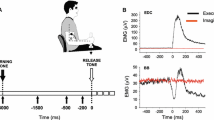
In SAS-alone trials, subjects reacted with a blink reflex that was recorded as a downward deflection in the vertical EOG at a mean onset latency of 48.6 ms (SD = 2.5 ms). No significant change was observed in the horizontal EOG (Fig. 1).
In control trials, the horizontal EOG signals accompanying saccades were recorded as a single straight line, consisting of a sharp deviation from the baseline followed by a slow return (Fig. 2a). No double saccades were observed in any trial. In test trials saccade onset was markedly shortened with respect to control trials, with no apparent changes in the latency of hand movement with respect to saccade end (Fig. 2b). Mean values are shown in Table 1 for all parameters measured in control and test trials. ANOVA showed that there were significant differences in saccade onset (F = 39.6; p < 0.0001) and saccade end (F = 29.9; p < 0.0001). No significant differences were found for saccade duration (F = 0.001; p = 0.9), saccade amplitude (F = 0.2; p = 0.7), or hand movement (F = 1.60, p = 0.2). The burst of EMG activity in the SCM was present in all test trials. The mean values for all individual trials pooled together were 46 ± 11 ms for onset latency and 2.1 ± 1.4 mV ms for response size. The large intra- and inter-individual variability in latency and amplitude of the response had a weak correlation with the percentage shortening of saccade onset latency for the same trial (Pearson’s correlation coefficient of −0.56 for onset latency and +0.64 for size).
The graphs are examples of recordings in control (a) and test (b) trials for saccade movements. They show a horizontal saccadic movement (upper trace), a hand movement (middle trace), and the EMG activity picked up from the left SCM (lower trace). Note the shortening of latency of the saccadic movement with no apparent changes in the latency of the hand movement with respect to the end of the saccade
Errors in the direction of hand movement occurred in all types of trials, but their number was largely variable among individuals, ranging from 0 to 3 in control trials and 0–4 in test trials. The mean percentage of error trials was larger in test than in control trials (t-test; p < 0.01). The percentage of errors made in catch trials (included in the data above) was larger in test than in control trials (with 0–2 errors out of the eight catch trials per subject interspersed among the control trials and 0–2 errors out of the two catch trials interspersed among the test trials). Hand movement reaction time was largely variable among individuals in error trials. Mean hand movement reaction time in test trials with errors was 211.7 ms (SD = 79.7 ms). These figures were significantly shorter than the mean values reported for trials with no apparent errors in Table 1 (unpaired t-test, p < 0.001).
Discussion
In this study we analysed the effects of a startle on the oculomotor system in preparation for a movement in an attempt to further understand the relationship between the startle reaction and the subcortical centres of eye movement control. Our results can be summarized in two main observations: (1) There was a speeding up execution of prepared saccadic movements made to a peripheral target when SAS was applied together with the visual target. (2) There were no differences between control and test trials in the execution of the secondary task, which instructions were only detected after saccade end and perception of target contents. In the following discussion, we will focus on how these results can be explained in the light of known physiology of eye movements and motor preparation.
Effects of startling auditory stimulus on saccade execution
Descriptions of movements observed in the startle reaction do not usually include ocular movements (Wilkins et al. 1986; Brown et al. 1991a; Chokroverty et al. 1992). Small and brief eye movements have been described with spontaneous blinking (Evinger et al. 1984) and also with reflex blinking to trigeminal nerve stimulation (Bour et al. 2000). Since blinking was indeed elicited by SAS in our subjects, we expected some small eye movements to occur. However, no eye movements were noticed in our study in the SAS-alone trials. It is possible that the superimposition of eyelid movement with the relatively small eye displacement of 1–2 mm (Bour et al. 2000) prevented the observation of any eye movement in the EOG. Recording the eye movements that could have been induced by the SAS in conditions of no preparation for a saccadic movement is an unresolved issue that would require a specific movement recording system. However, our results from the SAS-alone trials suggest that a SAS delivered with no preparation does not trigger an eye movement that could interfere with the recordings during execution of horizontal saccades. Furthermore, the observation of no differences regarding saccade duration and trajectory between control and test trials indicate that SAS did not induce significant changes on the intrinsic characteristics of the saccades.
It could be argued that the shortening of saccade onset in our subjects was related to the startle-induced reflex blinking. Gandhi and Bonadonna (2005) examined whether cessation of firing in omnipause neurons during blinking was sufficient to trigger saccades. In their study, when corneal air-puffs were delivered shortly after the cue to initiate movement, saccades occurred earlier in a linear relationship with blinking. However, this was not the case when reflex blinking overlapped with the cue (Gandhi and Bonadonna 2005). In a study in monkeys, Goossens and Van Opstal (2000) showed that reflex blinks induced by corneal air-puffs at onset of saccade dramatically changed the trajectory of the saccades. Modifications of saccades with blinking have also been described but with voluntary blinking (Rottach et al. 1998; Rambold et al. 2002). However, these observations do not apply to the results of our study. Blinking in our subjects occurred as a lateral manifestation of the startle reflex and caused no interference with the execution of the horizontal saccades. We believe that the saccade latency shortening observed in our study is independent of the startle-induced reflex blinking. However, it is possible that blinking contributed to accelerate saccade onset by disengaging gaze fixation.
Connections have been described between the reticular formation and the SC that could actually mediate the startle-induced saccade latency shortening by impinging on the executional centres for eye movements (Smit et al. 2005). This effect has been observed with limb movements in experiments involving simple reaction time tasks (Valls-Sole et al. 1999; Carlsen et al. 2003, 2004). In those instances, reaction time was so short that subcortical triggering of the motor program was favoured over cortical processing of sensory inputs (Valls-Sole et al. 1999). As with limb movements, the latency of eye movements performed in trials containing SAS was shortened with respect to control trials. Although the latency of saccade onset in test trials allows for the possibility of cortical processing, we think that the preparation of subcortical motor circuits plays the most important role for the shortening of saccade onset latency. Indeed, a high degree of motor preparation is an important requirement for the StartReact phenomenon to occur (Valls-Sole 2004; Kumru and Valls-Sole 2006). This may be the reason why the StartReact effect is less marked in choice than in simple reaction time paradigms (Valls-Sole 2004) and the startle reaction is larger in forced choice reaction time than in Go-noGo paradigms (Kumru et al. 2006). In our subjects, the percentage saccade onset latency shortening varied according to the size of the SCM burst, which is another argument favouring the relationship between the degree of subcortical motor preparation and the actual reaction time. We think that commands required for execution of a saccade are highly prepared and stored at a subcortical level, ready to be launched by an appropriate external trigger, as it is the case in most SRT paradigms (Henderson and Dittrich 1998).
Intentional visually guided saccades are likely generated in the frontal eye field (FEF), which neurons project not only to the deep layers of the SC but also to the pre-motor reticular formation of the brainstem (Pierrot-Deseilligny et al. 1995). In our paradigm, subjects had to perform an active disengagement from the fixation point before the saccade. Probably this involves cessation of activity in fixation-related cortical neurons of the FEF (Petit et al. 1995; Sommer and Wurtz 2000), de-activation of fixation neurons in the rostral SC (Gnadt et al. 1997), and dis-inhibition of saccade related neurons in SC (Munoz and Wurtz 1993; Munoz and Istvan 1998). It is possible that all mechanisms enabling fixation are interconnected, following a kind of corticotectal sequence that finalizes at the burst neurons of the brainstem reticular formation (Moschovakis 1996). If this is the case, external activation of the reticular formation could overcome the proposed steps and trigger the movement by actively disinhibiting the neurons in charge of movement execution. Premotor burst neurons of the brainstem reticular formation controlling horizontal saccades lie in the medial part of the nRPC (Horn et al. 1995), and a strong relationship between the nRPC and the SC has been thoroughly documented (Isa and Naito 1995; Zhao and Davis 2004).
Our data are consistent with the activation of a short-circuit at a site where the saccade is already fully programmed. Shortening of saccade onset latency takes place also in express saccades in paradigms making use of the gap effect (Munoz and Fecteau 2002; Coubard et al. 2004). Although the amount of shortening was significantly larger with the StartReact effect than with the gap effect, we should consider the mechanisms by which a saccade is speeded up in the gap effect in the discussion of possible explanations for our results (Sparks et al. 2000; Boulinguez et al. 2001). One of the opinions is that turning off the gaze fixation point causes disengagement of the fixation neurons of the SC (Munoz and Wurtz 1993; Moschovakis 1996). A similar disengagement from fixation could take place with reflex blinking occurring in the startle reaction or after startle-induced direct activation of the lateral portion of the SC, an area involved in coordination of eye and eyelid movements (Smit et al. 2005). Visually guided saccadic eye movements like those examined in our study were delayed after transcranial cortical magnetic stimulation (Priori et al. 1993), an observation pointing at a transient interruption of cerebral motor processing. However, the same stimulus did not affect express saccades which are thought to be mediated by subcortical structures without involvement of the cortex.
In our experiment, SAS could have induced a bypass of the cortical output to brainstem centres. The SC contains the structures that would make the generation of speeded-up saccades possible. A gradual build-up in activity takes place in neurons of the deep layers of the SC before execution of a saccade (Isa 2002). If activity in the deep layers of the SC is low when the visual input reaches the superficial layers, the visual input is relayed through cortical structures. However, in case of a high level of activity in the deep layers of the SC, inputs on the superficial layers could activate the deep layers and produce an express saccade (Isa 2002). A strong connectivity between superficial and deep layers has been already suggested by Behan and Appell (1992), Lee et al. (1997), and Doubell et al. (2003). These authors showed a strong staining of neurons of the deep layers of the SC after injection of a tracer in the neurons of the superficial layers. A similar mechanism could take place with auditory stimuli. It has been recently reported that, in monkeys, combined audiovisual stimuli induce reduced saccadic reaction times in comparison to unimodal stimuli (Bell et al. 2005). This was explained by an increase in premotor activity in neurons of the intermediate/deep layers of the SC, which should have the capability of integrating converging sensory inputs to influence the time of saccade initiation. Eye movement-related responses have been found in neurons of the ventral part of the human subthalamic nucleus in one experiment performed with the electrodes inserted for deep brain stimulation in eight patients with Parkinson’s disease (Fawcett et al. 2005). The role of such connections between the subthalamic nucleus and eye movements is not known but one of the possibilities is that they subserve a circuit projecting from the caudate to the substantia nigra pars reticulata (SNpr). The SNpr is known to exert a tonic inhibitory action on neurons of the intermediate layer of the SC, which is conveniently removed prior to saccade onset (Matsumura et al. 1992; Hikosaka et al. 2000). The activity found in the subthalamic nucleus could be modulating the output of the SNpr towards the SC as a manifestation of the basal ganglia control of eye and eyelid movements (Moschovakis 1996; Gnadt et al. 1997).
Accuracy of saccadic movements performed in startling auditory stimulus trials
The question of whether the movement executed is actually the movement intended is relevant not only for the physiology of the StartReact effect but also to provide further light on the relationship between movement and perception. Eye movements hold special characteristics that make them appropriate for the study of target accuracy. Therefore, by requesting the subjects to make a hand movement according to the in-target instructions, we aimed at finding out whether the endpoint of the horizontal saccade performed in test trials was modified or not with respect to a baseline condition and whether or not visual perception was delayed after SAS. The fact that the hand reaction time was similar in our subjects in control and test trials suggests that their perception after the saccadic movement was similar in both conditions, the only difference being the shorter onset and end of the saccade in test trials. This in turn indicates that the amplitude of the saccade should have been fully programmed at the level where the SAS activated the system. Our results indicate that the motor commands required for the execution of a saccade are already represented in the reticular formation at the time of presentation of the IS.
Errors were seen in both conditions, although they were more frequent in SAS trials. The error trials had a shorter HM onset than non-error trials. This is probably the consequence of movement precipitation, an effect that can be often seen in choice reaction time task paradigms (Valls-Solé 2004). We think that some of our subjects prepared the wrist motor set largely in advance for a rapid performance and, therefore, precipitation occurred as a consequence of excessive preparedness. It has generally been held that motor preparation does not occur in choice reaction time tasks (Frith and Done 1986; Carlsen et al. 2004). However, our findings suggest that subjects may prepare a response that they are likely predicting. Predictive preparation could form an optimal strategy for rapid responding, as the process of building up an unprepared response may be slower and more effortful than inhibiting a motor drive that is already prepared (Henderson and Dittrich 1998).
In conclusion, the fact that SAS induces a dramatic shortening of onset latency of externally guided saccades without modifying the intrinsic characteristics of the ocular movement suggests that saccades are mainly programmed at a level where SAS can trigger them. Such triggering may use a short-circuit bypassing some sensory processing but does not seem to affect the accuracy of target reaching. SAS-induced involuntary execution of prepared saccades does not allow for voluntary modification of gaze directionality but this does not seem to affect the time processing required for the analysis of the in-target signals.
References
Behan M, Appell PP (1992) Intrinsic circuitry in the cat superior colliculus: projections from the superficial layers. J Comp Neurol 315:230–243
Bell AH, Meredith MA, Van Opstal AJ, Munoz DP (2005) Crossmodal integration in the primate superior colliculus underlying the preparation and initiation of saccadic eye movements. J Neurophysiol 93:3659–3673
Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, Van Boxtel A (2005) Committee report: guidelines for human startle eyeblink electromyographic studies. Psychophysiology 42:1–15
Boulinguez P, Blouin J, Nougier V (2001) The gap effect for eye and hand movements in double-step pointing. Exp Brain Res 138:352–358
Bour LJ, Aramideh M, Ongerboer DE, Visser BW (2000) Neurophysiological aspects of eye and eyelid movements during blinking in humans. J Neurophysiol 83:166–176
Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD (1991a) New observations on the normal auditory startle reflex in man. Brain 114:1891–1902
Brown P, Day BL, Rothwell JC, Thompson PD, Marsden CD (1991b) The effect of posture on the normal and pathological auditory startle reflex. J Neurol Neurosurg Psychiatry 54:892–897
Carlsen AN, Hunt MA, Inglis JT, Sanderson DJ, Chua R (2003) Altered triggering of a prepared movement by a startling stimulus. J Neurophysiol 89:1857–1863
Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM (2004) Prepared movements are elicited early by startle. J Motor Behav 36:253–264
Chokroverty S, Walczak T, Hening W (1992) Human startle reflex: technique and criteria for abnormal response. Electroencephalogr Clin Neurophysiol 85:236–242
Collewijn H, Van Der Steen J, Steinman RM (1985) Human eye movements associated with blinks and prolonged eye closure. J Neurophysiol 54:11–27
Coubard O, Daunys G, Kapoula Z (2004) Gap effects on saccade and vergence latency. Exp Brain Res 154:368–381
Davis M, Gendelman DS, Tishler MD, Gendelman PM (1982) A primary acoustic startle circuit: lesion and stimulation studies. J Neurosci 2:791–805
Doubell TP, Skaliora I, Baron J, King AJ (2003) Functional connectivity between the superficial and deeper layers of the superior colliculus: an anatomical substrate for sensorimotor integration. J Neurosci 23:6596–6607
Evinger C, Shaw MD, Peck CK, Manning KA, Baker K (1984) Blinking and associated eye movements in human, guinea pig and rabbits. J Neurophysiol 52:323–329
Fawcett AP, Dostrovsky JO, Lozano AM, Hutchison WD (2005) Eye movement-related responses of neurons in human subthalamic nucleus. Exp Brain Res 162:357–365
Flaten MA, Nordmark E, Elden A (2005) Effects of background noise on the human startle reflex and prepulse inhibition. Psychophysiology 42:298–305
Frith CD, Done DJ (1986) Routes to action in reaction time tasks. Psychol Res 48:169–177
Gandhi NJ, Bonadonna DK (2005) Temporal interactions of air-puff-evoked blinks and saccadic eye movements: insights into motor preparation. J Neurophysiol 93:1718–1729
Gehricke JG, Ornitz EM, Siddarth P (2002) Differentiating between reflex and spontaneous blinks using simultaneous recording of the orbicularis oculi electromyogram and the electro-oculogram in startle research. Int J Psychophysiol 44:261–268
Gnadt JW, Lu SM, Breznen B, Basso MA, Henriquez VM, Evinger C (1997) Influence of the superior colliculus on the primate blink reflex. Exp Brain Res 116:389–398
Goossens HH, Van Opstal AJ (2000) Blink-perturbed saccades in monkey. I. Behavioral analysis. J Neurophysiol 83:3411–3429
Gribble PL, Everling S, Ford K, Mattar A (2002) Hand-eye coordination for rapid pointing movements. Arm movement direction and distance are specified prior to saccade onset. Exp Brain Res 145:372–382
Heide W, Koenig E, Trillenberg P, Kömpf D, Zee DS (1999) Electrooculography: technical standards and applications. Electroencephalogr Clin Neurophysiol Suppl 52:223–240
Henderson L, Dittrich WH (1998) Preparing to react in the absence of uncertainty: I. New perspectives on simple reaction time. Br J Psychol 89:531–554
Hikosaka O, Takikawa Y, Kawagoe R (2000) Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev 80:953–978
Horn AK, Buttner-Ennever JA, Suzuki Y, Henn V (1995) Histological identification of premotor neurons for horizontal saccades in monkey and man by parvalbumin immunostaining. J Comp Neurol 359:350–363
Isa T (2002) Intrinsic processing in the mammalian superior colliculus. Curr Opin Neurobiol 12:668–677
Isa T, Naito K (1995) Activity of neurons in the medial pontomedullary reticular formation during orienting movements in alert head-free cats. J Neurophysiol 74:73–95
Kofler M, Muller J, Reggiani L, Valls-Sole J (2001a) Influence of gender on auditory startle responses. Brain Res 921:206–210
Kofler M, Muller J, Reggiani L, Valls-Sole J (2001b) Influence of age on auditory startle responses in humans. Neurosci Lett 307:65–68
Kumru H, Valls-Sole J (2006) Excitability of the pathways mediating the startle reaction before execution of a voluntary movement. Exp Brain Res 169:427–432
Kumru H, Urra X, Compta Y, Castellote JM, Turbau J, Valls-Sole J (2006) Excitability of subcortical motor circuits in Go/noGo and forced choice reaction time tasks. Neurosci Lett (in press)
Landis C, Hunt WA (1939) The startle pattern. Farrar and Rinehart, New York
Lee PH, Helms MC, Augustine GJ, Hall WC (1997) Role of intrinsic synaptic circuitry in collicular sensorimotor integration. Proc Natl Acad Sci USA 94:13299–13304
Leintner DS, Powers AS, Hoffman HS (1980) The neural substrate of the startle response. Physiol Behav 25:291–297
Matsumura M, Kojima J, Gardiner TW, Hikosaka O (1992) Visual and oculomotor functions of monkey subthalamic nucleus. J Neurophysiol 67:1615–1632
Moschovakis AK (1996) The superior colliculus and eye movement control. Curr Opin Neurobiol 6:811–816
Munoz DP, Fecteau JH (2002) Vying for dominance: dynamic interactions control visual fixation and saccadic initiation in the superior colliculus. Prog Brain Res 140:3–19
Munoz DP, Istvan PJ (1998) Lateral inhibitory interactions in the intermediate layers of the monkey superior colliculus. J Neurophysiol 79:1193–1209
Munoz DP, Wurtz RH (1993) Fixation cells in monkey superior colliculus. I. Characteristics of cell discharge. J Neurophysiol 70:559–575
Nieuwenhuijzen PH, Schillings AM, Van Galen GP, Duysens J (2000) Modulation of the startle response during human gait. J Neurophysiol 84:65–74
Petit L, Tzourio N, Orssaud C, Pietrzyk U, Berthoz A, Mazoyer B (1995) Functional neuroanatomy of the human visual fixation system. Eur J Neurosci 7:169–174
Pierrot-Deseilligny C, Rivaud S, Gaymard B, Muri R, Vermersch AI (1995) Cortical control of saccades. Ann Neurol 37:557–567
Priori A, Berardelli A, Rothwell JC, Day BL, Marsden CD (1993) Some saccadic eye movements can be delayed by transcranial magnetic stimulation of the cerebral cortex in man. Brain 116:355–367
Rambold H, Sprenger A, Helmchen C (2002) Effects of voluntary blinks on saccades, vergence eye movements, and saccade-vergence interactions in humans. J Neurophysiol 88:1220–1233
Rambold H, El Baz I, Helmchen C (2005) Effect of blinks on saccades before smooth-pursuit eye-movement initiation. Ann NY Acad Sci 1039:563–566
Rottach KG, Das VE, Wohlgemuth W, Zivotofsky AZ, Leigh RJ (1998) Properties of horizontal saccades accompanied by blinks. J Neurophysiol 79:2895–2902
Smit AE, Zerari-Mailly F, Buisseret P, Buisseret-Delmas C, Vanderwerf F (2005) Reticulo-collicular projections: a neuronal tracing study in the rat. Neurosci Lett 380:276–279
Sommer MA, Wurtz RH (2000) Composition and topographic organization of signals sent from the frontal eye field to the superior colliculus. J Neurophysiol 83:1979–2001
Sparks D, Rohrer WH, Zhang Y (2000) The role of the superior colliculus in saccade initiation: a study of express saccades and the gap effect. Vision Res 40:2763–2777
Valls-Sole J, Rothwell JC, Goulart F, Cossu G, Munoz E (1999) Patterned ballistic movements triggered by a startle in healthy humans. J Physiol 516:931–938
Valls-Sole J (2004) Contribution of subcortical motor pathways to the execution of ballistic movements. Suppl Clin Neurophysiol 57:554–562
Wilkins DE, Hallett M, Wess MM (1986) Audiogenic startle reflex of man and its relationship to startle syndromes. A review. Brain 109:561–573
Yeomans JS, Li L, Scott BW, Frankland PW (2002) Tactile, acoustic and vestibular systems sum to elicit the startle reflex. Neurosci Biobehav Rev 26:1–11
Zhao Z, Davis M (2004) Fear-potentiated startle in rats is mediated by neurons in the deep layers of the superior colliculus/deep mesencephalic nucleus of the rostral midbrain through the glutamate non-NMDA receptors. J Neurosci 24:10326–10334
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castellote, J.M., Kumru, H., Queralt, A. et al. A startle speeds up the execution of externally guided saccades. Exp Brain Res 177, 129–136 (2007). https://doi.org/10.1007/s00221-006-0659-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0659-4