Abstract
We aimed to dissociate components in prism adaptation and its aftereffect by using prism adaptation training in healthy humans. Arm proprioceptive aftereffects are usually measured by indicating the subjective straight ahead direction with eyes closed (S). This measure however could be affected by other components besides proprioception, such as an efferent motor component and internal egocentric reference frame. Here we report a very long lasting proprioceptive shift, detected by two measuring methods, that is a component of the adaptation aftereffects to left wedge prism glasses. In order to minimize possible active motor components, arm passive proprioceptive midsagittal judgment was measured (P). The subject’s arm was passively brought from the right or left lateral position, and stopped by subjects’ verbal order. The results from these different measurements of midsagittal judgment were compared for 7 days after prism adaptation. Surprisingly, we found two distinctly separate aftereffects of proprioceptive shift depending on the directions of the passive arm movement. The shift of the midsagittal plane appeared only when tested from the left (Pl). This indicates that our strong prism adaptation procedure affected proprioception in a directionally biased way and not a spatially ubiquitous way. Further, the early aftereffect seen in active straight ahead pointing (S) was mostly similar to this biased shift in proprioception (Pl). However the long lasting aftereffect in straight ahead pointing was independently maintained up to day 7, when the passive proprioception had returned to pretest level. These results indicate that active straight ahead pointing (S) involves other components in addition to the passively measurable proprioceptive component. We suggest a late onset shift in the internal egocentric reference frame is involved in S. Possible neural mechanisms for these phenomena are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adapting to optically shifting prism glasses involves spatial adjustment of eye-hand coordination (Welch 1978, 1986; Redding and Wallace 1997). The adjustment is thought to have two main mechanisms: strategic and adaptive components (Redding and Wallace 1996, 1997; Redding et al. 2005). Initially, subjects cognitively correct their pointing using visual feedback from one movement to strategically adjust their pointing on the next movement, possibly in combination with automatic and/or cognitively guided on-line corrections during the pointing (Redding and Wallace 1996). Then, through repeated pointing at the correct location, neural networks are adaptively changed. When the glasses are removed after adaptation, subjects make opposite pointing errors, exposing a compensatory aftereffect thought to be unaffected by strategic components.
Subjective straight ahead pointing without vision has in the past been extensively used to measure ‘proprioceptive midsagittal judgment’ (e.g. Harris 1963; Wilkinson 1971; Redding and Wallace 1992). Harris (1963) investigated the changes after prism adaptation in visual, motor and proprioceptive components. He interpreted the shift in straight ahead pointing without vision as a proprioceptive shift. Active straight ahead pointing could, however, involve more cognitive top down control onto the felt sense of proprioceptive midsagittal plane, including for instance efferent motor control components evoked during active movement.
In order to measure purer proprioceptive midsagittal judgment (P), we tried to minimize the active motor control component of classical sagittal straight ahead pointing (S) by additionally measuring passive arm movements. The subject’s arm is brought passively by an experimenter from left (Pl) and right (Pr) lateral position and is verbally stopped at the subjective midline. In the past, Baily (1972) and Beckett (1980) used this method to assess prism adaptation aftereffect, and in particular they investigated the relationship between active or passive training and testing. In neither of these studies were the two different directions of passive arm judgments analysed separately. Recently, Girardi et al. (2004) reported stronger rightward than leftward directional bias after leftward 15° prism adaptation training in healthy human subjects, although their testing method differed from ours. They tested by using a haptic centering judgment when actively exploring a 30 cm circular plate presented at the middle in front of the chest. Chokron et al. (2004) reported that starting position bias causes right starting positions to generate more rightward shifts and left starting positions to generate more leftward shifts for midsagittal pointing in healthy subjects under normal conditions (i.e. without prism adaptation). This bias was opposite from the pretest measurement of bias seen in Girardi et al. study. Girardi et al. did not test lateral directional bias at the proprioceptive level to compare with haptic directional bias and they tested only straight ahead sagittal pointing which would not reveal the directional bias. Thus it is not clear if Girardi et al.’s bias originated at the internal representational level as they suggested, or at a lower proprioceptive level, with the haptic shift as a secondary effect. It would be informative if we can characterize the aftereffect spatial shift in the measurable sub-components, like directionality and sub-components of proprioception. We asked how much directionally biased proprioceptive shift could be generated in prism adaptation aftereffect.
Previously we reported that our strong prism adaptation procedure generated a very long lasting aftereffect in straight ahead pointing (Hatada et al. 2005). This aftereffect showed two waves with different time scales of hours and days. Here we further report that the shift in straight ahead pointing seen in this experiment could be dissociated into more than two components based on their aftereffect time course. The early aftereffect seen in the active (S) and in one of the two passive (Pl) midsagittal proprioceptive judgments was similar in magnitude for up to 1–2 days after adaptation. However, the long-lasting aftereffect in active straight ahead pointing was independently maintained for at least 7 days, at which point the shift in the passive proprioceptive component was no longer significantly different from pretest level. We suggest that a late onset shift in the internal egocentric reference frame is involved in the active straight ahead measurement, S. Furthermore, only when moving from the left (Pl) did the passive proprioceptive measure exhibit a shift of the perceived midsagittal plane. Therefore, we suggest that our prism adaptation generated a proprioceptive shift, affecting the passive measurement in a directionally biased way. The possible mechanisms involved in these aftereffects will be discussed.
Method
This paper reports data collected during a single prism adaptation experiment, in which multiple measures of the aftereffect were taken. Some of the data from that experiment were published elsewhere (Hatada and Rossetti 2004a, b; Hatada et al. 2005, 2006).
Apparatus
The same experimental set up was used both during the prism adaptation training and during the aftereffect measuring sessions (Hatada et al. 2006). The subject was seated at a fixed position relative to the measurement apparatus with head stabilized by a chin rest. The height and position of the chair was adjusted to bring the measurement table just below chest level for comfort. Pointing direction was measured using a touch tablet that registered the position of an index finger thimble (Rossetti et al. 1998). During all pointing tasks and prism adaptation training, the subject’s left hand rested on his left thigh. At the start of each pointing movement the right index finger rested on the table in front of the subject at lower chest level at an invisible position.
The prism adaptation training
Table 1 shows the prism adaptation training procedure (the full testing protocol is given in Hatada et al. 2005). Seven levels of left-shifting wedge glasses were used for this prism adaptation training (2, 4, 6, 8, 10, 12 and 15°, in order). The glasses were put on while the subject's eyes were closed. While first wearing the 2° glasses, the subject was asked to point at two fixed targets already marked on the apparatus board which were 10° right and left from the midsagittal point at 50 cm in front of the subject. The subject pointed ten times to each target with the right index finger at a comfortable speed. Pointing to the two targets was performed in a random order under the verbal instruction of the experimenter. After a total of 20 target pointing movements (right 10 times and left 10 times), there was a 5 s pause before the same training procedure was repeated. After the 40 pointing movements, the glasses were removed while the subject’s eyes were closed.
Then, with the next level of prism glasses, the above adaptation training was repeated. With the final 15° prism glasses, the adaptation training was repeated twice (i.e. a total of 80 pointing movements). Finally, the subject walked out from the laboratory in our institute while wearing 15° prism glasses for 45 min of whole body exposure during which he or she walked and pointed in the normal environment.
Measurements of prism adaptation aftereffects
There was a measurement session before adaptation training to provide baseline data (Table 1). These tests were performed with eyes closed, and using the right hand. The measured factors were, first, proprioceptive straight ahead judgement (P) from right and left sides (Pr and Pl), as described below and in Fig. 1; and second, active straight ahead pointing (S).
For proprioceptive judgements, the subject’s extended right hand was passively moved about 30° right or left from the midsagittal plane. First the hand was moved by the experimenter (at approximately 5°/s) from the right edge until the subject verbally stopped the passive movement in the subjective midsagittal plane. From that point the subject vertically lowered the hand onto the measuring board. Then the hand was moved from the left edge, by the experimenter who now stood on the left side of subject. The two opposite directions were measured 10 times each, giving a total of 20 measurements. After the proprioceptive midsagittal measurement, ten straight ahead pointing movements were performed: the subject pointed, at comfortable speed with the right index finger to the subjectively felt midsagittal plane on the apparatus board, without visual feedback.
After prism adaptation, aftereffects were measured at 0 h (immediately after the removal of the 15° prism glasses following the whole set of prism adaptation training as described above), and at 2, 4, 6 h and 1, 2, 3 and 7 days.
Subjects
Eight normal right handed subjects (3 females, 5 males, 22–45 years). In accordance with the French law, informed consent was gained individually in written form before their attendance for experiments.
Data analysis
Ten measurements of each component were averaged and analyzed using repeated measure ANOVA. Student’s t tests and Dunnett’s two-tailed post-hoc tests were performed.
Results
Firstly, we compared the passive directional measurements from two opposite lateral positions (Pr and Pl). The results unexpectedly revealed that, our prism adaptation affected the two directional proprioception in a separable, spatially biased way. Secondly, we compared the proprioceptive midsagittal shifts determined by the two measuring methods: straight ahead pointing (S) with the two passive proprioceptive measurements (Pr and Pl). One of the two passive proprioceptive directional measurements showed the same two wave pattern as was seen in straight ahead pointing (Hatada et al. 2005) with peaks at the same times.
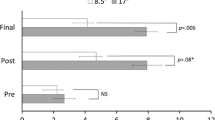
Figure 2 shows the absolute deviation from the center for the three measuring methods, the passive proprioceptive midsagittal judgments measured from opposite directions and straight ahead pointing, for comparing the magnitude and variability of the three measures at pre-test and post-test measurements times. Figure 3 shows the magnitude of deviation in each aftereffect with respect to its pre-test baseline comparing the three methods at each time point.
Absolute position of mean and standard error in midsagittal judgment by the two proprioceptive midsagittal plane judgments measured by passive arm movement from right (Pr) and left (Pl) lateral positions, and straight ahead pointing (S) for 7 days after prism adaptation procedure. Vertical bars indicate standard error. Asterisks indicate a significant shift from pretest measures within each measuring method by Dunnett’s post-hoc test. Dash non-significant, *P<0.05, **P<0.005. n=8
Comparisons between deviation from pretest measurements of mean and standard error in midsagittal judgment by straight ahead pointing (S) and the passive proprioceptive midsagittal plane judgments by two passive arm movement from right (Pr) and left (Pl) lateral positions for 7 days after prism adaptation procedure. Vertical bars indicate standard error. Asterisks indicate a significant difference between S, Pr and Pl tested by Dunnett’s post-hoc tests. Dash non-significant, *P<0.05, **P<0.005. n=8
Two passive proprioceptive measurements (Pr and Pl) show different shifts in adaptation aftereffect
Figure 2 shows the mean and SE of the passive proprioceptive midsagittal judgment measured from opposite directions. The pretest measurements of the two were unbiased, i.e. not significantly different from the center [Pr=−0.23°±0.75° (SE) and Pl=0.02°±1.46° (SE)]. In addition, at pretest the absolute positions of the two, Pr and Pl, were not significantly different from each other (t=0.24, P=0.82, student’ t test).
A two-way repeated-measure ANOVA with within-subject factors of measuring method (Pr, Pl) and time (pre-test, 0, 2, 4, 6 h and 1, 2, 3 and 7 days) revealed a significant main effect of time [F(8, 56)=7.49, P<0.001] and a significant interaction between the two factors [F(8, 56)=2.11, P<0.050]. The effect of measurement type (Pr, Pl) was near statistical significance [F(1, 7)=4.52, P=0.071]. Dunnett’s post-hoc test revealed that the magnitude of the shift of aftereffect was significantly different between Pr and Pl at 0 and 2 h, (P<0.001, 0.005, respectively; Fig. 3). Therefore these analyses suggest that the magnitude of the shifts seen in the aftereffect in Pr and Pl were significantly different from each other in the first wave (under 6 h) but not in the second wave (over 1 day).
For passive movement from the left (Pl), there were significant deviations from pre-test at two time points (Dunnett’s post-hoc tests): immediately after (0 h, P<0.001) and 3 days after (P<0.035) the prism adaptation procedure (Fig. 2). In contrast, the proprioceptive judgment from the right (Pr) did not show any significant difference between pre- and post-test during the entire 7 days of observation. Even immediately after adaptation, there was no significant difference from pre-test (Dunnett’s post-hoc test). The difference from pre-test was greatest after 3 days, but this was still non-significant (P>0.50; Fig. 2). Therefore, these results indicate that leftward moving passive proprioceptive midsagittal judgment from the right (Pr) was not significantly affected by our prism adaptation. Only the rightwards moving measure (Pl), from the left, was significantly affected.
Surprisingly, this suggests that our prism adaptation affected proprioception in a spatially biased manner depending on the direction of arm movement used to measure its aftereffects.
The relation between the aftereffects in straight ahead pointing (S) and the two directional proprioception measures (Pl and Pr)
Figure 3 shows the deviation from pretest of the three measurements (S, Pr and Pl). A two-way repeated-measures ANOVA with within-subject factors of measuring method (S, Pr, Pl) and time (0, 2, 4, 6 h and 1, 2, 3 and 7 days) showed a significant interaction effect between the two factors [F(14, 98)=9.41, P<0.019]. The three measuring methods showed a significant main effect [F(2, 14)=4.47, P<0.032]. Therefore, we analyzed the differences among the aftereffects in Pr, Pl, and S.
Dunnett’s post-hoc tests revealed the magnitudes of the shift of aftereffects were significantly different between S and Pr at 0 h, 2 h, 4 h and 2, 3 and 7 days (P<0.001, 0.005, 0.016, 0.020, 0.004, 0.001, respectively, indicated with asterisks in Fig. 3). However, the magnitudes of aftereffect in S and Pl were significantly different only after 7 days (P<0.013; Dunnett’s post-hoc test).
These results confirm first, in addition to the earlier analysis on Fig. 2, that the two opposite directions of passive proprioceptive midsagittal judgments shifted significantly differently after our prism adaptation in relation to the aftereffect in S. Secondly, the shift seen in the aftereffect in Pr was significantly different from the aftereffect in S. Thirdly, the magnitude of shift seen in the first wave and the early part of the second wave of the aftereffect in S (at under 3 days after adaptation) was not significantly different from the aftereffect in Pl. The second wave of S became significantly different from Pl for first time after 7 days delay.
Discussion
The results of our study revealed two main points. First, the passively measured proprioceptive aftereffects, Pl and Pr, were significantly shifted from each other. The prism adaptation procedure produced a significant rightward shift in Pl but, surprisingly, did not show any significant shift in Pr. In other words, the passive proprioceptive midsagittal judgment, measured by passive arm movements from opposing lateral positions, shifted in a significantly directionally biased manner and not in a ubiquitous manner. Second, comparison between S, Pl and Pr revealed that a similar two-wave pattern of aftereffect was seen in S and Pl with peaks at 0 h and 3 days. However, after 7 days the sustained aftereffect in the second wave of S became significantly different from both Pl as well as Pr.
After verifying the methods of our aftereffect measurement, we will next argue that peripheral sources are unlikely and discuss possible central mechanisms of plastic modification for both the immediate and long lasting modification of aftereffects, with respect to the behavioral characteristics we measured.
Validation of our method for measuring proprioceptive aftereffects
One may suggest that the lateral arm movement from the left side for measuring Pl is kinematically more similar than Pr to the midsagittal pointing performed during the prism adaptation. This could imply some contribution from context-dependent recall of the experimental testing conditions (reminiscence: Welch 1978 review). However, we believe these contextual effects should be minimal since the arm movements during adaptation involved pointing at two laterally displaced targets from a starting point in front of chest, while the test measurements were of different kinds for all the Pr, Pl and S. Pr and Pl were subjective midsagittal judgments made during passive lateral arm movements which are totally different kinematic movements to those during adaptation. S was kinematically more similar but was aimed at the subjective midsagittal 0° position, not pointing at two targets 10° lateral of the center. Further, as seen in Fig. 2, not all measures of aftereffect appear to be equally affected. For example only the 6 h measure of S lost significant aftereffect, while Pr did not show any significant aftereffect throughout the 7 days period. Note that the incremental exposure to prisms meant that the subjects were unaware of the adaptation process. Hence we did not believe recall will have greatly influenced our results.
It is possible that subjects may have been aware of the differential amplitude of movements required to go from right and left sides of the body towards the laterally shifted, but perceived straight ahead position and tried to correct (i.e. minimize) the difference in aftereffect measured in Pr and Pl. However, this would be likely to lead to averaging across Pr and Pl, rather than the effect we have seen, where there is no significant aftereffect in Pr (see also Fig. 1).
Other studies using proprioception by lateral passive midsagittal judgment, have not reported directional differences. Beckett (1980) measured proprioceptive shift by passive lateral arm movement from right and left, and showed a larger magnitude of aftereffect by active pointing than by passive judgment. Unfortunately he did not separately analyse the two different directions of passive arm judgments. In his study the prism adaptation training involved lateral direction pointing and not the near sagittal pointing as was the case of our study. Therefore the shift in active lateral during adaptation in Beckett’s study pointing could be more strongly associated with context specific aftereffects, instead of a proprioceptive adaptation.
More recently, Chokron et al. 2004 found a starting position bias, in which right starting positions (Pr) generated more rightward shifts and left starting positions (Pl) generated more leftward shifts for midsagittal pointing in normal subjects in their normal condition, without prismatic adaptation. The condition in our study that most closely resembles the Chokron et al. 2004 experiment is our pretest condition, which however did not show a significant difference between Pr and Pl. Their passive measurements were done at a speed of 2°/s, which is slow compared to our measurements at 5°/s. Note that the higher movement speed in our experiment will have the effect of magnifying, in terms of movement distance, any measurement error due to response time delay. For example, a 200 ms delay between proprioceptive perception of the midsagittal position and verbal response, at 5o/s, would lead to an error of 1o. Therefore, Chokron et al.’s results along with ours may not be directly comparable. However, the response time delays do not affect our conclusions since our data analysis is based on within-subject comparison between pre- and post-test measurements.
Early similarity and late difference between S and Pl aftereffects
In the first wave and the early second wave, the aftereffect seen in S showed a similar pattern to the aftereffect seen in Pl. This may suggest that the shift of aftereffect in S and the directional bias of aftereffect in the passively measurable shoulder proprioception (Pl) both involve the same neuronal networks, leading to early as well as late plasticity up to 1 or 2 days in our adaptation aftereffect. The neural mechanisms of long lasting aftereffect of prism adaptation with time courses in the ranges of hours and days have been explained in the literature with two separate mechanisms of cellular plasticity in neuronal cells: e-LTP (early long term plasticity, including potentiation, facilitation and depression) in hours and l-LTP (late long term plasticity) in days (e.g. Hatada et al. 2000, 2005; Kandel 2001). The first, e-LTP, is controlled by second messengers and kinases in cytoplasm within existing synapses. The second, l-LTP, depends on gene transcription and translation leading to stable morphological changes including new synaptic connectivity (Bailey and Kandel 1993; Kandel 2001). During the interval between e-LTP and l-LTP, adaptation-specific inputs at subsets of synapses can be maintained for a few hours via synapse-specific “tagging” (Martin and Kosik 2002).
The aftereffect in Pl became significant again at 3 days, coinciding with the peak time of S. Therefore the shifting patterns in S and Pl were still similar in this late stage, though the difference in the magnitudes of the shift in the two started to increase. The S aftereffect finally became significantly different from Pl after a long delay of 7 days. The sustained aftereffect shift in S after 7 days (Fig. 3) requires some other neural source to explain how it is maintained when the shift in Pl is not.
Possible sources for biased proprioceptive aftereffect at behavioural level
Most adaptation happens during outward arm movement towards the target, with visual feedback when the finger reaches the target position, while the returning inward arm movement does not give adaptive input since there is no visual feedback and subjects were not demanding high finger position accuracy. With increase in the strength of the wedge prisms, there would be some consistent pointing error.
However, if the small pointing errors occurring at the beginning of each increment of prism magnitude could generate the asymmetry of adaptation, we would expect that the proprioceptively measured aftereffect would only be leftward for movements from right, since each increment of prism shift causes overshooting to the left (though of a small magnitude). So the bias in reaching towards the left during adaptation could have been reflected in an aftereffect during movements towards the left (i.e. during Pr). However, the leftward overshooting during adaptation also causes subjects to correct rightwards from their leftward overshooting position. Our post-test proprioceptive bias was found only for rightward movements from the left (Pl). Therefore this aftereffect bias likely reflects the process of rightward correction, and not the leftward misreaching.
Peripheral components are unlikely sources for aftereffects
Motor efferent component
Our aftereffect measurement for S was not a ballistic movement but was done using subjectively comfortable speed of roughly 2 s per pointing movement Pr and Pl were measured using relatively slow speeds of roughly 5°/s. Lateral arm rotation at speeds of 5°/s means that subjects had enough time to feel the correct midsagittal position through afferent proprioceptive signals (Cordo et al. 1994). Pointing at subjectively comfortable speed means that subjects could correct their pointing until they felt subjectively satisfied. Therefore we think the measurement of Pl and Pr were accurate subjective measurements of the proprioceptive aftereffect in the shoulder (Pr and Pl were measured by shoulder movements) including muscles involved in the pointing movements used during prism adaptation.
Proprioceptive afferent component
The directional differences in the passive proprioceptive aftereffect may be thought to be related to arm kinematics. During the prism adaptation training, the subjects pointed to 10° lateral (left and right of centre) targets by outward arm movements starting from in front of their chest while their right hand, as mentioned above, might be expected to make the rightward corrective movements. During the passive proprioceptive aftereffect measurements, the measurement of Pl also involved the same rightward direction of movement as the outward training movements, whereas the measurement of Pr involved a leftward movement. These differences could then imply that the adaptation may have affected different sets of shoulder muscles for rightward and leftward movement.
From studies of elbow flexion/extension movement (Inglis and Frank 1990; Capaday and Coole 1981, 1983) it was shown that antagonist muscles play a greater role for accurate proprioception than agonist muscles. Muscle vibration is known to activate primary muscle spindle receptors (Burke et al. 1976b), which cause subjects to over-estimate muscle length and hence proprioceptive sense of angle of the joint. This effect was seen more by the vibration of antagonist than agonist muscles (Prochazka et al. 1979; Prochazka 1981; Inglis and Frank 1990) regardless of active/passive arm movement (Burke et al. 1976a, b).
However, although during the prism adaptation training the target was gradually optically shifted 15° leftward and the relation between visual perception and proprioceptive perception was separated 15° apart from each other, the physical positions of the two targets were unchanged and so the pointing posture should also not been changed. Therefore activity in shoulder muscle spindles as a peripheral origin for shifting proprioceptive perception is unlikely.
Active and passive components
Peripheral inputs, that are only available during active movements, could arise from Golgi tendon organs or some secondary spindles that are relatively insensitive to passive movements, but signal muscle tension and stretch when the muscle is actively contracting (Houk and Henneman 1967; Crago et al. 1982; Wei et al. 1986; Al-Falahe et al. 1990). Thus passive and active pointing could rely on different peripheral signals.
However, when we consider the results for the 7 days of observation, the early S and Pl measurements did not show significant differences. If a difference in peripheral signals caused by passive or active pointing per se had caused the significant behavioural difference after 7 days, it would be expected that this difference should have been apparent from the beginning of the aftereffect and not only after such a long delay. Therefore peripheral signals involved in differences between active and passive pointings are unlikely components for adaptation aftereffect.
CNS sources for aftereffects
Possible involvement of internal egocentric reference frame in straight ahead pointing
The difference between S and Pl after 7 days in Fig. 3 suggests that S consists of other components than simply measurable Pl and Pr. We therefore make the following suggestion, which by necessity must be speculative at this stage. The phenomena after 7 day could suggest the existence of an internal representational egocentric reference frame (IEREF) used in straight ahead pointing. IEREF has been suggested by studies in different conditions. Direct observation using PET has been reported for functional anatomy involved in the shift of egocentric space by caloric vestibular stimulation and neck muscle vibration (Bottini et al. 2001). Graziano et al. (1997) showed evidence for an abstract form of spatial coding directly from a monkey single-unit recording study. The vPM cells showed activity correlated to an internal representation of “object permanence” even when an object was no longer within the visual receptive field while the monkey believed the object was there, i.e. the activity of the neurons was related to “motor schema” (Rizzolatti et al. 1997).
Before each straight ahead pointing, subjects have to use IEREF to decide where to point, integrating the location of the midsagittal position into their movement plan. In contrast, before Pr and Pl measurement subjects do not need to use IEREF. Instead they judge when they feel they have reached the midsagittal point, by relying on afferent signals, and which are a part of perceptual proprioception itself. In the case of passive movements, subjects judge the midsagittal point directly from the proprioceptive afferent signals, there is no comparison to a planned movement guided by IEREF. The afferent proprioceptive signal is unlikely to change so much (since the kinematics of arm target pointing before and after adaptation should be the same), but the calibration of perceived proprioception in CNS has been adjusted by prism adaptation. Thus the same afferent signal is read differently due to an adjusted calibration after adaptation. The adjustment in the calibrator for Pl (CPl) could occur by interaction with other complex spatial codings during active prism adaptation. Therefore subjective sagittal straight ahead pointing at a comfortable speed will reflect a combination of shifts in the internal egocentric reference frame (IEREF), determining where the subject believes ‘straight ahead’ is, and shifts in the proprioceptive sense, determining where the subject feels his hand is pointing.
The relationship between IEREF and the calibrator of proprioception could be additive or independent. For example, Riley and Turvey (2001) studied the combined effect of 20 diopter prism adaptation in different directions, followed by distortion of the arm’s mass distribution for right arm pointing. The two kinds of modification showed independent and additive effects.
Possible neurobiological mechanisms of the coding shift
Immediate biased directional shift
We have argued above prism adaptation and its aftereffects are not caused by the changes in peripheral sensory and motor systems, so we next explore the possibility that the origin of the changes is in the central nervous system which codes the shifted relation between visual perception and proprioceptive perception during prism adaptation. There are reviews describing possible anatomical areas in the CNS (Redding et al. 2005; Redding and Wallace 2006). First, we discuss the directionally specific proprioceptive aftereffect, observed immediately after adaptation. Girardi et al. (2004) also reported a similar directionally biased rightward shift after 15° leftward prism adaptation in healthy subjects, using haptic centering task with 30 cm disk. Although their measuring methods differ from ours, the directional bias may be explained by the same passive mechanism since both used left-shifting prism adaptation with finger pointing at targets on a table.
A possible cause for this spatial bias in the shift could be asymmetry in the adaptation effect on the right and left cerebral cortical hemispheres. In a recent study, Butler et al. (2004) reported that reaching to a remembered position in the left hemispace without vision activated only the right hemisphere of the cerebral cortex, whereas the same task performed in the right hemispace activated both hemispheres. If we similarly assume that the proprioceptive maps for egocentric peri-personal left/right hemispace are separated into areas on the right/both cerebral cortical hemispheres respectively, then the leftward visual displacement produced by the prisms, which produces arm proprioceptive map activity largely in the left hemispace, would predominantly trigger adaptive realignment of eye-hand coordination in the right hemisphere. The proprioceptive map in the left hemisphere would then remain mostly unchanged since there were relatively few adaptation cues (i.e. pointing to targets) in the right visual hemispace. Since the Pl measurements used passive arm movements that start from the left hemispace, the proprioceptive judgment of the midsagittal plane in this case is based on the proprioceptive map in the right hemisphere. Since the map in the right hemisphere was strongly adapted during the prism adaptation, Pl measurements show shift of the proprioceptive midsagittal judgment. By the same reasoning, since the map in the left hemisphere was only weakly adapted during the prism adaptation, Pr measurements show no significant shift in the proprioceptive midsagittal judgment.
Late onset of IEREF aftereffect in the second wave
A possible mechanism for the late onset of a shift in IEREF after several days, causing the second wave of the aftereffect in S, could be seen in the following examples. Transfer between different CNS regions (e.g. cerebellum, hippocampus and prefrontal cortex) through dynamic interaction has been reported in the time range of late–long term plasticity (l-LTP), using spaced eye blink conditioning in rat (500 ms between conditional stimuli and unconditional stimuli; Takehara et al. 2003). Their results showed that memory consolidation required cerebellum throughout 4 weeks of study, but depended on medial prefrontal cortex more during the later period and hippocampus more during the earlier period. In mice, spatial memory depends crucially on hippocampus at day 1 but on parietal cortex, among others, at day 30 (Maviel et al. 2004). Maviel et al. suggested that parietal expression may develop for memory storage through cortico-cortical connections which require a long time to establish themselves. With this transfer process after “uploading” the information from one area to another, the information of the original area may no longer be required.
Hence, if the shifted spatial code is encoded in some areas of CNS, the shifted code could be transferred with some time delay, as reported in the examples above. A possible pathway could be from the cerebellum (Weiner et al. 1983; Martin et al. 1996; Baizer et al. 1999; Pisella et al. 2005), then through thalamus, directly to somatosensory cortex (Prud’Homme and Kalaska 1994; Naito et al. 2005) or indirectly via primary motor, pre-motor, SMA, parietal (Clower et al. 2001, 2005) and other network systems (Previc 1998).
Even after the decay of original direct coding of biased proprioception, the shift in transferred areas that receive inputs from more sensory pathways in the CNS (i.e. associative cortex regions) may have delayed decay due to the involvement of a greater number of network systems and so more complex integration. Therefore, decay of the aftereffect shift in different coding areas could be delayed for S (with its larger number of components and their interactions) with respect to Pl and Pr (with less number of components). These characteristics are described in the form of a summarized model (see details in Fig. A, Hatada et al. 2006).
Finally, the directional bias seen here might be specific to the direction (leftward visual shift) of the prisms used during adaptation, or it could be an asymmetrical bias that would be found using both directions of prismatic shift. Further work is needed to separate these possibilities.
Conclusions
Our prism adaptation procedure generated a two-wave pattern of decay followed by a long delayed development of aftereffect, evident in two measures of the midsagittal position by active pointing (S) and by judgment using passive arm movements (Pr and Pl). Interestingly, we found that the aftereffects of proprioceptive shift depended on the direction of arm movement (Pr and Pl). The shift of the midsagittal position appeared only in one of the two proprioceptive passive measures (Pl) indicating that proprioception is affected in a directionally biased way and not a spatially ubiquitous way. We also found different decay times in the aftereffect between active and passive proprioceptive measurements, suggesting that the shift of an internal egocentric reference frame is separated from the passively measurable proprioceptive shift and develops with a time delay of several days. Our results suggest that proprioceptive aftereffects can be measured better by passive arm movements, with two opposite lateral directionalities, than by active arm sagittal straight ahead pointing.
References
Al-Falahe NA, Nagaoka M, Vallbo AB (1990) Response profiles of human muscle afferents during active finger movements. Brain 113:325–346
Bailey C, Kandel ER (1993) Structural changes accompanying memory storage. Annu Rev Physiol 55:397–426
Baily JS (1972) Arm-body adaptation with passive arm movements. Percept Psychophys 12:39–44
Baizer JS, Kralj-Hans I, Glickstein M (1999) Cerebellar lesions and prism adaptation in macaque monkeys. J Neurophysiol 81:1960–1965
Beckett PA (1980) Development of the third component in prism adaptation: effects of active and passive movement. J Exp Psychol Hum Percept Perform 6:433–444
Bottini G, Karnath HO, Vallar G, Sterzi R, Frith CD, Frackowiak RS, Paulesu E (2001) Cerebral representations for egocentric space: Functional-anatomical evidence from caloric vestibular stimulation and neck vibration. Brain 124:1182–1196
Burke D, Hagbarth KE, Lofstedt L, Wallin BG (1976a) The responses of human muscle spindle endings to vibration of non-contracting muscles. J Physiol 261:673–693
Burke D, Hagbarth KE, Lofstedt L, Wallin BG (1976b) The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol 261:695–711
Butler AJ, Fink GR, Dohle C, Wunderlich G, Tellmann L, Seitz RJ, Zilles K, Freund HJ (2004) Neural mechanisms underlying reaching for remembered targets cued kinesthetically or visually in left or right hemispace. Hum Brain Mapp 21:165–177
Capaday C, Cooke JD (1981) The effects of muscle vibration on the attainment of intended final position during voluntary human arm movements. Exp Brain Res 42:228–230
Capaday C, Cooke JD (1983) Vibration-induced changes in movement-related EMG activity in humans. Exp Brain Res 52:139–146
Chokron S, Colliot P, Atzeni T, Bartolomeo P, Ohlmann T (2004) Active versus passive proprioceptive straight-ahead pointing in normal subjects. Brain Cogn 55:290–294
Clower DM, West RA, Lynch JC, Strick PL (2001) The inferior parietal lobule is the target of output from the superior colliculus, hippocampus, and cerebellum. J Neurosci 21:6283–6291
Clower DM, Dum RP, Strick PL (2005) Basal ganglia and cerebellar inputs to ‘AIP’. Cereb Cortex 15:913–920
Cordo P, Carlton L, Bevan L, Carlton M, Kerr GK (1994) Proprioceptive coordination of movement sequences: role of velocity and position information. J Neurophysiol 71:1848–1861
Crago PE, Houk JC, Rymer WZ (1982) Sampling of total muscle force by tendon organs. J Neurophysiol 47:1069–1083
Girardi M, McIntosh RD, Michel C, Vallar G, Rossetti Y (2004) Sensorimotor effects on central space representation: prism adaptation influences haptic and visual representations in normal subjects. Neuropsychologia 42:1477–1487
Graziano MS, Hu XT, Gross CG (1997) Coding the locations of objects in the dark. Science 277:239–241
Harris CS (1963) Adaptation to displaced vision: visual, motor, or proprioceptive change? Science 140:812–813
Hatada Y, Rossetti Y (2004a) Long-lasting prism-adaptation aftereffects: shift in open-loop midsagittal pointing involves more than just visual and proprioceptive components. Perception 33:Suppl:140
Hatada Y, Rossetti Y (2004b) Prism adaptation generates a very long lasting-directionally biased proprioceptive shift in healthy subjects. Soc neurosci Abstr 524:12
Hatada Y, Wu F, Sun ZY, Schacher S, Goldberg DJ (2000) Presynaptic morphological changes associated with long-term synaptic facilitation are triggered by actin polymerization at preexisting varicosities. J Neurosci 20:RC82
Hatada Y, Miall RC, Rossetti Y (2005) Two waves of a long-lasting after-effect of prism adaptation measured over 7 days. Exp Brain Res. E-pub on 18th Nov 2005
Hatada Y, Rossetti Y, Miall RC (2006) Long-lasting prism-adaptation after-effects reveal that shifts in vision and proprioception are independent. Exp Brain Res 1–14. DOI 10.1007/s00221-00609437-3
Houk J, Henneman E (1967) Responses of golgi tendon organs to active contractions of the soleus muscle of the cat. J Neurophysiol 30:466–481
Inglis JT, Frank JS (1990) The effect of agonist/antagonist muscle vibration on human position sense. Exp Brain Res 81:573–580
Kandel ER (2001) The molecular biology of memory storage: a dialogue between genes and synapses. Science 294:1030–1038 Review
Martin KC, Kosik KS (2002) Synaptic tagging – who's it? Nat Rev Neurosci 3:813–820
Martin TA, Keating JG, Goodkin HP, Bastian AJ, Thach WT (1996) Throwing while looking through prisms. II. Specificity and storage of multiple gaze-throw calibrations. Brain 119:1199–1211
Maviel T, Durkin TP, Menzaghi F, Bontempi B (2004) Sites of neocortical reorganization critical for remote spatial memory. Science 305:96–99
Naito E, Roland PE, Grefkes C, Choi HJ, Eickhoff S, Geyer S, Zilles K, Ehrsson HH (2005) Dominance of the right hemisphere and role of area 2 in human kinesthesia. J Neurophysiol 93:1020–1034
Pisella L, Rossetti Y, Michel C, Rode G, Boisson D, Pélisson D, Tilikete C (2005) Ipsidirectional impairment of prism adaptation after unilateral lesion of anterior cerebellum. Neurology 65:150–152
Previc FH (1998) The neuropsychology of 3-D space. Psychol Bull 124(2):123–164 Review
Prochazka A (1981) Muscle spindle function during normal movement. Int Rev Physiol 25:47–90
Prochazka A, Stephens JA, Wand P (1979) Muscle spindle discharge in normal and obstructed movements. J Physiol 287:57–66
Prud’Homme MJ, Kalaska JF (1994) Proprioceptive activity in primate primary somatosensory cortex during reaching movements. J Neurophysiol 72:2280–2301
Redding GM, Wallace B (1992) Effects of pointing rate and availability of visual feedback on visual and proprioceptive components of prism adaptation. J Mot Behav 24:226–237
Redding GM, Wallace B (1996) Adaptive spatial alignment and strategic perceptual-motor control. J Exp Psychol Hum Percept Perform 22:379–394
Redding GM, Wallace B (1997) Adaptive spatial alignment. Lawrence Erlbaum Associates, New Jersey
Redding GM, Wallace B (2006) Prism adaptation and unilateral neglect: review and analysis. Neuropsychologia 44:1–20
Redding GM, Rossetti Y, Wallace B (2005) Applications of prism adaptation: a tutorial in theory and method. Neurosci Biobehav Rev 29:431–444
Riley MA, Turvey MT (2001) Inertial constraints on limb proprioception are independent of visual calibration. J Exp Psychol Hum Percept Perform 27:438–455
Rizzolatti Fadiga L, Fogassi L, Gallese V (1997) The space around us. Science 277:190–191
Rossetti Y, Rode G, Pisella L, Farne A, Li L, Boisson D, Perenin MT (1998) Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature 395:166–169
Takehara K, Kawahara S, Kirino Y (2003) Time-dependent reorganization of the brain components underlying memory retention in trace eyeblink conditioning. J Neurosci 23:9897–9905
Wei JY, Simon J, Randic M, Burgess PR (1986) Joint angle signaling by muscle spindle receptors. Brain Res 370:108–118
Weiner MJ, Hallett M, Funkenstein HH. (1983) Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology 33:766–772
Welch RB (1978) Perceptual modification: adaptating to altered sensory environments. Academic press, New York
Welch RB (1986) Adaptation of space perception. In: Boff KR, Kaufman L, Thomas JR (eds) Handbook of perception and human performance, vol. 1: sensory processes and perception. Wiley, New York, pp 24.1–24.45
Wilkinson DA (1971) Visual-motor control loop: a linear system? J Exp Psychol 89:250–257
Acknowledgment
The authors would like to thank Gordon Redding, Kiyoshi Kurata and Ansgar Koene for their comments and suggestions. YH was partially supported by Gatsby charitable foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
R. Chris Miall and Yves Rossetti contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hatada, Y., Miall, R.C. & Rossetti, Y. Long lasting aftereffect of a single prism adaptation: directionally biased shift in proprioception and late onset shift of internal egocentric reference frame. Exp Brain Res 174, 189–198 (2006). https://doi.org/10.1007/s00221-006-0437-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-006-0437-3