Abstract
Several recent studies with transcranial magnetic stimulation (TMS) have demonstrated changes in motor evoked potentials (MEPs) in human limb muscles following modulation of sensory afferent inputs, but little is known about the regulation of the human tongue motor control. To test the effect of local anesthesia (LA) of the lingual nerve and topical application of capsaicin stimulation on tongue MEPs. Fourteen volunteers participated (21–30 years) in two randomized sessions; before, during a nerve block of the lingual nerve or topical capsaicin application (30 μl 5%) on the tongue, and after anesthesia or pain had subsided. EMG electrodes were placed on the tongue and the first dorsal interosseous (FDI) muscle (control). EMG signals were amplified, filtered (20 Hz–1 kHz), and sampled at 4 kHz (Nicolet, USA). TMS were delivered with a figure-of-eight coil (Magstim 200, UK). Scalp sites at which EMG responses were evoked in the relaxed tongue or FDI at the lowest stimulus strength were determined, i.e., motor threshold (T). MEPs were assessed using stimulus–response curves in steps of 10% T. Eight stimuli were presented at each stimulus level. The proximal hypoglossal nerve was activated by TMS delivered over the parieto-occipital skull distal to the right ear. Eight stimuli were delivered at 50% of maximum stimulator output. ANOVAs were used to analyze latency and peak-to-peak amplitudes. Capsaicin evoked mild pain (2.8±0.5), and a strong burning sensation (6.2±0.4) on 0–10 visual analogue scales. MEP amplitudes in tongue and FDI were not influenced by capsaicin (P>0.44) but by stimulus strength (P<0.001). MEP latencies in tongue (8.9±0.2 ms) and FDI (22.4±0.4 ms) were not affected by capsaicin (P>0.19). Hypoglossal nerve stimulation evoked a short-latency (3.6±0.9 ms) response (mean amplitude 65±9 μV); but was unaffected by capsaicin (P>0.54). LA did not have any effect on FDI MEPs but was associated with a significant facilitation of tongue MEPs at T+50% and T+60% about 50 min after the nerve block in the recovery phase. Also in this condition, the direct motor responses evoked by hypoglossal nerve stimulation remained constant. No direct effect of a strong burning sensation could be shown on peripheral or central corticomotor pathways to the relaxed tongue musculature, however, LA of the lingual nerve (cranial nerve V) seems able to induce a delayed change in corticomotor control of tongue musculature (cranial nerve XII) possibly related to unmasking effects at the cortical level but not completely excluding excitability changes at the brain stem level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transcranial magnetic stimulation (TMS) is a widely used, non-invasive and painless technique to study the corticomotor control and plasticity of the motor cortex (Abbruzzese and Trompetto 2002; Rothwell 1997). Relatively, few studies have used TMS to examine the control of the tongue musculature (Fadiga et al. 2002; Katayama et al. 2001; Meyer et al. 1997; Muellbacher et al. 1994, 1998, 2001) and virtually no studies have attempted to study the modulation of tongue motor evoked potentials (MEPs) (Svensson et al. 2003). A rapid and controlled coordination of sensory and motor function is essential for a number of complex orofacial behaviors involving the tongue musculature, such as swallowing, mastication, respiration and speech (Hiiemae and Palmer 2003; Sawczuk and Mosier 2001; Smith 1992) and it can be expected that the corticomotor pathways must adjust rapidly to changes in somatosensory inputs. However, the effect of, for example, sensory deprivation using local anesthetic nerve blocks or sensory perturbation with painful stimuli for the regulation of corticomotor pathways to the tongue musculature has not yet been described.
Transcranial magnetic stimulation studies on the corticospinal pathways have shown that ischemic nerve blocks generally are associated with immediate increases in MEP amplitudes (Brasil-Neto et al. 1992; Cohen et al. 1993; Ridding and Rothwell 1997; Werhahn et al. 2002; Ziemann et al. 1998a, b), although decreases in MEP amplitudes can be observed distal to the ischemia of the forearm or leg (Cohen et al. 1993). Recently, Duque et al. (2005) investigated the effect of digital anesthesia of the index finger and the thumb on the amplitude of MEPs in the first dorsal interosseous (FDI) and noted a dramatic decrease (29%) in maximal voluntary contraction possibly as a result of a lack of proper sensory feedback during the task. However, the amplitude of the FDI MEPs remained unchanged (Duque et al. 2005).
Transcranial magnetic stimulation studies on the effect of repetitive innocuous electrical stimulation of the peripheral nerves have consistently shown increases in MEP amplitudes in hand muscles (Miles 2005; Ridding et al. 2000) However, painful somatosensory stimulation with topical application of capsaicin on the skin significantly reduced the amplitude of the MEPs in the (FDI) muscle 20–30 min after the application of capsaicin and then progressively returned to baseline values (Farina et al. 2001). Another study on the effect of sensory perturbation using painful electrical stimulation of the arm showed an inhibition of the MEP amplitudes (Urban et al. 2004).
Few studies have addressed the issue of sensory deprivation or perturbation on MEPs in the orofacial region. Romaniello et al. (2000) investigated the effect of hypertonic saline-evoked muscle pain and capsaicin-evoked skin pain on the excitability of the trigeminal motor pathways using TMS (Romaniello et al. 2000). Muscle and skin pain did not induce significant effects on the amplitude or latency of the masseter MEPs, however, the masseter muscle requires a pre-contraction in order to obtain MEPs which may, in part, have masked an inhibitory effect. A recent TMS study showed that MEPs in the lower facial region were facilitated during anesthesia of the region whereas innocuous repetitive electrical stimulation was unable to modulate the MEP amplitudes (Yildiz et al. 2004). Nevertheless, the study by Yildiz et al. (2004) suggests the potential for a rapid modulation and plastic changes in the corticomotor control of orofacial muscles.
The aim of the present study was to test how sensory deprivation or perturbation of the sensory nerves of the tongue can affect the corticomotor control of tongue musculature using TMS and MEP recordings.
Materials and methods
Subjects
This study was carried out in 14 healthy volunteers (10 women and 4 men, right-handed) aged 20–31 years (mean age 23.6±3.0 years). The subjects reported no medical, physical or psychological problems. Informed consent was obtained in accordance with the guidelines of the Helsinki Declaration. The study was carried out in the Orofacial Pain Laboratory at the Department of Clinical Oral Physiology, University of Aarhus, Denmark with the approval of the Local Ethics Committee.
Recording of MEPs
The subjects sat upright and relaxed in a dental chair with the head supported by a headrest. EMG activity was recorded from the right side of the tongue and the right FDI muscle. Disposable self-adhesive silver chloride electrodes (Medtronic, USA, Type 9013S0211) were placed on the dorsal surface of the tongue (2–3 mm from midline, 10 mm from tongue tip) with an inter-electrode distance of 2 cm. Disposable surface electrodes (Medicotest, Denmark, Type 720-01 K) were placed over the FDI (muscle belly-caput metatarsale I). The EMG signals were amplified, filtered (20 Hz–1 kHz), sampled at 4 kHz (X, Nicolet Biomedical, Denmark) and stored on disk for off-line analysis. A total of 300-ms EMG activity was recorded with 100 ms pre-stimulus and 200 ms post-stimulus.
Transcranial magnetic stimuli (Magstim 200, The Magstim Co. Ltd., Whitland, UK, peak magnetic field=2 T) were delivered with a 5-cm diameter figure-of-eight coil to the left side of the scalp. The coil of the stimulator was oriented 45 obliquely to the sagittal midline so that the induced current flowed in a plane perpendicular to the estimated alignment of the central sulcus. Three markings on the coil helped to identify the position in relation to the scalp sites. The scalp sites at which EMG responses were evoked in the tongue or FDI muscles at the lowest stimulus strength were determined. The motor threshold was measured in the relaxed muscles with the use of a descending and ascending method of limits and was defined as the minimum stimulus intensity that produced five discrete MEPs clearly discernible on the monitor from background EMG activity (tongue MEP>5 μV and FDI MEP>50 μV).
The MEPs were assessed using stimulus–response curves (Devanne et al. 1997; Ridding and Rothwell 1997; Svensson et al. 2003). The curves were constructed in steps of 10% of motor threshold (T), from T−10% to T+60% or until a plateau in MEP amplitude was achieved. Eight stimuli were presented at each stimulus level with an inter-stimulus interval of about 15 s. Stimuli were delivered at sites over the scalp identified on a snugly fitting, flexible cap and related to the vertex (Cz) in accordance with the 10–20 electroencephalographic electrode placement system.
The proximal hypoglossal nerve was activated by transcranial magnetic stimuli delivered over the parieto-occipital skull distal to the right ear (Meyer et al. 1997). A series of eight stimuli were delivered at 50% of maximum stimulator output.
Experimental pain and anesthesia
Tonic pain was induced by capsaicin (30 μl 5%) applied topically in an adhesive bandage on the midline of the dorsum of the tongue, 15 mm from the tip. This procedure has previously been shown to cause a steady, burning type of pain (Baad-Hansen et al. 2003; Romaniello et al. 2000). Subjects were asked to rate the quality of the pain using a 10-cm visual analogue scale (VAS). TMS was undertaken when the subject felt a constant level of pain.
Anesthesia of the right side of the tongue was induced by local anesthetic block (Carbocaine, 0.5 ml, AstroZeneca) of the lingual nerve in the lingual mucosa 2 mm apical to the gingival margin in the right third mandibular molar region. Subjects were asked to rate pain in the tongue when pinched with tissue forceps on left and right sides 1 cm from the tip. TMS was performed when the subject felt no pain upon pinching on the right side of the tongue.
Protocol
The experiment was performed on two separate sessions. The order of experimental pain and anesthesia was randomized between sessions. The recording procedure was the same during both sessions. The tongue and FDI muscles were in resting condition throughout the procedures. MEPs were measured (1) immediately prior to experimental pain or anesthesia, (2) during constant pain or anesthesia and (3) after pain or anesthesia had subsided. The onset latency and peak-to-peak amplitude were measured on non-rectified, averaged MEPs.
Statistical analyses
The MEP onset latencies and amplitudes were compared using two-way ANOVAs with repeated factors: stimulus intensity and conditions (baseline, intervention, post-baseline). When appropriate, the ANOVAs were followed by post hoc Tukey tests to compensate for multiple comparisons. All data are presented as mean values and standard errors of mean. The level of significance was set at P<0.05.
Results
General characteristics
Transcranial magnetic stimulation applied to the scalp between 8–9 cm lateral and 2–3 cm anterior to the inter-auricular line produced clear MEP responses with latencies between 6 and 10 ms in the tongue musculature of all the subjects tested. The motor thresholds ranged between 31 and 56% output. The optimal scalp site for MEP responses with latencies between 18 and 26 ms in the FDI was 6–7 cm lateral and 1–2 cm anterior to the inter-auricular line. The motor thresholds for the FDI MEPs ranged between 30 and 70% output.
Transcranial magnetic stimulation at 50% output delivered over the parieto-occipital skull distal to the right ear in order to activate the proximal hypoglossal nerve produced short-latency responses in the tongue musculature with a latency in the range of 3.2–4.2 ms.
Effect of capsaicin
Topical application of capsaicin to the tongue-evoked mild pain (VAS=2.8±0.5), and a strong burning sensation (VAS=6.2±0.4). After recording of the MEPs and removal of the capsaicin plaster, it took 35±2 min (26–55 min) before the pain and burning had disappeared and the final series of MEP recordings could be done.
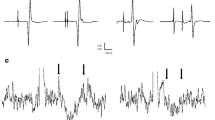
The motor threshold at baseline for tongue MEPs (43.9±2.7%) and FDI MEPs (39.9±2.2%) did not change significantly in response to the capsaicin application (ANOVA, P>0.204). The latency of the tongue (8.9±0.2 ms) and FDI MEPs (22.4±0.4 ms) was not influenced by condition or stimulus intensity (ANOVA, P>0.190). The amplitude of the tongue MEPs and FDI MEPs at baseline was unaffected by condition (ANOVA, P>0.436) but increased at higher stimulus intensities (ANOVA, P<0.001) (Fig. 1).
Amplitudes of MEPs in the tongue and FDI muscles (mean values, n=14) before, during and after application of capsaicin on the tongue (a, b) and local anesthesia (LA) (c, d). All stimulus–response curves show highly significant effects of stimulus intensity expressed in percentage of the motor threshold (T). *Indicate significantly larger MEPs after LA compared to before and during values (Tukey, P>0.05)
Transcranial magnetic stimulation applied to the hypoglossal nerve did not cause any significant changes in the baseline-to-peak amplitudes at baseline (65±9 μV) (ANOVA, P=0.650).
Effect of local anesthesia
Unilateral administration of local anesthesia (LA) on the right side was after 5–10 min associated with a significant reduction in the VAS scores to pinch-stimuli from 4.2±0.3 cm to 0.4±0.3 (ANOVA, P<0.001). The recovery of the pinch-evoked pain sensation (VAS=4.4±0.5) took 51±3 min (35–73 min).
In accordance with the capsaicin session, the motor thresholds at baseline for FDI MEPs (39.6±1.9%) did not change during the LA session (ANOVA, P>0.713), however, the motor thresholds for tongue MEPs during LA (44.9±1.8%) were slightly, but significantly higher than at baseline (43.7±1.9%) and at recovery (43.9±1.8%) (ANOVA, P=0.007).
The latency of the tongue MEPs in the LA session did not depend on condition or stimulus intensity (ANOVA, P>0.688). The latency of the FDI MEPs was unaffected by condition (ANOVA, P=0.702), but was 1–2 ms shorter at the highest stimulus intensities (ANOVA, P<0.001). The amplitudes of the FDI MEPs were significantly influenced by stimulus intensity (ANOVA, P<0.001) but not by condition (ANOVA, P=0.867). Also, the tongue MEPs were influenced by stimulus intensity (ANOVA, P<0.001) and with a significant interaction between stimulus intensity and condition (ANOVA, P=0.025). Thus, at T+50% and T+60% the tongue amplitudes at recovery were significantly higher than at baseline and during LA (Tukey, P<0.038) (Fig. 1).
Transcranial magnetic stimulation applied to the hypoglossal nerve in the LA session did not cause any significant changes in the baseline-to-peak amplitudes at baseline (59±7 μV) (ANOVA, P=0.834).
Discussion
Methodological considerations
Our MEP findings are consistent with recent data on the latency and stimulus–response functions of the MEPs elicited by TMS in the tongue musculature (Fadiga et al. 2002; Meyer et al. 1997; Muellbacher et al. 1994, 2001; Rodel et al. 2003; Svensson et al. 2003). For example, Muellbacher et al. (1994) studied the central and peripheral motor pathways to the lingual muscles using TMS. They found that TMS produced MEPs in the tongue musculature bilaterally with an average onset latency of 8.6–8.8 ms and with amplitudes in the range of 0.7–5.6 mV; the contralateral side having significantly larger amplitudes than the ipsilateral side. We chose only to record MEPs from the contralateral side and these amplitudes were smaller (Fig. 1). The difference in amplitude could be due a slight pre-contraction in the studies of Muellbacher and colleagues. In contradistinction, in the current study, subjects were asked to relax the tongue musculature as much as possible which, therefore, may have reduced the amplitudes of the MEPs.
We used stimulus–response curves as a measure of the cortical excitability of the tongue motor area. Other studies, including our own (Svensson et al. 2003), have also used cortical mappings where multiple sites on the scalp are systematically stimulated at constant intensity. However, the two techniques may be measuring very similar phenomena. The reason for this is that it is not possible to distinguish changes in the area of the motor output zone of constant excitability from changes in excitability of the zone of constant area (Ridding et al. 2000). Due to time restraints we therefore decided only to determine stimulus–response characteristics.
In the present study, MEPs in the FDI were used as a control and the findings that the amplitude of the MEPs increased and the latency decreased as the stimulus intensity was increased are in agreement with several other TMS studies in hand muscles (e.g., Burke et al. 1993; Kaneko et al. 1996). The latency gain at higher stimulus intensities may be due to the absence of D-waves at low stimulus intensities (see, e.g., Kaneko et al. 1996).
Effect of local anesthesia
The present finding that LA of the lingual nerve was associated with a significant facilitation of tongue MEPs is in general agreement with TMS studies on limb muscles that demonstrate increases in MEPs during ischemic blocks (Brasil-Neto et al. 1992; Cohen et al. 1993; Ridding and Rothwell 1997; Werhahn et al. 2002; Ziemann et al. 1998a, b). Furthermore, a recent study on MEPs in the lower facial muscles also showed a significant facilitation of MEPs during topical anesthesia of the region (Yildiz et al. 2004).
We noted a significant increase in motor threshold for tongue MEPs during the period with LA, however, this minor change (about 1% increase) was not associated with changes in the amplitudes of the tongue MEPs. The motor threshold for tongue MEPs did not differ between the baseline and the recovery phase of tongue anesthesia which may indicate that a general change in neuronal membrane excitability can be excluded as a major factor causing the observed increases in motor excitability (Ziemann et al. 1998a).
The additional finding that direct stimulation of the hypoglossal nerve was unaffected by the LA seems to support the notion that transient sensory deprivation may be associated with changes at the cortical level, and not at the spinal or brainstem level (Werhahn et al. 2002; Ziemann et al. 1998a, b). However, it should be noted that there is neuroanatomical evidence for direct connections between the trigeminal sensory nucleus and the ipsilateral hypoglossal nucleus, which potentially could influence the present findings (Zhang et al. 2001). Furthermore, a normal motor response to magnetic stimulation of the hypoglossal nerve may not entirely rule out excitability changes at the brain stem level due to the spread of the magnetic pulse and the relatively short distance between the cranial nerves and the motoneurons (Rösler et al. 1991). MEPs to electrical stimulation may therefore be required to exclude changes at the brain stem level (Di Lazzaro et al. 1998) and in fact, Werhahn et al. (2002) demonstrated that an ischemic nerve block caused marked excitability changes in hand muscles with TMS, but not with transmastoidal electrical stimulation indicating that the site of this effect is, indeed, cortical.
It has been suggested that when tonic afferent input is deprived, a number of neuroplastic changes can be triggered at the cortical level (Werhahn et al. 2002; Yildiz et al. 2004; Ziemann et al. 1998a, b). The explanation for such functional changes has been discussed extensively but is thought to involve an unmasking of previously silent neural connections (see, e.g., Weiss et al. 2004). The most likely mechanism for unmasking is disinhibition of the previously silent connections (Weiss et al. 2004). The timing of this potential cortical disinhibition and subsequent facilitation of MEPs seems to lag behind the onset of anesthesia because moderate levels of anesthesia can be detected after 15 min but this does not coincide with significant increases in MEPs in the lower facial muscles (Yildiz et al. 2004). Significant increases in MEPs can first be detected after 35 and 80 min after application of the topical anesthesia. Our findings also suggested that the increases in tongue MEPs occurred at a later stage following the local anesthetic block of the lingual nerve. It took between 35 and 73 min before the VAS scores of the pinch had returned to baseline values but we did not test other stimulus modalities and it may, therefore, be possible that some degree of LA was still present. Alternatively, a complete nerve block may have a different time course than topical anesthesia regarding the potential to trigger and maintain cortical disinhibition. Further studies will be required to describe the timing of the effects in more detail but such studies may only need to use high-intensity TMS stimuli because there appears to be no effect at lower intensities (Fig. 1). It could also be argued that a control injection would be required to test for natural variations in time but we argue that the FDI MEPs serve this function and remained remarkable stable over time. This is in accordance with our previous studies on facilitation of the tongue MEPs by a tongue-training task, but no changes in control FDI MEPs (Svensson et al. 2003).
Effect of capsaicin-evoked pain
The current study failed to demonstrate a significant effect of a robust burning sensation (VAS scores about 6), however, the actual painfulness of the capsaicin application was rated relatively low (VAS scores about 3). Thus, there is the possibility that stronger and more painful stimuli are required to effectively alter the corticomotor pathways. In the study of Farina et al. (2001), the topical application of capsaicin on the hand-evoked pain scores that never exceeded 3 on the VAS, which therefore suggests that the lack of changes in tongue MEPs in this study cannot be explained by stimulus intensity. Rather, these findings indicate that there are differences in the corticomotor integration of trigeminal and spinal sensory afferent inputs. Further support to this notion is derived from the TMS study on lower facial muscles and repetitive electrical stimulation for 10 or 30 min because this distinct transcutaneous stimulus evoked twitching of the lower facial muscles and a clear perception but no changes in MEPs when assessed up to 30 min after the stimulation (Yildiz et al. 2004). Finally, the lack of changes in tongue MEPs could be due to the variability of the TMS technique and low power. However, our set-up was capable of demonstrating an effect of LA in the same group of subjects and we have previously shown that 7 days with a 1-h tongue protrusion training task causes a significant facilitation of tongue MEPs in a group of 11 subjects (Svensson et al. 2003) and more recently, that a single day with a 1-h tongue protrusion task is sufficient to produce a significant facilitation of tongue MEPs (P. Svensson et al., submitted). The role of sensory inputs in the acquisition of orofacial motor skills and associated cortical plasticity also needs investigation since many face MI neurons receive orofacial sensory inputs (Martin et al. 1997; Murray and Sessle 1992; Yao et al. 2002a, b) and these inputs may be important, indeed perhaps crucial, during motor learning. Further studies are warranted to examine the potential influence of, for example, simultaneous painful input on the tongue training-evoked cortical plasticity of tongue MEPs.
In conclusion, no direct effect of a strong burning sensation could be shown on peripheral or central corticomotor pathways to the relaxed tongue musculature, however, LA of the lingual nerve (cranial nerve V) seems able to induce a delayed change in corticomotor control of tongue musculature (cranial nerve XII) possibly related to unmasking effects at the cortical level but not completely excluding excitability changes at the brain stem level.
References
Abbruzzese G, Trompetto C (2002) Clinical and research methods for evaluating cortical excitability. J Clin Neurophysiol 19:307–321
Baad-Hansen L, Jensen TS, Svensson P (2003) A human model of intraoral pain and heat hyperalgesia. J Orofac Pain 17:333–340
Brasil-Neto JP, Cohen LG, Pascual-Leone A, Jabir FK, Wall RT, Hallett M (1992) Rapid reversible modulation of human motor outputs after transient deafferentation of the forearm: a study with transcranial magnetic stimulation. Neurology 42:1302–1306
Burke D, Hicks R, Gandevia SC, Stephen J, Woodforth I, Crawford M (1993) Direct comparison of corticospinal volleys in human subjects to transcranial magnetic and electrical stimulation. J Physiol 470:383–393
Cohen LG, Brasil-Neto JP, Pascual-Leone A, Hallett M (1993) Plasticity of cortical motor output organization following deafferentation, cerebral lesions, and skill acquisition. Adv Neurol 63:187–200
Devanne H, Lavoie BA, Capaday C (1997) Input–output properties and gain changes in the human corticospinal pathway. Exp Brain Res 114:329–338
Di Lazzaro V, Oliviero A, Profice P, Saturno E, Pilato F, Insola A, Mazzone P, Tonali P, Rothwell JC (1998) Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr Clin Neurophysiol 109:397–401
Duque J, Vandermeeren Y, Lejeune TM, Thonnard JL, Smith AM, Olivier E (2005) Paradoxical effect of digital anaesthesia on force and corticospinal excitability. Neuroreport 16:259–262
Fadiga L, Craighero L, Buccino G, Rizzolatti G (2002) Speech listening specifically modulates the excitability of tongue muscles: a TMS study. Eur J Neurosci 15:399–402
Farina S, Valeriani M, Rosso T, Aglioti S, Tamburin S, Fiaschi A, Tinazzi M (2001) Transient inhibition of the human motor cortex by capsaicin-induced pain. A study with transcranial magnetic stimulation. Neurosci Lett 314:97–101
Hiiemae KM, Palmer JB (2003) Tongue movements in feeding and speech. Crit Rev Oral Biol Med 14:413–429
Kaneko K, Kawai S, Fuchigami Y, Shiraishi G, Ito T (1996) Effect of stimulus intensity and voluntary contraction on corticospinal potentials following transcranial magnetic stimulation. J Neurol Sci 139:131–136
Katayama T, Aizawa H, Kuroda K, Suzuki Y, Kikuchi K, Kimura T, Hashimoto K, Yahara O (2001) Cortical silent period in the tongue induced by transcranial magnetic stimulation. J Neurol Sci 193:37–41
Martin RE, Murray GM, Kemppainen P, Masuda Y, Sessle BJ (1997) Functional properties of neurons in the primate tongue primary motor cortex during swallowing. J Neurophysiol 78:1516–1530
Meyer BU, Liebsch R, Roricht S (1997) Tongue motor responses following transcranial magnetic stimulation of the motor cortex and proximal hypoglossal nerve in man. Electroencephalogr Clin Neurophysiol 105:15–23
Miles TS (2005) Reorganization of the human motor cortex by sensory signals: a selective review. Clin Exp Pharmacol Physiol 32:128–131
Muellbacher W, Mathis J, Hess CW (1994) Electrophysiological assessment of central and peripheral motor routes to the lingual muscles. J Neurol Neurosurg Psychiatry 57:309–315
Muellbacher W, Artner C, Mamoli B (1998) Motor evoked potentials in unilateral lingual paralysis after monohemispheric ischaemia. J Neurol Neurosurg Psychiatry 65:755–761
Muellbacher W, Boroojerdi B, Ziemann U, Hallett M (2001) Analogous corticocortical inhibition and facilitation in ipsilateral and contralateral human motor cortex representations of the tongue. J Clin Neurophysiol 18:550–558
Murray GM, Sessle BJ (1992) Functional properties of single neurons in the face primary motor cortex of the primate. II. Relations with trained orofacial motor behavior. J Neurophysiol 67:759–774
Ridding MC, Rothwell JC (1997) Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalogr Clin Neurophysiol 105:340–344
Ridding MC, Brouwer B, Miles TS, Pitcher JB, Thompson PD (2000) Changes in muscle responses to stimulation of the motor cortex induced by peripheral nerve stimulation in human subjects. Exp Brain Res 131:135–143
Rodel RMW, Laskawi R, Markus H (2003) Tongue representation in the lateral cortical motor region of the human brain as assessed by transcranial magnetic stimulation. Ann Otol Rhinol Laryngol 112:71–76
Romaniello A, Cruccu G, McMillan AS, Arendt-Nielsen L, Svensson P (2000) Effect of experimental pain from trigeminal muscle and skin on motor cortex excitability in humans. Brain Res 882:120–127
Rösler KM, Schmid UD, Hess CW (1991) Transcranial magnetic stimulation of the facial nerve: where is the actual excitation site? Electroencephalogr Clin Neurophysiol 43:362–368
Rothwell JC (1997) Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J Neurosci Methods 74:113–122
Sawczuk A, Mosier KM (2001) Neural control of tongue movement with respect to respiration and swallowing. Crit Rev Oral Biol Med 12:18–37
Smith A (1992) The control of orofacial movements in speech. Crit Rev Oral Biol Med 3:233–267
Svensson P, Romaniello A, Arendt-Nielsen L, Sessle BJ (2003) Plasticity in corticomotor control of the human tongue musculature induced by tongue-task training. Exp Brain Res 152:42–51
Urban PP, Solinski M, Best C, Rolke R, Hopf HC, Dieterich M (2004) Different short-term modulation of cortical motor output to distal and proximal upper-limb muscles during painful sensory nerve stimulation. Muscle Nerve 29:663–669
Weiss T, Miltner WH, Liepert J, Meissner W, Taub E (2004) Rapid functional plasticity in the primary somatomotor cortex and perceptual changes after nerve block. Eur J Neurosci 20:3413–3423
Werhahn KJ, Mortensen J, Kaelin-Lang A, Boroojerdi B, Cohen LG (2002) Cortical excitability changes induced by deafferentation of the contralateral hemisphere. Brain 125:1402–1413
Yao D, Yamamura K, Narita N, Martin RE, Murray GM, Sessle BJ (2002a) Neuronal activity patterns in primate primary motor cortex related to trained or semiautomatic jaw and tongue movements. J Neurophysiol 87:2531–2541
Yao D, Yamamura K, Narita N, Murray GM, Sessle BJ (2002b) Effects of reversible cold block of face primary somatosensory cortex on orofacial movements and related face primary motor cortex neuronal activity. Somatosens Mot Res 19:261–271
Yildiz N, Yildiz S, Ertekin C, Aydogdu I, Uludag B (2004) Changes in the perioral muscle responses to cortical TMS induced by decrease of sensory input and electrical stimulation to lower facial region. Clin Neurophysiol 115:2343–2349
Zhang J, Luo P, Pendlebury WW (2001) Light and electron microscopic observations of a direct projection from mesencephalic trigeminal nucleus neurons to hypoglossal motoneurons in the rat. Brain Res 917:67–80
Ziemann U, Corwell B, Cohen LG (1998a) Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci 18:1115–1123
Ziemann U, Hallett M, Cohen LG (1998b) Mechanisms of deafferentation-induced plasticity in human motor cortex. J Neurosci 18:7000–7007
Acknowledgments
The authors wish to thank Aarhus University Research Foundation and the Danish Medical Research Council for their support of the present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Halkjaer, L., Melsen, B., McMillan, A. et al. Influence of sensory deprivation and perturbation of trigeminal afferent fibers on corticomotor control of human tongue musculature. Exp Brain Res 170, 199–205 (2006). https://doi.org/10.1007/s00221-005-0199-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0199-3