Abstract
A preferred target for parkinsonian tremor alleviation is the ventrolateral (VL) thalamus. The goal of the present study is to determine how lesions involving the presumed cerebellar and pallidal recipient areas of the “motor” thalamus would alter the tremor and motor behavior of ten patients with Parkinson’s disease (PD). Tremor amplitude, power dispersion (a measure of sharpness of the power spectrum of tremor), and power distribution were quantified using a laser displacement sensor prior to, and a week after, VL thalamotomy. As well, the impact of surgery on tremor seen during movement was quantified in a manual-tracking (MT) task. Tremor-induced noise (a measure of the amount of tremor present during movement) and ERROR (difference between subject’s performance and target) were quantified. Finally, bradykinesia was assessed with a rapid alternating movement (RAM) task. Duration, range, and amplitude irregularity of wrist pronation–supination cycles were computed. Both motor tasks were quantified using a highly sensitive forearm rotational sensor. Healthy age-matched control subjects were also tested. Magnetic resonance images with an integrated atlas of thalamic nuclei were used to confirm lesion location. Results show that the lesions were centered upon the posterior portion of the ventral lateral (VLp) nucleus of the thalamus, included the posterior part of the ventral lateral anterior nucleus (VLa), and extended posteriorly to encroach upon the most rostral sector of the sensory ventral posterior nucleus (VPLa). VL thalamotomy significantly decreased tremor amplitude in all cases. Power dispersion was increased significantly so that it became similar to that of control subjects. Changes in power distribution indicate that thalamotomy selectively targeted PD tremor oscillations. Tremor detected during the MT task was also markedly decreased, becoming similar to that of controls. Patients also showed significant decrease in ERROR during MT. RAM duration and range were not significantly modified by the surgery, and patients’ performance remained impaired compared to healthy control subjects. Collectively, these results suggest that lesions involving the presumed “cerebellar” and “pallidal” recipient sectors of the motor thalamus do not worsen bradykinesia, suggesting that neural circuits other than the pallido-thalamo-cortical loop may be involved in slowness of movement in PD. A review of alternate pathways is presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Voluntary motor behavior results from activity in the motor cortices, with output to the brainstem and spinal cord, and to subcortical targets that include “motor sectors” of the basal ganglia and thalamus (Wichmann and DeLong 2003 for review). Dopamine probably plays an important role in allowing the basal ganglia to selectively focus motor programs, inhibiting unwanted movements, and allowing expression of desired movements implemented by cortical and cerebellar networks (cf. Mink 1996; Wichman and Delong 2003). In Parkinson’s disease (PD), progressive cell loss in dopaminergic and non-dopaminergic systems results in a spectrum of motor manifestations including bradykinesia, rigidity, postural imbalance, and tremor at rest. The current basal ganglia model suggests that bradykinesia, or impaired rapid voluntary movement, results from excessive tonic and abnormal phasic inhibitory activity of basal ganglia outputs to the thalamus and brainstem (Miller and DeLong 1988; DeLong 1990; Filion and Tremblay 1991). It is postulated that altered firing patterns within internal segment of the globus pallidus-thalamo-cortical neural network create “neural noise”, which is in turn transmitted by the thalamus to the motor cortices, resulting in “primary bradykinesia” (Berardelli et al. 2001).
Ventrolateral (VL) thalamotomy is an effective treatment for PD tremor (Ohye et al. 1982; Nagaseki et al. 1986; Ohye 2000). Early work suggested that lesions in the motor thalamus may improve accuracy of fine movement (Perret 1968; Perret et al. 1970), probably as a consequence of tremor relief. The impact of thalamotomy on fast repetitive movements is controversial; ranging from improvement, no effect, or even worsened performance (cf. Krayenbühl et al. 1963; Perret et al. 1970; Van Buren et al. 1973; Van Manen et al. 1984; Velasco et al. 1986; Walker 1982; van Someren et al. 1993; Okun and Vitek 2004). The current basal ganglia model suggests that thalamic lesions in PD patients may produce deleterious effects on rapid alternating movement (RAM) performance due to impaired thalamo-cortical excitatory drive, with reduced activation of motor cortices (Obrist et al. 1975; Boecker et al. 1997). On the other hand, thalamotomy in PD may improve motor performance by reducing cortical transmission of neural noise generated by disorganized pallido-thalamic activity that yokes voluntary behavior to abnormal tremor frequencies (Volkmann et al. 1996). The earlier work however is limited by lack of detail on lesion location using postoperative imaging, and the use of mainly qualitative rather than stringent quantitative measures of motor performance.
Here, we quantified the effects of VL lesions on voluntary motor behavior in PD. We determined the extent of lesions on MRI, and defined the “cytoarchitectonic extent” of lesions using a novel MRI-atlas integration technique (St-Jean et al. 1998; Atkinson et al. 2002; Finnis et al. 2003). In previous work, we presented details of lesion volume and lesion extent. We reported that lesions involved both the cerebellar and pallidal recipient sectors of the VL thalamus, and encroached upon the “proprioceptive shell” in the most rostral part of the ventral posterior nucleus (VP) (Atkinson et al. 2002). We now quantify the changes in tremor amplitude, power dispersion, and power distribution in the frequency spectrum. As well, tremor-induced noise and ERROR were assessed during a manual-tracking (MT) task. Finally, the duration, range, and amplitude irregularity of wrist pronation–supination cycles were determined during a RAM task.
Materials and methods
Subjects
Ten consecutive patients who underwent unilateral VL thalamotomy for tremor predominant PD were tested (age range 45–75, mean age 65.3±9.40 SD). Patients had mainly unilateral tremor that was medically intractable and disabling. Patients with advanced bilateral disease and/or with l-Dopa-related complications generally underwent subthalamic deep brain stimulation in our Center (Strafella et al. 2003). In addition, ten healthy control subjects matched for age (±5 years), gender, and handedness were asked to participate in the study (age range 50–78, mean age 66.5±9.74 SD). Patients underwent clinical evaluation using the United Parkinson’s Disease Rating Scale (UPDRS) 2 days prior to, and 7 days after, the surgical procedure while off medication for at least 12 h. Concurrently with clinical evaluation, quantitative measurements of tremor, manual tracking (MT), and RAM were performed on the contralateral side of the proposed lesion. As for healthy control subjects, quantification of tremor characteristics, RAM and MT were done with the same time frame between test–retest, and on the same side as their respective matched patient. The Montreal Neurological Institute and Hospital Institutional Ethics Review Board approved the study, and all procedures were performed with informed written consent obtained from the subjects.
Surgical procedure and assessment of lesion location
Terminology for thalamic nuclei of Hirai and Jones (1989) is used in the present paper. Homologies with Hassler’s (1982) terminology have been recently reviewed (Atkinson et al. 2002). The surgical procedure, target localization techniques and lesion mapping have been previously described in detail elsewhere (Bertrand 1966a, b; Bertrand et al. 1969; St-Jean et al. 1998; Atkinson et al. 2002). In brief, a stereotactic frame was attached to the patient’s head. The target was localized using stereotactic MR imaging, and stereotactic ventriculography was also used for intra-operative image guidance. A deformable volumetric atlas of the thalamus and basal ganglia was integrated with the patient’s stereotactic MR scan for intra-operative guidance (St-Jean et al. 1998). An automated non-linear warping algorithm was used to produce the transformation of a model atlas-integrated MR volume to each patient’s MR scan. Target localization within the VL thalamic nucleus was then further confirmed. In addition, the localization of the target was established in the patient who was awake using a curved retractable monopolar stimulation electrode to localize the neighboring sensory thalamus and the motor fibers within the internal capsule (Bertrand 1966a, b; Bertrand et al. 1969; St-Jean et al. 1998; Atkinson et al. 2002). Tailored lesions were performed in the VL thalamic nucleus using a loop-shaped leukotome manufactured to allow extrusion based on a millimeter scale (St-Jean et al. 1998). Prior to generating the lesion, the position of the adjacent somatosensory thalamus, motor fibers of the internal capsule, and a 3-D model of the proposed lesion were all visualized on a computerized platform using the MR-integrated stereotactic atlas (St-Jean et al. 1998).
Lesion volume and location were confirmed postoperatively using MR images obtained within 24 h of surgery in the ten patients. First, individual lesions were manually segmented on a pixel-by-pixel basis, using the native T1-weighted MR volume of individual patients, and hence producing individual lesion volumes. Average lesion location was then determined by taking each patient’s MR images and warping it into a common MR image reference space which also contained an integrated computerized version of the atlas of Schalterbrand and Wahren, as described in detail in our previous work (St-Jean et al. 1998; Atkinson et al. 2002). The transformation resulted in a probabilistic map that was used to assess the average lesion location.
Assessment of tremor
Tremor of the index finger was recorded using a laser displacement sensor (LDS 90/40, DynaVision, Dynamic Control System, Vancouver, Canada). The tremor recording method is described in detail elsewhere (Duval et al. 2000, 2001, 2004). In brief, the subject’s arm rested comfortably on a foam-padded support, with the elbow flexed at approximately 100°. A palm support was adjusted so as to allow isolation of the index finger. During recording, subjects were asked to raise their index finger to a horizontal position. A small piece of white cardboard was placed on the fingernail in order to provide an accentuated reflective surface for the laser beam. Three 60-s recordings were made with a sample rate of 2 kHz. Tremor data was subsequently reduced to 100 Hz using a moving average. The voltage signal from the laser sensor was subsequently transformed into millimeters. Each tremor recording of a particular subject was then separated into 5-s periods. Next, three tremor characteristics were computed on each 5-s period and subsequently averaged together: (a) tremor amplitude (root mean square), and (b) power dispersion, which represent the width of a frequency band containing 68% of the power centered at the median power frequency (Beuter et al. 1999). Also, the power distribution was assessed (Duval et al. 2000, 2001) by calculating the relative power percentage of four frequency bands: (1) 0–3.5 Hz, probably related to slow mechanical-reflex components; (2) 3.6–7.5 Hz, in which major PD pathological oscillations are usually found; (3) 7.6–12.5 Hz, in which the centrally generated component of physiological tremor is believed to be located; and (4) 12.6–16.5 where higher frequency components of physiological tremor may be found. Using the power spectrum of tremor velocity (Duval and Jones 2005) obtained from each 5-s period, the sum of power within each frequency band was divided by the total power, providing the power distribution in percentage (%). All 5-s periods of a particular trial were subsequently averaged together, yielding the averaged power distribution. This qualitative assessment method provides a means to determine where the power is located in the frequency spectrum.
Assessment of tremor during movement
Tremor during movement was assessed while subjects performed a MT task using an angular displacement sensor. Three trials lasting 1 min each, separated by a 1-min rest, were recorded. A 1-min practice session was allowed prior to testing. Subjects were seated comfortably in front of a computer monitor. They were asked to pronate and supinate their forearm while holding a ball. This ball controlled the length of a horizontal line displayed on the computer monitor. Patients were asked to match the length of that line with the length of a computer-generated target line, also displayed on the monitor. The target line changed length and velocity (frequency between 0.25 and 1 Hz, and amplitude ranging between 20° and 120°, respectively). Although the target movement remained similar between trials, it was practically impossible for the subjects to anticipate changes of either amplitude or frequency, due to the irregular nature of the target line displacement. The voltage signal from the angular displacement sensor was subsequently transformed into degrees. Two characteristics of MT performance were quantified. First, a tremor-induced noise index (TNI) was computed in order to detect the presence of tremor during the pronation–supination movements. A power spectrum was computed on the once differentiated MT signal from the subject (angular velocity). Then, the relative power percentage was calculated (sum of power divided by total power) within the 3.5–7 Hz band, which contains the power related to parkinsonian tremor. Since differentiation of angular displacement signals to angular velocity accentuates higher frequency components (i.e., PD tremor in the present case), the detectable tremor in the signal would increase the relative power percentage within the 3.5–7 Hz band. As tremor became relatively more predominant in the pronation–supination performance of patients, their TNI score increased accordingly. Second, an ERROR was computed in order to quantify the accuracy of subjects. The target line generated by the computer was subtracted from the patients’ performance. Then, the mean of the remaining signal (absolute value in degrees) was computed, obtaining an ERROR in degrees per second. A higher ERROR score indicates that patients were less accurate in performing the MT task.
Assessment of bradykinesia and hypokinesia
Performance of RAM was quantified using a pronation–supination task. RAM is often used as a direct measure of slowness of movement (Okada and Okada 1983; Beuter et al. 1999; Duval et al. 2001). The RAM recording method is described in detail elsewhere (Duval et al. 2001). In brief, subjects remained seated and their elbow rested on the foam-padded support. They were instructed to perform pronation–supination movements with the largest excursion possible, and as fast as possible, for 7 s while holding a small handball connected to the angular displacement sensor. Recording began immediately after subjects initiated movements so as to exclude movement initiation from analysis. Four trials were recorded and RAM data was sampled at 2 kHz. Voltage signal from the angular displacement sensor was subsequently transformed into degrees. The trials were separated by a 1-min rest period. Three RAM characteristics were computed: (a) mean duration of a full cycle of pronation–supination in seconds. A higher value indicates slower movement, or bradykinesia. (b) Mean angular displacement over a full cycle of pronation–supination in degrees. A lower value indicates low amplitude movements, or hypokinesia. Finally, (c) RAM cycle amplitude irregularity score. The latter was obtained by calculating the standard deviation (SD) of the linear envelope from the normalized pronation–supination trace (mean=0 and SD=1). A high value means more variability in RAM amplitude, and hence a more irregular performance.
Statistical analysis
An analysis of variance (ANOVA), more specifically a factorial design (Group × Test), with repeated measure on the last factor (Test) and a P-value threshold set at 0.05 was used to determine statistical differences. Newman–Keuls post hoc analysis was performed to determine which comparisons yield statistical significance between pre- and post-surgical values, as well as comparisons between post-surgical and pre- or post-test values from controls (Kirk 1968).
Results
Lesion location
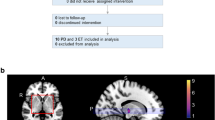
Table 1 includes the description of patients and clinical evaluation. Post-surgical MRI showed that the average lesion volume was 320.6 mm3 ± 72 SD. Figure 1 shows that lesions included the VLp, and also included a part of the VLa. Furthermore, all lesions encroached upon the most rostral portion of the ventral posterior lateral sensory thalamus (VPLa), at a border zone between VL and VP. Thus, the lesions included significant parts of the presumed cerebellar and pallidal territories, and encroached upon a “shell region” in the most rostral VP thought to contain proprioceptive inputs in primates (cf. Atkinson et al. 2002; Stepniewska et al. 2003). The lesion spared the intralaminar nuclei, including the adjacent centromedian, and also excluded the “associative” and “limbic-related” territories of the thalamus.
Digitized atlas of thalamic nuclei integrated with model MR image (in a) and probabilistic map of lesion position of the ten patients superimposed on atlas (horizontal view, in b). Also shown, a sagittal (in c) and coronal view (in d) of the probabilistic map. The red section of the averaged lesion indicates the location common to all lesions. Note that the lesion included the VLp, extended into the VLa, and encroached upon the most rostral part of the VP (“shell area”). The internal capsule is spared. PU putamen, CN caudate nucleus, GP globus pallidus, VLa ventral lateral anterior, VLp ventral lateral posterior, CM centromedian, IC internal capsule, VPL ventral posterior lateral, VPm ventral posterior medial
Tremor
Figure 2 shows the collective results for tremor amplitude, power dispersion, and power distribution (mean ± standard error). ANOVA indicates that there were Group (patients vs controls, F=32.62, P<0.05), Test (pre vs post, F=26.14, P<0.05), and Group × Test interactions (F=26.35, P<0.05) for tremor amplitude. More specifically, post hoc analyses show that tremor amplitude of patients decreased significantly after the surgical procedure (P<0.05, top-left), but remained statistically larger than control subjects’ tremor amplitude (P<0.05). Group (F=60.95, P<0.05), Test (F=8.69, P<0.05), and Group × Test interactions (F=8.27, P<0.05) were also found for power dispersion. Post hoc analyses show that the power dispersion was much wider after the surgical procedure (P<0.05, top-right), regaining values statistically similar to that seen in the control group (P>0.05). The power distribution of post-surgical tremor was not the same as controls (bottom): power percentage within the 7.6–8.5 Hz band did not return to values seen in the control group. Qualitative assessment of the power distribution shows that the power percentage within the 3.6–7.5 Hz band decreased in favor of the 0–3.5 Hz band postoperatively.
Changes in tremor amplitude (top-left), power dispersion (top-right), and power distribution (bottom). Note that the power within the 3.6–7.5 Hz band decreased post-surgery, in favor of the 0–3.5 Hz band. The power did not increase much within the 7.6–12.5 Hz band. A star (*) indicates that post hoc analyses revealed a significant difference between pre- and post-surgical scores, ** would indicate significant differences between post-surgical and pre- or post-test scores from controls. Error bars are the standard error
Tremor during movement
Figure 3 shows the individual and collective TNI (top) and ERROR (bottom) scores for MT. ANOVA indicates that there were Group (F=25.55, P<0.05), Test (F=51.37, P<0.05), and Group × Test interaction (F=45.15, P<0.05) for TNI. Post hoc analyses show that there was a dramatic decrease of the amount of tremor detected during MT (P<0.05). In fact, post-test TNI scores became statistically similar to those of control subjects’ TNI scores (P>0.05). As for ERROR, Group (F=22.66, P<0.05), Test (F=9.31, P<0.05), and Group × Test interaction was also found (F=6.90, P<0.05). Post hoc analyses show that despite a significant decrease in post-surgical ERROR (P<0.05), values from patients remained statistically higher than controls (P<0.05). Figure 4 shows an example of tremor during movement, and the impact of the surgical procedure on that tremor.
Individual and collective results for manual-tracking TNI (top) and ERROR scores (bottom). There was a significant reduction of TNI post-surgery. In fact, post hoc revealed that post-surgical scores were statistically similar to that of controls (P>0.05). A significant reduction was also noted for ERROR, but post-surgical values were significantly different from controls (P<0.05). A star (*) indicates that post hoc analyses showed a significant difference between pre- and post-surgical scores, ** indicates differences between post-surgical and pre- or post-test scores from controls
This figure shows the pre- (top) and post-surgery (middle) MT from patient no. 5, as well as MT from her matched control subject (bottom). Tremor is noticeably present during MT pre-surgery. As a result, her accuracy seemed to be affected. In this particular case, the TNI score would be high because of the presence of tremor during movement. In the post-surgery condition, tremor was no longer present and her accuracy increased accordingly. However, the patient’s movement sometimes lagged behind the target, contrary to her matched control. TNI and ERROR scores for each sample trace are indicated in the upper right of each plot. TNI tremor noise index, ER ERROR
Bradykinesia and hypokinesia
Figure 5 shows the individual and collective results for RAM duration, range, and amplitude irregularity. ANOVA shows that there was a Group effect for RAM duration (F=105.8, P<0.05), but not for Test (F=4.24, P>0.05) or Group × Test interaction (F=2.14, P>0.05). Despite a slight reduction (near 7% improvement) in movement time for patients, post hoc analyses show that no significant change occurred between pre- and post-surgery performance (P>0.05), and that the performance of patients remained well below that of controls (P<0.05). Control subjects also showed a 4% improvement post-test. These results suggest a negligible practice effect for both groups. Group effect was found for RAM range (F=50.72, P<0.05), but there was no Test (F=1.73, P>0.05) or Group × Test interaction (F=1.25, P>0.05). Post hoc analyses show that no significant change occurred between pre- and post-surgery performance (P>0.05). No effect was found for RAM irregularity for either Group (F=2.29, P>0.05) or Group × Test interaction (F=2.27, P>0.05). However a Test effect was found (F=6.93, P>0.05). Post hoc analysis showed that RAM irregularity score was decreased significantly (P<0.05), regaining statistically similar values to controls (P>0.05).
Individual and collective results for RAM duration (top), range (middle), and irregularity scores (bottom). RAM duration and range were not modified by the surgical procedure. However, there was a decrease in RAM irregularity due to a reduction observed in two patients (indicated by arrows). This reduction was not however statistically significant upon post hoc analysis (P>0.05). A star (*) indicates that post hoc analyses show a significant difference between pre- and post-surgical scores, ** would indicate differences between post-surgical and pre- or post-test scores from controls
Discussion
Collectively, the results indicate that VL thalamotomy markedly reduced tremor amplitude in PD, confirming that the thalamus is an essential component of PD tremor generation and/or propagation. Tremor during movement was also reduced, and movement accuracy improved accordingly. RAM performance did not worsen after thalamotomy, suggesting that the motor thalamus may not be directly implicated in slowness of repetitive movements seen in PD. We discuss these findings in the context of the traditional basal ganglia model and current controversies regarding the function of the basal ganglia.
Lesion location
We previously analyzed our thalamotomy lesions from the postoperative MR images of multiple subjects in a single MRI space including an integrated “cytoarchitectonic” atlas of thalamic nuclei (St-Jean et al. 1998; Atkinson et al. 2002). In the present paper, we used the Hirai and Jones (1989) terminology to describe thalamic nuclei. In the current discussion, we indicate the homologous terminology of Hassler (1982) in brackets where it may be useful to the reader. The previous analysis, as well as the current work, revealed that our leukotome generated lesions are relatively large, and include the posterior part of the VL nucleus (VLp: may include Hassler’s Voi, Vim, Zim, Dim), the posterior portion of the VLa (may include Hassler’s Voa and Vop), and an interface zone between the VLp and ventral posterior lateral nucleus (VPL), rostral to the cutaneous sensory thalamus, which comprises a thin shell containing cells with kinesthetic response properties (VPLa: may include Hassler’s Vcae and Zc) (Albe-Fessard et al. 1967; Bertrand 1972; Hardy et al. 1979; Jones and Friedman 1982; Lenz et al. 1990; Atkinson et al. 2002; Stepniewska et al. 2003). The posterior limits of the lesion was defined physiologically by evoking sensory responses in the VP by macrostimulation using a curved retractable monopolar electrode, defining the anterior border of “core area” containing the sensory cutaneous thalamus. The lateral limit was defined using a similar technique, mapping motor responses in the internal capsule (Duerden et al. 2003) so as to include the lateral part of the VL, but avoiding encroachment in the internal capsule. Volumetric analysis (Atkinson et al. 2002) indicated that our lesions are generally large compared to other MRI-based lesions reported in the literature, using the more commonly used radiofrequency technique (Tomlinson et al. 1991). The area of functional inactivation following radiofrequency generated coagulation may however extend beyond that seen on MRI, and is dependent on the time period after imaging (Tomlinson et al. 1991). The use of a tailored physical lesion generated using a loop-shaped leukotome, and creation of a 3-D “virtual lesion” modeled into the patient’s stereotaxic space with an MRI-atlas integrated computerized platform (St-Jean et al. 1998), also allowed for generation of a well-defined thalamic lesion rostral to the sensory thalamus and medial to and mainly bordering the internal capsule.
It is useful to subdivide the motor thalamus into cerebellar, thalamic, and even nigral territories, which are largely separate (Macchi and Jones 1997; Krack 2002; Percheron 2004). Of necessity, these territories are based mainly on information available in non-human primate, and therefore are inferential when homologized on the basis of cytoarchitecture to human. The thalamotomy lesions presented in the present study, analyzed in more detail in our previous work (Atkinson et al. 2002), included significant portions of the presumed “cerebellar” (VLp) and “pallidal” (VLa) recipient areas of the motor thalamus, and encroached upon the somatosensory thalamic “shell,” an area that likely contains proprioception-related thalamic units (Poggio and Mountcastle 1963; Albe-Fessard et al. 1967; Maendly et al. 1981; Jones et al. 1982; Kaas et al. 1984; Lenz et al. 1990; Atkinson et al. 2002; Stepniewska et al. 2003). The goal of the lesions was indeed to include more posterior territories, anterior to the sensory cutaneous thalamus, in order to improve tremor, and with deliberate extension into the pallidal territory, in order to achieve a salutary effect on rigidity (Cooper et al. 1963; Smith 1967; Ohye 1986; van Someren 1993; Atkinson et al. 2002). With respect to other thalamic recipients of basal ganglia territories relevant to motor function, the lesions mainly excluded the centromedian nucleus, and more medial thalamic territories that receive “nigral” inputs (Illinsky et al. 1985; Sadikot et al. 1992; Sidibé et al. 1997; Percheron 2004).
It is important to note that the territorial organization of the human thalamus has not been fully defined, and work on territorial organization with respect to afferents relies largely on cytoarchitectonic homology using data from monkeys and chemical anatomy. Indeed the organization in monkey also remains somewhat controversial (Atkinson et al. 2002; Krack et al. 2002; Percheron 2004 for reviews). For example the extent of the “pallidal territory,” although mainly rostral and lateral to the “cerebellar” territories, varies in different tracing studies (Kuo and Carpenter 1973; DeVito and Anderson 1982; Ilinsky and Kultas-Ilinsky 1987; Sidibé et al. 1997; Percheron 2004), and partially interdigitates with the “cerebellar” territory (Mehler 1971; Percheron 1977; Asanuma et al. 1983; Orioli and Strick 1989). Using current techniques, given interdigitation and passing fibers, it is therefore virtually impossible to lesion exclusively “pallidal,” “cerebellar”, or “nigral” parts of the VL or VA nuclei of the human thalamus.
Based on intra-operative physiological localization (anterior to the cutaneous sensory thalamus; medial to the motor fibers of the internal capsule), MRI-atlas integrated lesion analysis presented here, and a more extensive probabilistic lesion analysis in multiple subjects published previously (Atkinson et al. 2002), our lesions can be considered to include parts of both the cerebellar territory and pallidal territories. Given the dorsal and rostral thalamic extent of these territories, it is unlikely that any one of these territories is lesioned completely. The lesions may also include a part of the nigral territory, but since they are placed laterally within the thalamus, we expect that they involve predominantly cerebellar and pallidal territories. Furthermore, pallidal and cerebellar afferents to the intralaminar nuclei including the CM remain intact (Sadikot et al. 1992, Atkinson et al. 2002). On the other hand, given that the lesions involve more ventral and lateral parts of the thalamus, the functional effect on different thalamic motor territories may be more extensive than the nuclear extent of lesions, since afferent systems are likely interrupted.
Effect of thalamotomy on tremor
The exact pathophysiological mechanism that underlies tremor in PD remains undetermined. The predominant theory suggests that degeneration of dopaminergic, or even non-dopaminergic systems, results in an oscillatory network that involves the motor circuit of the basal ganglia, the cerebellum, the thalamus, and the somatosensory and motor cortices (Filion 1979; Filion and Tremblay 1991; Paré et al. 1990). VL thalamotomy resulted in marked reduction of tremor amplitude. Power dispersion was much higher in the power spectrum of tremor after thalamotomy, becoming quite similar to that seen in the control group. This indicates that the surgical procedure eliminated PD-related oscillations that previously dominated the tremor power spectrum. In healthy elderly subjects, tremor oscillations may include mechanical limb perturbations such as ballistocardiac thrust and respiration (Van Buskirk et al. 1966; Yap and Boshes 1967; Marsden et al. 1969), resonance frequency of the limb (Stiles and Randall 1967), or occur as a result of local reflex mechanisms (Marsden 1984). In addition, the power spectrum of physiological tremor may include oscillations of supraspinal origin: the 8–12 Hz component (Elble 1995; Köster et al. 1998; Duval et al. 2000). An increase of power dispersion indicates that multiple oscillations were now present in the tremor power spectrum, as seen in healthy subjects. In the present study, the power percentage within the 3.6–7.5 Hz band decreased only in favor of the 0–3.5 Hz band after the surgical procedure. This result is in agreement with previous work showing that the 8–12 Hz component fails to emerge after VL thalamotomy (Duval et al. 2000). In a different group of patients, it was recently shown that VL thalamotomy may also impact upon the centrally driven components of physiological tremor located at higher frequencies (i.e., 16–30 Hz) (Duval et al. 2005). The present results emphasize the key role played by the VL thalamus in the propagation of neural oscillations that underlie tremor.
Effect of thalamotomy on tremor during movement
Results show a marked decrease of tremor-induced noise during MT. In fact, post-surgery TNI scores were statistically similar to TNI scores of control subjects. As for ERROR, a major improvement of accuracy was seen post-surgery, even though scores from patients remained statistically higher than control subjects. ERROR observed in patients may originate from two distinct sources; first, tremor itself is a deviation from target, hence increasing the ERROR score. Second, the involuntary muscle contraction associated with tremor could interfere with the voluntary muscle contractions needed to perform the task. In any case, elimination of tremor did improve accuracy in these patients. This particular result emphasizes the positive functional impact of thalamotomy on movement accuracy, and supports previous findings (cf. Perret 1968; Perret et al. 1970). The fact that bradykinesia and hypokinesia were not altered in patients (see below) may explain why patient’s accuracy remained lower than controls; patients may have had difficulty in adapting to changes in target velocity.
Impact of VL thalamotomy on bradykinesia
Ventrolateral thalamotomy did not alter the underlying bradykinesia present in patients. No significant change between pre- and post-surgery scores for RAM duration and range were found, and patients’ scores remained lower than control subjects’ scores. Accordingly, it is reasonable to assume that the interference created by tremor oscillations had little effect on motor output associated with fast repetitive movements. RAM irregularity was significantly decreased after thalamotomy to reach values similar to those of controls. Of note, two patients with high pre-surgery RAM amplitude irregularity scores contributed significantly to the higher group average. We hypothesized that high tremor amplitude may be a potential cause of RAM irregularity. Interestingly, however, both these patients showed pre-surgery tremor amplitudes similar to the median, and were not the worst cases of tremor. Consequently, reduction of tremor amplitude cannot be used to explain improvements in RAM amplitude irregularity scores. The source of the improvement remains to be determined. Of course, all the aforementioned observations are based on performance assessments made 7-days post-surgery. Although clinical observations suggest stable long-term effects of thalamotomy on tremor, long-term follow-up with detailed quantitative evaluation may provide more complete information on possible re-organization of motor behavior after thalamic surgery (Ohye et al. 1985).
The classical model of the basal ganglia predicts that pathological hyperactivity in the subthalamic nucleus occurs as a consequence of degeneration of the nigrostriatal pathway, resulting in inhibition of the “motor thalamus” due to excessive and disordered activity in the inhibitory pallido-thalamic pathway (Miller and DeLong 1988; DeLong 1990; Filion and Tremblay 1991). It is proposed that the resulting inhibition of thalamo-cortical drive in the sensorimotor cortex results in bradykinesia (DeLong 1990; Wichmann and DeLong 2003). In keeping with this prediction, therapeutic interventions aimed at reducing abnormal activity in the internal pallidal segment (Lozano et al. 1995) or in the subthalamic nucleus (Strafella et al. 2003; Benabid 2003); both significantly improve these symptoms.
Based on previous evidence (Obrist et al. 1975; Boecker et al. 1997), it may be hypothesized that a lesion in the VL thalamus would worsen RAM performance by further reducing thalamo-cortical drive, especially when pallido-thalamic circuits are implicated. Our results clearly show that RAM performance was not modified by the surgical procedure, even though lesions encroached upon the VLa, a presumed pallidal recipient area of the thalamus. Accordingly, the notion that a reduction of thalamo-cortical activity is solely responsible for slowness of movement in PD is likely to be incorrect. Other neural pathways must therefore be considered in order to define the anatomical substrate for bradykinesia. Fast repetitive voluntary movements may utilize parts of the brain external to the thalamic motor circuit. For example, the basal ganglia, especially when abnormal, may relegate some movement-related functions to the classical pyramidal system. Motor processing during voluntary movements in patients with PD could preferentially entrain cortico-cortical mechanisms involving motor and premotor areas for processing, with output via cortico-spinal and cortico-bulbar systems.
Alternatively, an extra-thalamic basal ganglia output system may be important in generation of bradykinesia in PD. The mesencephalic motor area, including the pedunculopontine nucleus (PPN), receives significant afferent projections from motor and non-motor components of the basal ganglia (Shink et al. 1997; Parent and Cossette 2001). This system would not be impaired following a lesion in the motor circuit of the thalamus, explaining a lack of effect of motor thalamotomy on bradykinesia. Indeed, severity of parkinsonian symptoms appears to correlate with neuronal loss in the PPN (Zweig et al. 1989). Furthermore, destruction of PPN neurons in primates leads to a bradykinetic parkinsonian state (Kojima et al. 1997; Aziz et al. 1998; Pahapill and Lozano 2000). The importance of an extra-thalamic motor circuit for bradykinesia is further emphasized by improvement of bradykinesia by pallidotomy or subthalamic stimulation where pallidal-brainstem pathways may also play a role (Aziz 1998). Other alternative pathways implicated in the pathophysiology of bradykinesia that would not necessarily require thalamic circuitry may include dopaminergic input to the supplementary motor areas of the frontal lobe, or non-dopaminergic pathways including efferent projections from brainstem noradrenergic, cholinergic, or serotonergic systems that also degenerate in PD (Hornykiewicz and Kish 1987; Zweig et al. 1989; Jellinger 2002; Braak et al. 2003).
Other sectors of the thalamus that were not included in thalamotomy for tremor may also be involved in bradykinesia. For example, work in MPTP lesioned primates suggests that the substantia nigra pars reticulata shows patterns of disordered and hyperactive neuronal discharge similar to those seen in the internal pallidum (Wichmann et al. 1999). In the present study, the nigral recipient component of the ventral thalamus (Ilinsky et al. 1985) is spared following VL thalamotomy. Furthermore, the lesions in VLp and VLa are partial. These spared components may therefore allow for persistent bradykinesia; however, a partial lesion explanation is less likely since there was virtually no change in bradykinesia following thalamotomy. Finally, non-motor components of the thalamus including areas that receive inputs from associative and limbic circuits of the basal ganglia (cf. Vogt et al. 1987; Sadikot et al. 1992) may be implicated in the production of bradykinesia, with ultimate involvement of associative and limbic components of the frontal lobe (Mogenson and Yang 1991; Brown and Pluck 2000; Paus 2001). Interestingly, bradykinesia is not a well-defined phenomenon after focal thalamic lesions such as stroke, providing further evidence for alternate extrathalamic substrates (Lee and Marsden 1994; Bhatia and Marsden 1994).
Conclusion
Results presented in the present paper demonstrate that lesions in the motor thalamus markedly reduced PD tremor, but had little effect on bradykinesia. These results suggest that lesioning the presumed cerebellar recipient and pallidal recipient areas of the thalamus does not worsen either bradykinesia or hypokinesia. The results suggest that reduction in thalamo-cortical drive alone is not sufficient to explain slowness of movement seen in patients with PD. Alternative neural circuits to the traditional pallido-thalamo-cortical loop may be implicated in slowness of movement in PD.
References
Atkinson JD, Collins L, Bertrand G, Peters TM, Pike BG, Sadikot AF (2002) Optimal location of thalamotomy lesions for tremor associated with Parkinson’s disease: a probabilistic analysis based on postoperative magnetic resonance imaging and integrated digital atlas. J Neurosurg 96:854–866
Aziz TZ, Davies L, Stein J, France S (1998) The role of descending basal ganglia connections to the brain stem in parkinsonian akinesia. Br J Neurosurg 12:245–249
Benabid AL (2003) Deep brain stimulation for Parkinson’s disease. Curr Opin Neurobiol 13:696–706
Berardelli A, Rothwell JC, Thompson PD, Hallet M (2001) Pathophysiology of bradykinesia in Parkinson’s disease. Brain 124:2131–2146
Bertrand G (1966a) Stimulation during stereotactic operations for dyskinesias. J Neurosurg 24:419–428
Bertrand G (1966b) Localization of lesions. J Neurosurg 24:446–448
Bertrand G, Jasper S, Wong A, Matthews G (1969) Microelectrode recording during stereotactic surgery. Clin Neurosurg 16:328–355
Beuter A, Edwards R, deGeoffroy A, Mergler D, Hundnell K (1999) Quantification of neuromotor function for detection of the effects of manganese. Neurotoxicology 20(2–3):355–366
Bhatia KP, Marsden CD (1994) The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain 117(Pt 4):859–876
Boecker H, Wills AJ, Ceballos-Baumann A, Samuel M, Thomas DG, Marsden CD, Brooks DJ (1997) Stereotactic thalamotomy in tremor-dominant Parkinson’s disease: an H2 (15)O PET motor activation study. Ann Neurol 41:108–111
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24(2):197–211
Brown RG, Pluck G (2000) Negative symptoms: the pathology of motivation and goal-directed behaviour. Trends Neurosci 23(9):412–417
Van Buren JM, Li CL, Shapiro DY, Henderson WG, Sadowsky DA (1973) A qualitative and quantitative evaluation of parkinsonians three to six years following thalamotomy. Confin Neurol 35(4):202–235
Van Buskirk C, Wolbarcht ML, Stecher K Jr (1966) The non nervous causes of normal physiologic tremor. Neurology 16:217–220
DeLong MR (1990) Primate models of movement disorders of basal ganglia origin. Trends Neurosci 13(7):281–285
Duval C, Jones J (2005) Amplitude assessment of oscillations associated with high frequency components of physiological tremor: impact of loading and signal differentiation. Exp Brain Res 163(2):261–266
Duval C, Panisset M, Bertrand G, Sadikot AF (2000) Evidence that ventrolateral thalamotomy may eliminate the supraspinal component of both pathological and normal physiological tremors. Exp Brain Res 132(2):216–222
Duval C, Panisset M, Sadikot AF (2001) The relationship between physiological tremor and the performance of rapid alternating movements in healthy elderly subjects. Exp Brain Res 139:412–418
Duval C, Sadikot AF, Panisset M (2004) The detection of tremor during slow alternating movements performed by patients with early Parkinson’s disease. Exp Brain Res 154(3):395–398
Duval C, Strafella AP, Sadikot AF (2005) The impact of vetrolateral thalamotomy on high frequency components of tremor. Clin Neurophysiol 116(6):1391–1399
Elble RJ (1995) Mechanisms of physiological tremor and relationship to essential tremor. In: Findley LJ, Koller WC (eds) Handbook of tremor disorders. Dekker, New York, pp 51–62
Filion M (1979) Effects of interruption of the nigrostriatal pathway and of dopaminergic agents on the spontaneous activity of globus pallidus neurons in the awake monkey. Brain Res 178:425–441
Filion M, Tremblay L (1991) Abnormal spontaneous activity of globus pallidus neurons in monkey with MPTP-induced parkinsonism. Brain Res 547:142–151
Finnis KW, Starreveld YP, Parrent AG, Sadikot AF, Peters TM (2003) Three-dimensional database of subcortical electrophysiology for image-guided stereotactic functional neurosurgery. IEEE Trans Med Imaging 22(1):93–104
Hassler R (1982) Architectonic organization of the thalamic nuclei. In: Schaltenbrand G, Walker AR (eds) Stereotaxy of the human brain Anatomical Physiological and clinical application, 2nd edn. Theime, Stuttgart, pp 140–180
Hirai T, Jones EG (1989) A new parcellation of the human thalamus on the basis of histochemical staining. Brain Res Brain Res Rev 14(1):1–34
Hornykiewicz O, Kish SJ (1987) Biochemical pathophysiology of Parkinson’s disease. Adv Neurol 45:19–34
Ilinsky IA, Jouandet ML, Goldman-Rakic PS (1985) Organization of the nigrothalamocortical system in the rhesus monkey. J Comp Neurol 236(3):315–330
Jellinger KA (2002) Recent developments in the pathology of Parkinson’s disease. J Neural Transm Suppl 62:347–376
Kirk RE (1968) Experimental design: procedures for the behavioral sciences, 1st edn. Brooks-Cole, Monterey
Kojima J, Yamaji Y, Matsumura M, Nambu A, Inase M, Tokuno H, Takada M, Imai H (1997) Excitotoxic lesions of the pedunculopontine tegmental nucleus produce contralateral hemiparkinsonism in the monkey. Neurosci Lett 226(2):111–114
Köster B, Lauk M, Timmer J, Winter T, Guschlbauer B, Glocker FX, Danek A, Deuschl G, Lucking CH (1998) Central mechanisms in human enhanced physiological tremor. Neurosci Lett 241(2–3):135–138
Krayenbühl H, Siegfried J, Yasargil MG (1963) Résultats tardifs des opérations stéréotaxiques dans le traitement de la maladie de Parkinson. Rev Neurol (Paris) 108:485–494
Lee MS, Marsden CD (1994) Movement disorders following lesions of the thalamus or subthalamic region. Mov Disord 9(5):493–507
Lozano AM, Lang AE, Galvez-Jimenez N, Miyasaki J, Duff J, Hutchinson WD, Dostrovsky JO (1995) Effect of GPi Pallidotomy on motor function in Parkinson’s disease. Lancet 346:1383–1387
Van Manen J, Speelman JD, Tans RJJ (1984) Indications for surgical treatment of Parkinson’s disease after levodopa therapy. Clin Neurol Neurosurg 86:207–212
Marsden CD (1984) Origins of normal and pathological tremor. In: Findley LJ, Calpiledo R (eds) Movement disorders: tremor. Macmillan, London, pp 165–182
Marsden CD, Meadows JC, Lange GW, Watson RS (1969) The role of ballistocardiac impulse in the genesis of physiological tremor. Brain 92:647–662
Miller WC, DeLong MR (1988) Parkinsonian symptomatology. An anatomical and physiological analysis. Ann N Y Acad Sci 515:287–302
Mink JW (1996) The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol 50(4):381–425
Mogenson GJ, Yang CR (1991) The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. In: Napier TC, Kalivas PW, Hanin I (eds) The basal forebrain: anatomy to function. Advances in experimental medicine and biology, vol 295. Plenum Press, New York, p 267
Nagaseki Y, Shibazaki T, Hirai T, Kawashima Y, Hirato M, Wada H, Miyazaki M, Ohye C (1986) Long-term follow-up results of selective VIM-thalamotomy. J Neurosurg 65:296–302
Obrist WD, Thompson HK Jr, Wang HS, Wilkinson WE (1975) Regional cerebral blood flow estimated by 133-xenon inhalation. Stroke 6(3):245–256
Ohye C, (2000) Use of selective thalamotomy for various kinds of movement disorders, based on basic studies. Stereotact Funct Neurosurg 75:54–65
Ohye C, Hirai T, Miyazaki M, Shibazaki T, Nakajima H (1982) VIM thalamotomy for the treatment of various kinds of tremor. Appl Neurophysiol 45:275–280
Ohye C, Shibasaki T, Hirai T, Kawashima Y, Hirato M, Matsumura M (1985) Plastic change of thalamic organization in patients with tremor after stroke. Appl Neurophysiol 48:288–292
Okada M, Okada M (1983) A method for quantification of alternate pronation and supination of forearms. Comput Biomed Res 16(1):59–78
Okun MS, Vitek JL (2004) Lesion therapy for Parkinson’s disease and other movement disorders: update and controversies. Mov Disord 19(4):375–389
Pahapill PA, Lozano AM (2000) The pedunculopontine nucleus and Parkinson’s disease. Brain 123(Pt 9):1767–1783
Paré D, Curro’Dossi R, Steriade M (1990) Neural basis of the parkinsonian resting tremor: a hypothesis and its implications for treatment. Neuroscience 35:217–226
Parent A, Cossette M (2001) Extrastriatal dopamine and Parkinson’s disease. Adv Neurol 86:45–54
Paus T (2001) Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci 2(6):417–424
Perret E (1968) Simple motor performance of patients with Parkinson’s disease before and after surgical lesion in the thalamus. J Neurol Neurosurg Psychiatry 31:284–290
Perret E, Eggenberger E, Siegfried J (1970) Simple and complex finger movement performance of patients with Parkinsonism before and after a unilateral stereotaxic thalamotomy. J Neurol Neurosurg Psychiatry 33(1):16–21
Sadikot AF, Parent A, Smith Y, Bolam JP (1992) Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a light and electron microscopic study of the thalamostriatal projection in relation to striatal heterogeneity. J Comp Neurol 320:228–242
Shink E, Sidibe M, Smith Y (1997) Efferent connections of the internal globus pallidus in the squirrel monkey: II Topography and synaptic organization of pallidal efferents to the pedunculopontine nucleus. J Comp Neurol 382(3):348–363
Sidibe M, Bevan MD, Bolam JP, Smith Y (1997) Efferent connections of the internal globus pallidus in the squirrel monkey: I Topography and synaptic organization of the pallidothalamic projection. J Comp Neurol 382(3):323–347
van Someren EJ, van Gool WA, Vonk BF, Mirmiran M, Speelman JD, Bosch DA, Swaab DF (1993) Ambulatory monitoring of tremor and other movements before and after thalamotomy: a new quantitative technique. J Neurol Sci 117(1–2):16–23
Stepniewska I, Sakai ST, Qi HX, Kaas JH (2003) Somatosensory input to the ventrolateral thalamic region in the macaque monkey: potential substrate for parkinsonian tremor. J Comp Neurol 455(3):378–395
Stiles RN, Randall JE (1967) Mechanical factors in human tremor frequency. J Appl Physiol 23(3):324–330
St-Jean P, Sadikot AF, Collins L, Clonda D, Kasrai R, Evans AC, Peters TM (1998) Automated atlas integration and interactive three-dimensional visualization tools for planning and guidance in functional neurosurgery. IEEE Trans Med Imaging 17(5):672–680
Strafella AP, Dagher A, Sadikot AF (2003) Cerebral blood flow changes induced by subthalamic stimulation in Parkinson’s disease. Neurology 60(6):1039–1042
Velasco F, Velasco M, Ogarrio C, Olvera A (1986) Neglect induced by thalamotomy in humans: a quantitative appraisal of the sensory and motor deficits. Neurosurgery 19(5):744–751
Vogt BA, Pandya DN, Rosene DL (1987) Cingulate cortex of the rhesus monkey: I Cytoarchitecture and thalamic afferents. J Comp Neurol 262(2):256–270
Volkmann J, Joliot M, Mogilner A, Ioannides AA, Lado F, Fazzini E, Ribary U, Llinas R (1996) Central motor loop oscillations in parkinsonian resting tremor revealed by magnetoencephalography. Neurology 46(5):1359–1370
Walker AE (1982) Stereotaxic surgery for tremor. In: Schaltenbrand G, Walker AE (eds) Stereotaxy for the human brain: anatomical Physiological and clinical applications. Thieme, Stuttgart, pp 515–521
Wichmann T, DeLong MR (2003) Pathophysiology of Parkinson’s disease: the MPTP primate model of the human disorder. Ann N Y Acad Sci 991:199–213
Wichmann T, Bergman H, Starr PA, Subramanian T, Watts RL, DeLong MR (1999) Comparison of MPTP-induced changes in spontaneous neuronal discharge in the internal pallidal segment and in the substantia nigra pars reticulata in primates. Exp Brain Res 125(4):397–409
Yap CB, Boshes B (1967) The frequency and pattern of normal tremor. Electroencephalogr Clin Neurophysiol 22:197–203
Zweig RM, Jankel WR, Hedreen JC, Mayeux R, Price DL (1989) The pedunculopontine nucleus in Parkinson’s disease. Ann Neurol 26:41–46
Acknowledgments
Christian Duval is supported by the Natural Sciences and Engineering Research Council of Canada (NSERC), Canada Foundation for Innovation (CFI), Ontario Innovation Trust (OIT), and Parkinson Society Canada. Antonio P. Strafella and Abbas F. Sadikot are supported by Operating Grants from the Canadian Institute for Health Research (CIHR, AS, AFS) and the Parkinson Society of Canada (AFS). Abbas F. Sadikot is supported by a Senior Scientist Award of the Fonds de Recherche en Santé du Quebec (FRSQ).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Duval, C., Panisset, M., Strafella, A.P. et al. The impact of ventrolateral thalamotomy on tremor and voluntary motor behavior in patients with Parkinson’s disease. Exp Brain Res 170, 160–171 (2006). https://doi.org/10.1007/s00221-005-0198-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0198-4