Abstract
Since specific benzodiazepine (Bz) binding sites have been found in the vision and oculomotor control areas of the central nervous system (CNS), the fast phases of optokinetic nystagmus (OKN) should be affected by Bz administration. In this study, we examine the effects of Bzs on OKN fast phases under closed- and open-loop experimental conditions. Six normal subjects participated in the experiments. The eye movements were measured by the magnetic field, search coil technique, 90 min after diazepam or placebo administration. The study was performed in a randomized, double-blind fashion. After diazepam, the mean amplitude (MAmp) and mean peak velocity (MVel) of OKN fast phases decreased significantly under both experimental conditions. The percentage decreases in MAmp and MVel under the open-loop condition were significantly larger than those under the closed-loop condition. The results indicate that the fast phases of OKN could sensitively reflect the pharmacodynamic effects of Bzs on the CNS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Benzodiazepines (Bzs) are a class of drugs widely prescribed in the treatment of various physical and emotional disorders (Blackwell 1973; Fraser 1998; Woods et al. 1992). The high density of Bz binding sites located in the frontal and occipital cortices, the cerebellar floculus and vermis and brainstem areas (Braestrup et al. 1977; Möhler et al. 1978; Speth et al. 1978), are known to participate in vision and oculomotor control (Leigh and Zee 1999; Rothenberg and Selkoe 1981; Roy-Byrne et al. 1993). In recent years, a growing body of literature has described the utility of saccades and optokinetic nystagmus (OKN) as a surrogate measure of Bz pharmacodynamics (Roy-Byrne et al. 1993; Tian et al. 2003; de Visser et al. 2003). Previous investigations showed that Bz administration would decrease voluntary saccade accuracy, peak velocity, and peak acceleration (Ball et al. 1991; Masson et al. 2000; Rothenberg and Selkoe 1981), and prolong voluntary saccade latency (Fafrowicz et al. 1995; Masson et al. 2000; Roy-Byrne et al. 1993), especially when disparity stimuli are applied (Wang et al. 2005). However, saccade metrics may be affected by fatigue, attention, or adaptation (Abrams et al. 1992; Bollen et al. 1993; Leigh and Zee 1999; Schmidt et al. 1979). In contrast to saccades, which are usually voluntary, OKN is a series of involuntary eye movements consisting of a tracking slow phase followed by a resetting fast phase (Collewijn 1981). Recently, we examined the effects of Bzs on the slow phases of OKN, and found a significant decrease in the gain of open-loop OKN (Tian et al. 2003; Wei et al. 1998). Since the brainstem areas and mesencephalon, where Bz binding sites are densely distributed (Braestrup et al. 1977; Möhler et al. 1978; Speth et al. 1978), are involved in the generation of OKN fast phases (Anastasio 1997; Cohen and Henn 1972; Leigh and Zee 1999), it is a reasonable assumption that the OKN fast phases might also be an effective Bz pharmacodynamic measure.
The regular OKN eye movement tracking the stimulus motion serves to stabilize the retinal image of the moving stimulus. The effect of this involuntary eye movement response is to provide negative feedback to the input to reduce the image slip on the retina. Due to the effect of deep negative feedback, the closed-loop OKN response is very stable, even when some lesions occurred in its pathway. Therefore, eliminating the negative feedback (setting open-loop condition) could give a more direct insight into the changes of OKN system (Collewijn 1981). In the present experiments, building upon our previous work (Tian et al. 2002, 2003; Wei et al. 1998; Yang et al. 2000), we investigated the changes in amplitude and peak velocity of OKN fast phases under closed- and open-loop experimental conditions after diazepam administration.
Methods
Subjects
Six normal volunteers [age range 20–29 years (24.3±3.3 years, mean ± SD), and weight 65–75 kg (70.4±3.8 kg, mean ± SD)] served as subjects. None had prior experience with Bzs or any other sedative-hypnotics, and none had taken any medication for at least 1 month. All subjects abstained from coffee, tea, and alcohol for more than 12 h preceding the experiment. Data were discarded on one subject who appeared to be overly sensitive to Bzs, and was unable to produce a continuous and regular OKN after diazepam administration.
Written informed consent was obtained from each subject prior to the study, after all procedures and risks were explained. The experiment was approved by the Institutional Review Board at the Shanghai Institutes for Biological Sciences, and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Stimuli and apparatus
Computer-generated moving gratings were presented on a monitor located 57 cm in front of the observer and subtended a visual angle of 36° (H) × 27° (V). The luminances of the black-and-white vertical stripes were 0.06 and 3.53 cd/m2, respectively, with spatial frequency of 3.6°/cycle. Four horizontal velocities of 1.68, 3.375, 6.75, and 13.5°/s were employed both under closed- and open-loop conditions.
Under the closed-loop condition, the horizontal velocity of the stimuli remained constant. Under the open-loop condition, the horizontal velocity of eye movements was calculated in real-time and added to the computer-generated signals controlling the movement of the gratings. Thus, the negative feedback was eliminated, and a constant retinal slip velocity was maintained (Tian et al. 2002, 2003; Wei et al. 1998; Yang et al. 2000).
Experimental procedure
For each subject, a single dose of 5 mg diazepam or placebo (Vitamin B1) was administered orally on an empty stomach. The study was performed in a double-blind, randomized fashion. On each test day, the OKN experiment started about 90 min after the drug administration. Each subject returned for a second session, following an interval of at least 1 week.
The subjects sat in a dimly lit room with the head stabilized by a chin rest. They were instructed not to make any voluntary eye movements but to stare at the screen and allow involuntary responses to occur. At the beginning of each trial, calibration of eye movements was performed. Leftward and rightward OKN were recorded under closed- and open-loop conditions. The order of the two conditions was randomized. Each trial took 30 s, and the experiment was limited to 30 min.
Eye movement measurement
Eye movements were recorded using the magnetic field, search coil technique (Collewijn et al. 1975; Robinson 1963). An annulus of silicone rubber with an induction coil (Skalar Medical BV, The Netherlands) was placed on the subject’s right eye after topical anesthesia with oxybuprocaine hydrochloride 0.4%. Eye movement signals were sampled and stored on a computer for off-line analysis. Simultaneously, eye movements were displayed on another monitor for on-line monitoring (Tian et al. 2002, 2003; Wei and Sun 1998; Yang et al. 2000).
Data analysis
A computer program developed in our lab (Wei and Sun 1998; Tian et al. 2002) was used to calculate the amplitude and velocity of eye movements. The mean amplitude (MAmp) and mean peak velocity (MVel) of OKN fast phases were calculated for each subject and each stimulus velocity. All fast phases associated with blinks were rejected.
The changes in MAmp and MVel of OKN fast phases for all subjects were analyzed by a multiway factorial analysis of variance (ANOVA) with drug treatment (diazepam versus placebo), experimental condition (open-loop versus closed-loop), and stimulus velocity (1.68, 3.375, 6.75, and 13.5°/s) as fixed factors, and subjects as the random factor. The data for leftward and rightward OKN were averaged together for statistical analysis due to the fact that no significant differences were found between the two directions in our experiments.
Results
The MAmp of closed- and open-loop OKN fast phases after placebo or diazepam is shown in Fig. 1a. ANOVA analysis showed that MAmp of OKN fast phases decreased significantly after diazepam treatment (F(1,4)=15.3, P<0.02). A significant interaction was also found between experimental condition (open-loop versus closed-loop) and drug treatment (diazepam versus placebo) (F(1,4)=14.5, P<0.02). The percentage changes in MAmp of OKN fast phases after diazepam are shown in Fig. 1b. The percentage decreases in MAmp under the open-loop condition were significantly larger than those under the closed-loop condition (F(1,4)=23.4, P<0.01). Similarly, the MVel of OKN fast phases also decreased significantly after diazepam (F(1,4)=17.3, P<0.02, Fig. 1c), and the percentage decreases in MVel under the open-loop condition were significantly larger than those under the closed-loop condition (F(1,4)=25.6, P<0.01, Fig. 1d). The results suggest that MAmp and MVel of OKN fast phases explicitly reflect the pharmacodynamic effects of Bzs on the central nervous system (CNS), and that the measures are much more sensitive under the open-loop condition.
The mean amplitude (MAmp) and mean peak velocity (MVel) of OKN fast phases after diazepam versus placebo. a MAmp of OKN fast phases, empty symbols for the closed-loop condition, solid symbols for the open-loop condition, single symbol represents one subject, each shape of symbol corresponding to one stimulus velocity: 1.68°/s (circle), 3.375°/s (triangle), 6.75°/s (square), and 13.5°/s (diamond). The diagonal line represents no difference between the diazepam and placebo. b Percentage changes in MAmp after diazepam for four different stimulus velocities, empty bars for the closed-loop condition, hatched bars for the open-loop condition. Error bars are standard errors. c MVel of OKN fast phases. d Percentage changes in MVel after diazepam. e The changes in the peak velocities of OKN fast phases for four amplitude ranges. Symbols for each amplitude range: 2.5±0.25° (circle), 5.0±0.5° (triangle), 7.5±0.75° (square), and 10.0±1.0° (diamond). f Percentage changes in the peak velocity of OKN fast phases for the four amplitude ranges; symbols same as (b)
We also compared the peak velocities of OKN fast phases after diazepam with those of the same amplitudes after placebo. Due to the small fast-phase amplitudes in the present experiments, it is not suitable to perform a curve fitting for a large amplitude range. We selected fast phases in the amplitude range of 2.5±0.25°, 5.0±0.5°, 7.5±0.75°, and 10.0±1.0°, and compared their peak velocities between diazepam and placebo administration. A multiway factorial ANOVA was used with drug treatment (diazepam versus placebo), amplitude range, and experimental condition (open-loop versus closed-loop) as fixed factors, and subjects as the random factor. ANOVA analysis showed that, for the four amplitude ranges, peak velocity decreased significantly after diazepam, compared with those after placebo (F(1,4)=32.43, P<0.005, Fig. 1e). The results suggest that there is a direct effect on the fast-phase OKN by the Bz administration. The percentage decreases in peak velocity under the open-loop condition were not significantly different from those of the same amplitude ranges under the closed-loop condition (F(1,4)=0.01, P=0.9, Fig. 1f).
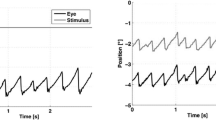
Sample traces of OKN eye movements are illustrated in Fig. 2. Compared to placebo, the diazepam dramatically decreased the amplitude and peak velocity of OKN fast phases.
Discussion
Optokinetic nystagmus eye movements are the results of a complex visuo-oculomotor transformation process which involves many structures at the cortical as well as cerebellar and brainstem level (Collewijn 1981; Ilg 1997). In the present experiments, our own placebo data agree with previous investigations (Henriksson et al. 1981; Gavilán and Gavilán 1984; Tian et al. 2002, 2003). As a classical representative of Bzs, diazepam acts on potentiate gamma amino butyric acid, which is a major inhibitory neurotransmitter in the CNS (Montarolo et al. 1979). Neurophysiologic studies showed that Bzs bind densely in these oculomotor areas, especially in the pons (Braestrup et al. 1977; Möhler et al. 1978; Speth et al. 1978), where cells responsible for fast-phase generation are located (Anastasio 1997; Cohen and Henn 1972). The observed impairment of OKN fast phases in the present experiments might result from the diazepam-induced depression in these CNS areas.
The fast phase of OKN is a resetting response to the slow-phase tracking movement (Kanayama et al. 1987; Leigh and Zee 1999). The amplitudes of fast phases are correlated with those of slow-phase components (Pyykkö and Dahlen 1985). Therefore, a portion of decreases in MAmp and MVel of OKN fast phases in the present experiments may result from the impairment of slow-phase amplitudes (or gain). On the other hand, the decreases may also arise from the direct effects of diazepam on the fast-phase areas involved (Braestrup et al. 1977; Möhler et al. 1978; Speth et al. 1978). The peak velocities significantly decreased after diazepam, compared with those of the same amplitudes after placebo, which clearly demonstrates a direct effect of diazepam on the rapid eye movements in the present study. Further analyzing each subject and amplitude range also showed that the peak velocities significantly decreased (all P<0.05), except for one subject for the amplitude range of 10.0±1.0° under the closed-loop condition (t 14=0.57, P=0.29, it might be due to the small quantity of fast phases in this amplitude range for this subject). Therefore, the decreases in MAmp and MVel reflect the Bz effects with both direct influence on the fast phases and indirect influence through modulating the slow-phase OKN.
The MAmp and MVel of OKN fast phases decreased significantly after diazepam administration, but the decreases were impaired under the closed-loop condition due to the deep negative feedback control (Collewijn 1981; Tian et al. 2003). By contrast, under the open-loop condition, both the amplitude and peak velocity of OKN fast phases decreased more significantly after diazepam when the effect of deep negative feedback was eliminated. The results suggest that MAmp and MVel, especially under the open-loop condition, are sensitive to Bz effects on the CNS.
In Fig. 1, panels a and c, although data points from low-stimulus velocities appeared near the diagonal line under the closed-loop condition (such as empty circles, 1.68°/s, empty triangles, 3.375°/s in Fig. 1a), the diazepam-induced decreases in MAmp and MVel were significant (F(1,4)=8.0, P<0.05 for MAmp; F(1,4)=9.3, P<0.04 for MVel, closed-loop condition). Further analysis by ANOVA revealed that all the percentage decreases in MAmp and MVel were independent of stimulus velocities (F(3,12)=0.5, P=0.67 for MAmp; F(3,12)=1.3, P=0.31 for MVel, closed-loop condition), i.e., there was no significant difference between the low velocities of stimulus and high velocities for the effects of diazepam on MAmp and MVel. Similarly, as shown in panel e, the peak velocity of OKN fast phases decreased significantly for each amplitude range after diazepam, and the percentage decreases in the peak velocities were independent of fast-phase amplitude (F(3,12)=1.9, P=0.19, closed-loop condition; and F(3,12)=0.9, P=0.46, open-loop condition).
In summary, the present study suggests that the measurement of changes in OKN fast phases, especially under the open-loop condition, is a new approach to approximate the effects of Bzs on the CNS. In addition, due to the involuntary nature of OKN, the method could be applied even for examining non-cooperative subjects and animals.
References
Abrams RA, Dobkin RS, Helfrich MK (1992) Adaptive modification of saccadic eye movements. J Exp Psychol Hum Percept Perform 18:922–933
Anastasio TJ (1997) A burst-feedback model of fast-phase burst generation during nystagmus. Biol Cybern 76:139–152
Ball DM, Glue P, Wilson S, Nutt DJ (1991) Pharmacology of saccadic eye movements in man. 1. Effects of the benzodiazepine receptor ligands midazolam and flumazenil. Psychopharmacology 105:361–367
Blackwell B (1973) Psychotropic drugs in use today. The role of diazepam in medical practice. JAMA 225:1637–1641
Bollen E, Bax J, van Dijk JG, Koning M, Bos JE, Kramer CG, van der Velde EA (1993) Variability of the main sequence. Invest Ophthalmol Vis Sci 34:3700–3704
Braestrup C, Albrechtsen R, Squires RF (1977) High densities of benzodiazepine receptors in human cortical areas. Nature 269:702–704
Cohen B, Henn V (1972) The origin of quick phases of nystagmus in the horizontal plane. Bibl Ophthalmol 82:36–55
Collewijn H (1981) The optokinetic system. In: Zuber BL (ed) Models of oculomotor behavior and control. CRC Press, Boca Raton, pp 111–139
Collewijn H, van der Mark F, Jansen TC (1975) Precise recording of human eye movements. Vision Res 15:447–450
de Visser SJ, van der Post JP, de Waal PP, Cornet F, Cohen AF, van Gerven JM (2003) Biomarkers for the effects of benzodiazepines in healthy volunteers. Br J Clin Pharmacol 55:39–50
Fafrowicz M, Unrug A, Marek T, van Luijtelaar G, Noworol C, Coenen A (1995) Effects of diazepam and buspirone on reaction time of saccadic eye movements. Neuropsychobiology 32:156–160
Fraser AD (1998) Use and abuse of the benzodiazepines. Ther Drug Monit 20:481–489
Gavilán C, Gavilán J (1984) Computerized study of the velocity of the rapid eye movements. Clin Otolaryngol 9:191–194
Henriksson NG, Hindfelt B, Pyykkö I, Schalén L (1981) Rapid eye movements reflecting neurological disorders. Clin Otolaryngol 6:111–119
Ilg UJ (1997) Slow eye movements. Prog Neurobiol 53:293–329
Kanayama R, Kato I, Nakamura T, Koike Y (1987) The fast-phase velocity of optokinetic nystagmus in central nervous system disorders. Acta Otolaryngol 104:392–399
Leigh RJ, Zee DS (1999) The neurology of eye movements, 3rd edn. Oxford University Press, New York
Masson GS, Mestre DR, Martineau F, Soubrouillard C, Brefel C, Rascol O, Blin O (2000) Lorazepam-induced modifications of saccadic and smooth-pursuit eye movements in humans: attentional and motor factors. Behav Brain Res 108:169–180
Möhler H, Okada T, Heitz P, Ulrich J (1978) Biochemical identification of the site of action of benzodiazepines in human brain by 3H-diazepam binding. Life Sci 22:985–995
Montarolo PG, Raschi F, Strata P (1979) Interactions between benzodiazepines and GABA in the cerebellar cortex. Brain Res 162:358–362
Pyykkö I, Dahlen AI (1985) Intrabeat relationship of postrotatory nystagmus in normal subjects. Acta Otolaryngol (Stockh) 99:74–82
Robinson DA (1963) A method of measuring eye movement using a scleral search coil in a magnetic field. IEEE Trans Biomed Eng 10:137–145
Rothenberg SJ, Selkoe D (1981) Specific oculomotor deficit after diazepam. I. Saccadic eye movements. Psychopharmacology 74:232–236
Roy-Byrne PP, Cowley DS, Radant A, Hommer D, Greenblatt DJ (1993) Benzodiazepine pharmacodynamics: utility of eye movement measures. Psychopharmacology 110:85–91
Schmidt D, Abel LA, Dell’Osso LF, Daroff RB (1979) Saccadic velocity characteristics: intrinsic variability and fatigue. Aviat Space Environ Med 50:393–395
Speth RC, Wastek GJ, Johnson PC, Yamamura HI (1978) Benzodiazepine binding in human brain: characterization using (3H) flunitrazepam. Life Sci 22:859–866
Tian J, Yang Q, Wei M, Terdiman J, Sun F (2002) Neostigmine-induced alternations in fast phase of optokinetic responses in myasthenic ocular palsies. J Neurol 249:867–874
Tian J, Wei M, Liang P, Sun F (2003) Effects of diazepam on closed- and open-loop optokinetic nystagmus (OKN) in humans. Exp Brain Res 152:523–527
Wang C, Tong J, Sun F (2005) Effects of diazepam on the latency of saccades for luminance and binocular disparity defined stimuli. Exp Brain Res 163:246–251
Wei M, Sun F (1998) The alternation of optokinetic responses driven by moving stimuli in humans. Brain Res 813:406–410
Wei M, Yang Q, Tian J, Shen Y, Sun F (1998) The effect of diazepam on human optokinetic nystagmus in open loop conditions. In: Abstract of the 8th Chinese symposium on biophysics. Biophysical Society of China, Chengdu, China, May 17–22, 312 pp
Woods JH, Katz JL, Winger G (1992) Benzodiazepines: use, abuse and consequences. Pharmacol Rev 44:151–347
Yang Q, Wei M, Sun F, Tian J, Chen X, Lu C (2000) Open-loop and closed-loop optokinetic nystagmus (OKN) in myasthenia gravis and nonmyasthenic subjects. Exp Neurol 166:166–172
Acknowledgments
This work was partially supported by the National Basic Research Program of China (G1999054000) and by the National Natural Science Foundation of China. The authors are grateful to Dr. Faisal Karmali for checking the language of the paper, and Lingyu Chen, Xinzhen Zhao, and Zhi Li for their help in the experiments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, C., Tian, J., Liang, P. et al. Diazepam-induced changes of optokinetic nystagmus fast phase. Exp Brain Res 167, 446–450 (2005). https://doi.org/10.1007/s00221-005-0176-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0176-x