Abstract
The purpose of the present study was to determine whether glutamate-induced mechanical sensitization of the masseter muscle in human volunteers involves activation of peripheral N-methyl-d-aspartate (NMDA) receptors. Healthy male volunteers (n=18) participated in this randomized, two-session study. During each session, the volunteers received two injections into the right masseter muscle. An initial injection of glutamate (1 M, 0.2 ml) alone was followed 30 min later by a second injection of glutamate alone or glutamate combined with ketamine (10 mM). Pressure pain threshold (PPT) was assessed over the right masseter muscle at and 2 cm above the injection site, as well as over the right temporalis muscle and left masseter muscle prior to the first injection. The PPT was reassessed at all four sites every 5 min from 10 to 30 min after the second injection and once again 60 min after the second injection. Glutamate-evoked muscle pain, pain area and the sensory pain response index of the McGill pain questionnaire were all significantly reduced by co-injection of ketamine. The mean PPT values were significantly decreased by ~10%, 10, 15 and 25 min after injection of glutamate, but only over the site of injection. Co-injection of ketamine with glutamate also completely blocked the glutamate-induced mechanical sensitization 15 min post-injection as compared with glutamate alone. The lack of spread of mechanical sensitization outside the area of glutamate injection is consistent with the view that glutamate-induced mechanical sensitization results from a peripheral mechanism. The attenuation of glutamate-induced mechanical sensitization by ketamine suggests that this effect is mediated, in part, through activation of peripheral NMDA receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Evidence suggests that elevation of glutamate concentration in deep tissues such as muscles and joints is associated with painful disorders. An association between pain and elevated glutamate concentrations in the synovial fluid of arthritis sufferers and in the tendon tissues of volunteers suffering from “Jumpers knee” and tennis elbow has been made (Alfredson et al. 2000, 2001; Alfredson and Lorentzon 2002; Carlton et al. 2003). Injection of glutamate into the human masseter muscle evokes pain, decreases pressure pain threshold (PPT) and enhances the amplitude of the jaw-stretch reflex (Cairns et al. 2001, 2003b, c; Svensson et al. 2003; Wang et al. 2004). Since muscle pain, localized mechanical sensitivity and alterations in jaw-stretch reflexes are also found in patients suffering from temporomandibular disorders (TMD), it has been speculated that elevated tissue levels of glutamate may be a contributing factor to the development and maintenance of pain in these disorders (Cairns et al. 2001; Svensson et al. 2003). The source of glutamate in these various tissues remains to be defined; however, it has been suggested that glutamate levels may be elevated secondary to plasma extravasation into damaged tissues or as a result of vesicular release of glutamate from the terminal endings of nerve fibers that innervate the affected tissue (Lawand et al. 1997; Cairns et al. 1998; Carlton et al. 1998; deGroot et al. 2000; Lam et al. 2005).
Animal models have shown that injection of glutamate into the masseter muscle can both activate and sensitize putative masseter nociceptors (Cairns et al. 2001, 2002, 2003b). These studies have revealed that glutamate exerts its effects on masseter muscle afferent fibers in part through activation of peripheral N-methyl-d-aspartate (NMDA) receptors. We have recently demonstrated that glutamate-evoked muscle pain in human volunteers can be attenuated by co-injection of the NMDA receptor antagonist ketamine, which indicates that this experimentally induced muscle pain is also mediated through activation of peripheral NMDA receptors (Cairns et al. 2003b) The purpose of the present study was to determine whether glutamate-induced mechanical sensitization of the masseter muscle in human volunteers involves activation of peripheral NMDA receptors.
Materials and methods
Volunteers
Eighteen healthy men (mean age 27±1 year) without signs or symptoms of TMD (Dworkin and LeResche 1992) volunteered to participate in this study, which was undertaken at the Orofacial Pain Laboratory, Center for Sensory–Motor Interaction, Aalborg University, Denmark. The study was approved by the local Ethics Committee (Counties of Nordjylland and Viborg, Denmark) and conducted in accordance with the Helsinki Declaration. Informed consent was obtained from all the volunteers.
Pressure pain thresholds
In agreement with previously detailed methodology (Svensson et al. 2003), a pressure algometer (Somedic, Sweden) was used to measure PPTs in response to deep stimuli applied to the masseter muscles and temporalis muscle. The volunteers were asked to keep their jaw at rest and not to clench their teeth. Pressure was applied to the muscle at a rate of 30 kPa/s with a 1 cm diameter probe and volunteers pushed a button when their PPT was reached. PPT (kPa) was determined from a single measurement at each time point.
Experimental protocol
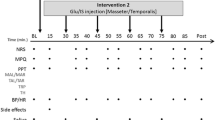
The experimental protocol is illustrated in Fig. 1. PPTs were collected from the injection site and 2 cm above the injection site in the right masseter muscle, the right temporalis muscle and the left masseter muscle. Our previous work has found that repeated injection of 1.0 M glutamate at 25 min intervals into the masseter muscle significantly decreases PPTs in human volunteers (Svensson et al. 2003). In the present study, an initial injection of a sterile solution of glutamate (0.2 ml; 1.0 M; ~pH 7.2) was made after collecting baseline PPTs from these sites. This was followed 30 min later by a second injection of glutamate alone or glutamate and ketamine (10 mM; ~pH 7.0; Ketalar, Park Davis). Volunteers attended two sessions at a minimum interval of 1 week between sessions. The order of injection of glutamate alone or with ketamine was randomized between the two sessions and neither the examiners nor the volunteers were aware of the contents of the second injection.
The volunteers continuously scored pain intensity on a 10 cm electronic visual analog scale (VAS) with the lower extreme marked “no pain” and the upper extreme marked “most pain imaginable”. Peak pain (the highest VAS score), duration of pain (time required for the VAS to return to “no pain” after injection) and overall pain (area under the VAS-time curve) were calculated from the recorded VAS data in accordance with previously detailed methods (Cairns et al. 2001, 2003b, c; Svensson et al. 2003; Wang et al. 2004). We have previously reported, based on the VAS data from 14 of these volunteers, that 10 mM ketamine reduces glutamate-evoked but not hypertonic saline-evoked masseter muscle pain but has no effect when injected by itself on masseter muscle PPTs (Cairns et al. 2003b). Volunteers also filled in the McGill pain questionnaire and were asked to draw their perceived distribution of pain on a map that had a picture of the profile of the face, approximately 10 min after the second injection of glutamate and just prior to the first post-injection assessment of PPTs. Pain drawings were subsequently digitized with the aid of a computer program and specialized hardware (ACECAD, model D9000 1 digitizer, Taiwan) to calculate the area of perceived pain in arbitrary units (a.u.).PPTs from the various sites were assessed every 5 min for the period from 10 to 30 min as well as at 60 min after the second injection.
Statistics
A one-way repeated measures ANOVA was employed to determine whether the injection of glutamate significantly decreased PPT values at the various time points post-injection. A paired t test was used to compare PPT values at selected times post-injection between the glutamate alone and the glutamate plus ketamine sessions. Pearson correlation was employed to assess whether there were significant interactions between any of the pain parameters (peak, duration, overall pain, McGill pain score or area of pain drawing) and the relative change in mechanical threshold after the second injection of glutamate. Mean (± SEM) values are reported in the text and figures.
Results
Pain
The peak pain (P=0.005), duration of pain (P<0.001) and overall pain (P=0.001) were all significantly reduced after injection of glutamate and ketamine when compared with glutamate alone (Fig. 2). The pain area drawings (in a.u.) were significantly (P=0.011) smaller after injection of glutamate with ketamine than after injection of glutamate alone (Fig. 2). The most commonly used McGill pain questionnaire adjectives and the mean pain response index scores are shown in Table 1. There was a significant difference in the sensory PRI when the result of glutamate alone was compared with the result of glutamate with ketamine. This was also reflected in a decreased usage of the most common adjectives and a significant decrease in the number of words chosen.
Pressure pain threshold
The mean baseline PPT values prior to injection of glutamate were as follows: right masseter muscle injection site 276±17 kPa (range 193–469 kPa); right masseter muscle 2 cm above injection site 298±18 kPa (range 167–494 kPa); right temporalis 327±23 kPa (range 188–499 kPa); left masseter muscle 274±15 kPa (range 189–388 kPa). As a result of the large inter-individual variability in baseline PPTs, post-injection PPTs have been normalized to the pre-injection baseline in Fig. 3 to better illustrate the effect of injection of substances into the masseter muscle. The mean PPT values obtained over the site of injection in the right masseter muscle were significantly decreased at 10, 15 and 25 min after the second injection of glutamate (Fig. 3). There was no significant effect of glutamate injection on the PPT measured over the right masseter muscle 2 cm above the injection site, over the right temporalis muscle or in the left masseter muscle.
The line and scatter plots illustrate the PPT relative to pre-injection baseline after the second injection at the various sites. Significant decreases in the PPT after injection of glutamate alone were limited to the site of masseter injection (+P<0.05, repeated measures ANOVA and Holm–Sidak post hoc tests). At the site of masseter injection, co-administration of ketamine with glutamate significantly attenuated glutamate-induced decreases in PPT 15 min post-injection (*P<0.05, paired t test)
There was a significant inverse correlation (r=−0.48, P=0.043) between the overall pain area and the relative decrease in PPT at the injection site, 15 min post-injection. No other significant correlations were identified.
The mean baseline PPT values prior to injection of ketamine with glutamate were as follows: right masseter muscle injection site 267±12 kPa (range 182–413 kPa); right masseter muscle 2 cm above injection site 296±12 kPa (range 169–364 kPa); right temporalis 306±15 kPa (range 211–431 kPa); left masseter muscle 247±10 kPa (range 188–328 kPa). Co-injection of ketamine with glutamate appeared to completely attenuate glutamate-induced mechanical sensitization at the injection site at all three time points; however, the difference between the two treatments reached significance (P=0.039) only at the 15 min time point.
Discussion
The present study showed that repeated injection of glutamate into the masseter muscle evoked muscle pain and significantly decreased masseter muscle PPTs at the site of injection for up to 20 min post-injection. These data are consistent with our previously reported findings (Svensson et al. 2003). Data obtained from the McGill pain questionnaire suggest that the quality of this pain does not appear markedly different from that described previously for injection of hypertonic saline into the masseter muscle (Svensson et al. 1995). Despite the fact that volunteers have reliably included large areas of the right masseter muscle as well as the temporalis muscle in their pain drawings after glutamate injection (Cairns et al. 2001), there was no significant effect of glutamate on the PPTs 2 cm rostral to the site of injection or in the temporalis muscle. This result implies that glutamate-induced mechanical sensitization is principally a local phenomenon and is limited to a small area near the site of the injection.
Co-injection of ketamine with glutamate into the human masseter muscle also prevented the development of glutamate-induced mechanical sensitization at the site of injection. A single injection of 1.0 M (10 μl) glutamate into the masseter muscle decreases the mechanical threshold of rat masseter muscle afferent fibers for prolonged periods (Cairns et al. 2002, 2003a). Co-injection of the mixed NMDA and non-NMDA receptor antagonist kynurenic acid prevents the development of glutamate-induced masseter muscle afferent fiber sensitization (Cairns et al. 2002, 2003a). Recently, it has been determined by magnetic resonance spectroscopy that glutamate is rapidly cleared from the site of injection in the masseter muscle with a half-life of approximately 2 min (Gambarota et al. 2005). Such rapid clearance of the injected glutamate suggests that only a brief elevation of intramuscular glutamate concentration is sufficient to trigger a cascade of events within the muscle tissue that alters the response properties of muscle afferent fibers. One possibility is that the influx of calcium, which would be predicted to occur upon peripheral NMDA receptor activation, produces long-term alterations in the sensitivity of mechano-nociceptors, for example, via a mechanism of calcium-mediated receptor phosphorylation (Salter and Kalia 2004). Glutamate can also activate G-protein-coupled peripheral metabotropic glutamate (mGlu) receptors, which are present on some primary afferent fibers (Zhou et al. 2001). Activation of the mGlu1 and mGlu5 receptor subtypes, which increase intracellular calcium levels through the phospholipase C cascade pathway, has been demonstrated to cause a prolonged period of mechanical sensitization of the skin (Zhou et al. 2001). Activation of peripheral glutamate receptors also results in the release of neuropeptides such as calcitonin gene-related peptide (CGRP) and substance P from other deep tissues (Jackson and Hargreaves 1999; McRoberts et al. 2001). Glutamate-induced mechanical sensitization of cutaneous tissue is enhanced by substance P and reduced by neurokinin receptor antagonists (Carlton et al. 1998; Beirith et al. 2003). Although the present results suggest that activation of peripheral NMDA receptors alone may be sufficient to induce mechanical sensitization upon injection of glutamate into the masseter muscle, they do not exclude the possibility of a contribution by some or all of these other mechanisms.
Co-injection of the NMDA receptor antagonist ketamine with glutamate significantly attenuated glutamate-evoked pain responses, decreased pain areas and reduced the sensory PRI of the McGill pain questionnaire. The effect of ketamine on glutamate-evoked pain intensity and duration is consistent with our earlier reported findings (Cairns et al. 2003a). Pain area has been found to be positively correlated with pain intensity when hypertonic saline infusion is employed to evoke tibialis muscle pain and can be significantly reduced by systemic administration of ketamine (0.3–0.5 mg/kg) (Graven-Nielsen et al. 1997, 2000; Schulte et al. 2003). Taken together with the results of the present study, it is proposed that the measurement of pain area can provide an additional sensitive means of assessing the efficacy of analgesic agents.
Several recent studies have examined the relationship between the concentration of extracellular glutamate in deep tissues and pain in conditions such as tennis elbow, chronic shoulder pain and even headache (Alfredson et al. 2000, 2001; Ashina et al. 2003; Rosendal et al. 2004). Glutamate levels are higher in the trapezius muscles of patients suffering from ongoing pain and tenderness in these muscles than they are in healthy controls (Rosendal et al. 2004). In these patients, it has been found that the glutamate level was positively correlated with ongoing pain intensity and inversely correlated with PPT. Similar findings of a correlation between pain and glutamate concentrations in the extensor carpi radialis brevis tendon have been reported in patients with tennis elbow (Alfredson et al. 2000). In contrast, other work has not identified an increase in glutamate in painful regions of skeletal muscles (Ashina et al. 2003). This difference may reflect the various mechanisms whereby tissue glutamate could become elevated under natural and experimental conditions. After tissue damage, glutamate levels may become elevated as a result of cytosolic release from injured or dying cells, secondary to plasma extravasation due to local changes in vascular permeability or as a result of neurogenic mechanisms wherein glutamate is released from the terminal endings of nerve fibers that innervate the affected tissue (Lawand et al. 1997; Cairns et al. 1998; Carlton et al. 1998; deGroot et al. 2000; Lam et al. 2005). In the absence of obvious tissue damage, neurogenic release of glutamate may be the principal means of increasing tissue glutamate levels (deGroot et al. 2000) and it remains to be determined under what conditions this may occur. Our findings that peripheral administration of an NMDA receptor antagonist can attenuate both glutamate-evoked pain and glutamate-induced mechanical sensitization suggest that ketamine and other NMDA receptor antagonists may prove useful as a pharmacological probe to help assess the extent to which elevated tissue glutamate levels contribute to the development and maintenance of chronic deep tissue pain in a number of clinical conditions.
References
Alfredson H, Lorentzon RJ (2002) Chronic tendon pain: no signs of chemical inflammation but high concentrations of the neurotransmitter glutamate. Implications for treatment? Curr Drug Targets 3:43–54
Alfredson H, Ljung BO, Thorsen K, Lorentzon R (2000) In vivo investigation of ECRB tendons with microdialysis technique—no signs of inflammation but high amounts of glutamate in tennis elbow. Acta Orthop Scand 71:475–479
Alfredson H, Forsgren S, Thorsen K, Lorentzon RJ (2001) In vivo microdialysis and immunohistochemical analyses of tendon tissue demonstrated high amounts of free glutamate and glutamate NMDAR1 receptors, but no signs of inflammation, in Jumper’s knee. Orthop Res 19:881–886
Ashina M, Stallknecht B, Bendtsen L, Pedersen JF, Schifter S, Galbo H, Olesen J (2003) Tender points are not sites of ongoing inflammation–in vivo evidence in patients with chronic tension-type headache. Cephalalgia 23:109–116
Beirith A, Santos AR, Calixto JB (2003) The role of neuropeptides and capsaicin-sensitive fibres in glutamate-induced nociception and paw oedema in mice. Brain Res 969:110–116
Cairns BE, Sessle BJ, Hu JW (1998) Evidence that excitatory amino acid receptors within the temporomandibular joint region are involved in the reflex activation of the jaw muscles. J Neurosci 18:8056–8064
Cairns BE, Hu JW, Arendt-Nielsen L, Sessle BJ, Svensson P (2001) Sex-related differences in human pain and rat afferent discharge evoked by injection of glutamate into the masseter muscle. J Neurophysiol 86:782–791
Cairns BE, Gambarota G, Svensson P, Arendt-Nielsen L, Berde CB (2002) Glutamate-induced sensitization of rat masseter muscle fibers. Neuroscience 109:389–399
Cairns BE, Gambarota G, Dunning PS, Mulkern RV, Berde CB (2003a) Activation of peripheral excitatory amino acid receptors decreases the duration of local anesthesia. Anesthesiology 98:521–529
Cairns BE, Svensson P, Wang K, Hupfeld S, Graven-Nielsen T, Sessle BJ, Berde CB, Arendt-Nielsen L (2003b) Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophysiol 90:2098–2105
Cairns BE, Wang K, Hu JW, Sessle BJ, Arendt-Nielsen L, Svensson P (2003) The effect of glutamate-evoked masseter muscle pain on the human jaw-stretch reflex differs in men and women. J Orofacial Pain 17:317–325
Carlton SM, Zhou S, Coggeshall RE (1998) Evidence for the interaction of glutamate and NK1 receptors in the periphery. Brain Res 790 160–169
Carlton SM, McNearney TA, Cairns BE (2003) Peripheral glutamate receptors: novel targets for analgesics? In: Dostrovsky JO, Carr DB, Koltzenburg M (eds) Proceedings of the 10th world congress on pain, progress in pain research and management, vol 24. IASP Press, Seattle, pp 125–139
deGroot J, Zhou S, Carlton SM (2000) Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. NeuroReport 11:497–502
Dworkin SF, LeResche L (1992) Research diagnostic criteria for temporomandibular disorders. Review, criteria, examinations and specifications, critique. J Craniomandib Disord Facial Oral Pain 6:301–355
Gambarota G, Philippens M, Cairns BE, Dong XD, Renema WKJ, Heerschap A (2005) MRS assessment of glutamate clearance in a novel masticatory muscle pain model. NMR Biomed 6:345–351
Graven-Nielsen T, Arendt-Nielsen L, Svensson P, Jensen TS (1997) Quantification of local and referred muscle pain in humans after sequential i.m. injections of hypertonic saline. Pain 69:111–117
Graven-Nielsen T, Aspegren Kendall S, Henriksson KG, Bengtsson M, Sorensen J, Johnson A, Gerdle B, Arendt-Nielsen L (2000) Ketamine reduces muscle pain, temporal summation, and referred pain in fibromyalgia patients. Pain 85:483–491
Jackson DL, Hargreaves KM (1999) Activation of excitatory amino acid receptors in bovine dental pulp evokes the release of iCGRP. J Dent Res 78:54–60
Lam DK, Sessle BJ, Cairns BE, Hu JW (2005) Neural mechanism of temporomandibular joint and masticatory muscle pain: a possible role for peripheral glutamate receptor mechanisms. Pain Res Manag 10:145–152
Lawand NB, Willis WD, Westlund KN (1997) Excitatory amino acid receptor involvement in peripheral nociceptive transmission in rats. Eur J Pharmacol 324:169–177
McRoberts JA, Coutinho SV, Marvizon JC, Grady EF, Tognetto M, Sengupta JN, Ennes HS, Chaban VV, Amadesi S, Creminon C, Lanthorn T, Geppetti P, Bunnett NW, Mayer EA (2001) Role of peripheral N-methyl-d-aspartate (NMDA) receptors in visceral nociception in rats. Gastroenterology 120:1737–1748
Rosendal L, Larsson B, Kristiansen J, Peolsson M, Sogaard K, Kjaer M, Sorensen J, Gerdle B (2004) Increase in muscle nociceptive substances and anaerobic metabolism in patients with trapezius myalgia: microdialysis in rest and during exercise. Pain 112: 324–334
Salter MW, Kalia LV (2004) Src kinases: a hub for NMDA receptor regulation. Nat Rev Neurosci 5:317–328
Schulte H, Graven-Nielsen T, Sollevi A, Jansson Y, Arendt-Nielsen L, Segerdahl M (2003) Pharmacological modulation of experimental phasic and tonic muscle pain by morphine, alfentanil and ketamine in healthy volunteers. Acta Anaesthesiol Scand 47:1020–1030
Svensson P, Arendt-Nielsen L, Houe L (1995) Sensory-motor: interactions of human experimental unilateral jaw muscle pain: a quantitative analysis. Pain 64:241–249
Svensson P, Cairns BE, Wang K, Hu JW, Graven-Nielsen T, Arendt-Nielsen L, Sessle BJ (2003) Glutamate-evoked pain and mechanical allodynia in the human masseter muscle. Pain 101:221–227
Wang K, Sessle BJ, Svensson P, Arendt-Nielsen L (2004) Glutamate evoked neck and jaw muscle pain facilitate the human jaw stretch reflex. Clin Neurophysiol 115:1288–1295
Zhou S, Komak S, Du J, Carlton SM (2001) Metabotropic glutamate 1alpha receptors on peripheral primary afferent fibers: their role in nociception. Brain Res 913:18–26
Acknowledgments
The Danish National Research Foundation, the Danish Dental Association and the U.S. National Institutes for Health (DE15420) supported the present research. BEC and BJS are recipients of Canada Research Chairs. The authors would also like to thank Ms. Mandeep Mann for her help in the preparation of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
All experiments were performed at The Orofacial Pain Laboratory, Center for Sensory–Motor Interaction, Aalborg University, 9220 Aalborg, Denmark.
Rights and permissions
About this article
Cite this article
Cairns, B.E., Svensson, P., Wang, K. et al. Ketamine attenuates glutamate-induced mechanical sensitization of the masseter muscle in human males. Exp Brain Res 169, 467–472 (2006). https://doi.org/10.1007/s00221-005-0158-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0158-z