Abstract
This investigation is one in a series of studies that address the possibility of stroke rehabilitation using robotic devices to facilitate “adaptive training.” Healthy subjects, after training in the presence of systematically applied forces, typically exhibit a predictable “after-effect.” A critical question is whether this adaptive characteristic is preserved following stroke so that it might be exploited for restoring function. Another important question is whether subjects benefit more from training forces that enhance their errors than from forces that reduce their errors. We exposed hemiparetic stroke survivors and healthy age-matched controls to a pattern of disturbing forces that have been found by previous studies to induce a dramatic adaptation in healthy individuals. Eighteen stroke survivors made 834 movements in the presence of a robot-generated force field that pushed their hands proportional to its speed and perpendicular to its direction of motion — either clockwise or counterclockwise. We found that subjects could adapt, as evidenced by significant after-effects. After-effects were not correlated with the clinical scores that we used for measuring motor impairment. Further examination revealed that significant improvements occurred only when the training forces magnified the original errors, and not when the training forces reduced the errors or were zero. Within this constrained experimental task we found that error-enhancing therapy (as opposed to guiding the limb closer to the correct path) to be more effective than therapy that assisted the subject.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It is appealing to consider using machines for the rehabilitation of brain-injured patients. Machine-assisted training can be highly accurate, can be sustained for very long periods of time, can measure progress automatically, and can produce a wide range of forces or motions. Repetitive practice of the impaired limb has been shown to be beneficial in improving functional ability (Wolf et al. 1989; Taub et al. 1993, 1999; Taub 2000). Beyond the recommended therapy that strengthens and stretches (Delisa and Gans 1993) lies the possibility of neurofacilitation, or neuromuscular re-education through techniques that incorporate our knowledge of the neural circuitry. An important question is which method, among the repertoire of possibilities, is best for motor recovery? Encouraging research (Patton et al. 2001a, b; Dancausea et al. 2002; Patton and Mussa-Ivaldi 2003; Takahashi and Reinkensmeyer 2003) suggests one method may be adaptive training, in which the natural adaptive tendencies of the nervous system are used to facilitate motor recovery. This paper investigates a critical question of the feasibility of adaptive training: Do stroke survivors preserve their ability to adapt? We also address the question of which type of training forces are best and whether the effects of adaptation last after the forces are removed.
There have been a few promising preliminary studies on neurorehabilitation using mechatronic and robotic devices. A two-degree-of-freedom (DOF) robot manipulator (similar to the one used in this study) was used to train stroke survivors in shoulder and elbow movement by moving the hand and forearm of the patient in the horizontal plane (Krebs et al. 1998b, 2000; Volpe et al. 2000, 2001). This is an assistive form of therapy that guides the arm along the desired path and is different from the strategy presented in this paper. Clinical testing of assistive training has been underway for several years, and results have shown improved patient performance (Krebs et al. 1998b) with benefits lasting more than 3 years (Krebs et al. 1999b). This training has led to increased clinical scores and greater gains in proximal arm strength and greater recovery of functional independence (Volpe et al. 2000).
An industrial robot (Puma 560) was also used to apply forces to the paretic limbs of stroke survivors through a customized forearm attachment (Burgar et al. 2000; Lum et al. 2002). The robot could move the limb to a target, applying spring-like forces toward the target or mirror the contralateral limb movements. These results provide convincing evidence that supplemental robotic therapy can improve recovery. They do not, however, indicate which type of robotic treatment offers the greatest advantage. Previous approaches have attempted to mimic the actions of the therapist by using a robot to apply error-decreasing, assisting forces. In fact, Kahn and colleagues (Kahn et al. 2001) suggested that reducing errors during reaching training with a robotic device does not provide any added benefit compared to repetitive reaching training where errors were allowed. This paper tests an alternative approach that enhances error and can only be implemented by a computer-controlled device.
Interestingly, several theories have been proposed for clinical treatment. Some sources suggest that providing manual guidance during reaching may facilitate rehabilitation (Bobath 1978). Other theories advocate using a component of resistance in a direction opposite to movement during diagonal reaching patterns (Voss et al. 1985). Although these approaches are in some ways mutually exclusive, their efficacy has not been tested objectively, and the most effective rehabilitation algorithm(s) have yet to be determined. New techniques are currently being explored. For example, one possible technique is to provide assistance by guiding (pulling) the hand toward the desired trajectory (Volpe et al. 1999; Lum et al. 2002). Another possible technique is to provide resistance by either opposing the hand as it moves (Stein et al. 2004), or by imposing forces that amplify the error. The latter method is justified by the observation that movement error is likely to be a driving signal for adaptation and learning (Rumelhart et al. 1986; Lisberger 1988; Kawato 1990; Dancausea et al. 2002). In a walking study, subjects significantly reduced the time required to predict the applied force field by approximately 26% when the field was transiently amplified (Emken and Reinkensmeyer 2005). Others have also emphasized augmented or amplified error in the therapeutic process. (Winstein et al. 1999; Brewer et al. 2004; Emken and Reinkensmeyer 2005). However, for such an error-enhancing, adaptive technique to work, the patient’s ability to adapt must be preserved following the injury.
Recent studies of motor adaptation in healthy individuals have demonstrated the excellent potential of the natural adaptive process in altering motor patterns. When people are repeatedly exposed to a robot-generated force field applied to the hand (forces as a function of hand position and/or hand velocity) that systematically disturbs limb motion, they are able to recover their original kinematic patterns over a short period of practice (Shadmehr and Mussa-Ivaldi 1994). Subjects do this by cancelling the disturbance with an appropriate preplanned pattern of forces. This is a form of feedforward control that is revealed by characteristic after-effects: when the disturbing force field is unexpectedly removed, subjects make erroneous movements in directions opposite to the perturbing forces. Adaptation and its related after-effects have been demonstrated for different types of force fields, ranging from simple position-, velocity-, and acceleration-dependent force fields (Bock 1990; Flash and Gurevitch 1992; Shadmehr and Mussa-Ivaldi 1994; Gandolfo et al. 1996; Conditt et al. 1997) to Coriolis forces caused by moving in a rotating room (Lackner and DiZio 1994) to skew-symmetric “curl” fields that produce forces in a direction perpendicular to the velocity of the hand (Gandolfo et al. 1996). Similar results have also been observed after manipulations of visual perception that altered the visual feedback of movement (Held and Freedman 1963; Miall et al. 1993; Pine et al. 1996; Krakauer et al. 1999).
More recent studies support the view that subjects adapt by learning the appropriate internal model of the perturbing force field rather than learning an appropriate temporal sequence of muscle activations (Gandolfo et al. 1996; Conditt et al. 1997). Using this internal model, subjects are able to predict the effects of the external field along a desired movement and use a feedforward control strategy (also called anticipatory control) (Hemami and Stokes 1982; Ghez 1991). Modeling techniques have been successful in predicting both how the arm is disturbed by a force field and the after-effects of training (Shadmehr and Mussa-Ivaldi 1994; Kawato and Wolpert 1998; Bhushan and Shadmehr 1999). If this is true, one possible method for rehabilitation may be to use models to design the appropriate force field that will result in beneficial after-effects. Again, this would only work if stroke survivors can adapt. Furthermore, after-effects would also have to be permanently retained for this approach to have rehabilitative significance.
There are several recent studies providing encouraging evidence that the ability to adapt and exhibit after-effects is preserved following cortical stroke. Reaching in individuals with stroke is characterized by errors that reflect their poor ability to manage the interaction torques (Beer et al. 2000). Aspects of reaching in cortical stroke survivors resembles that of cerebellar patients (Bastian et al. 1996). Adaptation and after-effects in stroke survivors can be observed in the oculomotor (Weiner et al. 1983) and limb-motor systems (Raasch et al. 1997; Dancausea et al. 2002). In fact, prism adaptation has been shown to trigger the recovery from hemispatial neglect following stroke (Rossetti et al. 1998). Stroke-related damage in the sensorimotor areas appears to effect the processes underlying the control and execution of motor skills but not the learning of those skills (Winstein et al. 1999). Recently, stroke survivors showed the ability to adapt to small elbow motions that were disturbed by a spring-like force disturbance (Dancausea et al. 2002). However, severely affected individuals used atypical correction strategies. Another recent study evaluated the ability to adapt to a sideways force during forward motions and found that the after-effects were less evident in stroke survivors with more severe impairment (Takahashi and Reinkensmeyer 2003). Furthermore, preliminary studies in our laboratory on stroke survivors have revealed that the after-effects may persist longer when the after-effects resemble healthy unperturbed movements (Raasch et al. 1997; Patton et al. 2001a, b;).
While conventional skill learning, such as learning to play a musical instrument, requires more conscious attention in order to achieve a goal, neuromotor adaptation has been argued to be closely related to procedural learning and thus to be a form of implicit learning (Krebs et al. 2001). Hence, these learning mechanisms may offer an effective alternative to conventional methods of rehabilitation. Implicit learning takes place without awareness of what has been taught (Squire 1986), and often does not require complete conscious attention. One example is procedural motor learning of a motor sequence that is embedded in a seemingly random set of movements (Fitts 1964; Squire 1986; Gomez Beldarrain et al. 1999; Seidler et al. 2002). Another example is sensory-motor adaptation observed in force field paradigms (Krebs et al. 2001). Following unilateral stroke, recent evidence suggests that learning is facilitated by providing explicit information about the task that can enhance implicit motor learning (Boyd and Winstein 2001, 2003). If one could demonstrate that stroke subjects readily adapt to force training, and that beneficial after-effects persist, a new family of rehabilitation strategies would emerge.
To begin exploring the possibility of exploiting implicit learning mechanisms for poststroke rehabilitation, this paper explores the features of motor adaptation in chronic stroke survivors during the execution of planar multijoint movements that are disturbed by a force field. We studied six movement directions using a two-joint planar robotic device, exposing hemiparetic stroke survivors and healthy age-matched controls to a force field that is commonly known to induce unequivocal adaptation in healthy individuals. We found that stroke survivors do adapt, albeit at a diminished level compared to healthy controls, and this capacity to adapt was not related to clinical scores of motor impairment. Furthermore, we found that final improvements were most evident when the training forces magnified rather than reduced the original error. This study provides encouraging evidence that adaptive training could provide an effective supplement to conventional therapy. This research was presented in preliminary form at the Society for Neuroscience meeting, 2001 (Patton et al. 2001b).
Methods
Experiments
Research was approved by the Northwestern University Internal Review Board to conform to ethical standards laid down in the 1964 Declaration of Helsinki and federal mandates that protect research subjects. Before beginning, each subject signed a consent form that conformed to these Northwestern University guidelines. Twenty-seven stroke survivors, aged 30–72 years (mean age 51), and four healthy controls, aged 32–61 years (mean age 47) volunteered to participate (Table 1). All stroke participants were in the chronic stage, having suffered a stroke 16–173 months prior to the experiment. Our exclusion criteria were: 1) bilateral impairment, 2) severe sensory deficits in the limb, 3) aphasia, cognitive impairment or affective dysfunction that would influence the ability to comprehend or to perform the experiment, 4) inability to provide an informed consent, and 5) other current severe medical problems.
Eight additional subjects were recruited whose data did not reach final analysis (not shown in Table 1). Of these, one chose to abort the experiment due to his own frustration (SA15); one healthy subject chose to quit because of time constraints (SA23); two healthy controls and three stroke patients had lost data due to technical problems with the robotic device or data collection (SA26, SA30, SA31, SA39, and SA 44); one stroke subject had such a poor elbow movement that she was not capable of completing the experiment (SA41).
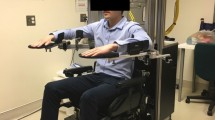
Subjects held the free-extremity (here referred to as the “endpoint”) of a DOF robot (Fig. 1) described elsewhere (Conditt et al. 1997; Scheidt et al. 2000). Endpoint forces and torques were monitored with a six-degree-of-freedom load cell fixed to the handle of the robot (Assurance Technologies Inc., model F/T Gamma 30/100). The robot was equipped with position encoders that were used to record the angular position of the two robotic joints with a resolution exceeding 20 arc/s of rotation (Teledyne Gurley, model 25/045-NB17-TA-PPA-QAR1S). The position, velocity and acceleration of the handle were derived from these two signals. Two torque motors were used to apply programmed forces to the hands of the subjects (PMI Motor Technologies, model JR24M4CH).
Subjects positioning and the experimental apparatus. Two brushed DC torque motors (PMI model JR24M4CH, Kolmorgen Motion Technologies, NY, USA) control forces at a handle via a 4-bar linkage. Rotational digital encoders (model 25/045-NB17-TA-PPA-QAR1S, Teledyne-Gurley, Troy, NY, USA) report absolute angular position, and a 6-axis force/torque sensor reports the interface kinetics (Assurance Technologies, Inc., TI F/T Gamma 30/10, NC, USA). A PC acquires the signals and controls torque
Subjects were seated so that the center of the range of targets — lying approximately at the center of their reachable workspace — was aligned with the shoulder, in the proximal-distal direction (y-axis) (Fig. 1, right). The experiment involved only the hemiparetic limbs of the stroke subjects, and this corresponded to nondominant limbs in 17 of the 27 subjects (See Table 1). Subjects were asked to reach visual targets so that they made a series of random 10 cm movements to the vertices of a triangle. If subjects had difficulty in reaching the vertices of the triangle, we adjusted their chair position. To avoid fatigue, their elbow and forearm rested on a lightweight frictionless linkage (Fig. 1, left), and they could choose to rest between movements (subjects rarely rested longer than a few seconds every hundred movements). We controlled for a peak speed of 0.288 m/s by giving subjects feedback at the end of each movement using colored dots and auditory tones to let subjects know if they were going too fast, too slow, or within a range of ±0.05 m/s. Consequently, subjects’ speeds remained roughly constant throughout the entire experiment.
All subjects performed a total of 834 movements (trials), broken down into the following experimental phases:
-
Unperturbed familiarization: 60 movements (approximately 5 min) to become familiar with the system and with the task of moving the manipulandum.
-
Unperturbed baseline: 30 movements (approximately 2 min) to establish a baseline pattern of reaching movements.
-
Learning: 372 movements (approximately 25 min) with constant exposure to the “curl” force field, governed by:
$$ {\textbf{F}} = {\left[ {\begin{array}{*{20}c} {0} & {\lambda } \\ {{ - \lambda }} & {0} \\ \end{array} } \right]}\,{\textbf{\ifmmode\expandafter\dot\else\expandafter\.\fi{x}}} $$where F is the vector of forces, \( {\textbf{\ifmmode\expandafter\dot\else\expandafter\.\fi{x}}} \) is the vector of instantaneous velocity, and λ is the gain. With this type of field, the forces are always orthogonal to the velocity of the hand and form either a clockwise or counterclockwise circulating pattern in the space of hand velocities. This phase of 372 trials was subdivided into a block of 30 trials (five in each direction) at the beginning and end of training for statistical analyses. After a second series of 240 trials, there was a rest period of approximately 1 min while data collection equipment was reset, followed by a block of 72 trials and then the final 30 trials (see Figs. 3 and 4).
During the learning phase, half of the subjects experienced λ = 20 N·s/m (corresponding to clockwise forces), and half experienced λ = −20 N·s/m (corresponding to counterclockwise forces) (c.f. Table 1).
Some of these subjects also served as their own controls as subjects in the zero-force group (Table 1). Prior to experiencing any force field, some visited the lab and performed the same experiment without any forces applied. These subjects were used to determine if practicing the motion alone (without any forces) led to a beneficial outcome.
-
After-effects: 240 movements (approximately 16 min), with random, intermittent removal of the force field for one in eight of the trials (catch trials) to determine the after-effects.
-
Training refresher: Twelve movements (approximately 1 min), identical to the learning Phase.
-
Washout: 120 movements (approximately 8 minutes), all without forces.
Subjects were also required to take breaks (approximately 1–2 min) after movements 307 and 650 so that our data collection equipment could be reset. The movements in each direction were divided equally in each phase. At all times during the experiment, an additional set of “background” torques was generated to remove the inertial effects of the robot arm linkage, resulting in the feeling of movement on a slippery surface when the force field was not present. Motion and force data were collected at 100 Hz.
Analysis
We restricted our focus in this study to the earliest parts of movements for two reasons. First, stroke survivors often make excessively large corrections later in their movements that may depend on earlier errors (Krebs et al. 1999a; Beer et al. 2000). Second, we were primarily interested in the early phase of the movement that best reflects the operation of a feedforward controller based on an internal model of the arm/environment dynamics. Our measure, the initial direction error, reflected this early phase of movement by forming a vector from the start point to 25% of the distance to the target (2.5 cm). This corresponded to approximately the first 200–300 ms of movement that, if there were no error corrections at the end of the movement, would last about 1.1 s. Positive error corresponded to a counterclockwise rotation from the actual trajectory to the desired trajectory, and zero corresponded to a straight-line to the target. Initial direction error was used for testing our hypotheses on the feedforward controller and also was found to be highly correlated with the perpendicular distance measure used in other adaptation studies (Conditt et al. 1997; Scheidt and Rymer 2000; Thoroughman and Shadmehr 2000).
To quantify adaptation, we established the Adaptation capacity, defined as the average shift in initial direction from unperturbed baseline trials to the after-effects catch trials. All hypotheses were tested using an alpha level of 0.05. We tested for a shift in initial direction from baseline to after-effects and for the tendency of the after-effects to disappear in the washout phase.
Results
We found that both the healthy and the stroke subjects demonstrated a clear ability to adapt when these subjects moved their hands in the force field. The force field significantly disturbed hand movement (Fig. 2b). Initial direction error did not significantly decrease after 330 movements of practice (Fig. 2; compare b to c). After-effects were evident when the forces were removed (Fig. 2d). These results were evident as a group as well (Fig. 3), with a significant shift in initial direction error from baseline to after-effects. As anticipated, we detected no significant change in any subjects’ movement speeds across the phases of the experiment, although peak speeds were slightly but significantly below target for stroke subjects (average of 0.218 m/s, P<0.05) but not for healthy subjects (average of 0.279 m/s). Speeds for some movements were as high as twice the target, resulting in peak forces in these extreme cases as high as 10 N. The maximum speeds 5th, 50th, 95th, and 99th percentiles for stroke patients were: 0.101, 0.201, 0.361, and 0.469 m/s, respectively, and for healthy they were 0.091, 0.255, 0.437, and 0.469 m/s, respectively.
Movement paths for a mildly impaired stroke subject in successive phase of the experiment. For clarity, the starting points of the triangle pattern in Fig. 1 were shifted to display all starting points at the center. The ideal trajectories are the bold dotted lines, the average trajectories are represented as bold solid lines, and individual trajectories are thin lines. The subject first performs without force disturbances (a), and then experiences a prolonged training (b and c). The training forces are then turned off intermittently in catch trials to test for after-effects (d). Finally, subject moves for 120 movements without forces, and the after-effects “wash out.” Results from the final 15 movements of the washout phase are shown in (e). Arrows indicate the movement direction that showed the largest error in the baseline trials. This movement error was amplified by the force field during training, but resulted in a reduction of error following training (d) that was sustained until the end of the experiment (e)
Group results for stroke survivors showing error between the actual trajectory and a straight-line movement. Each block of data along the horizontal axis represents a successive phases of the experiment. The training phase in which the force field was applied to the subject are indicated by the light shading in the background. Baseline is compared to after-effects to test our hypothesis that the method shifts trajectories toward the desired, resulting in a significant reduction in error. Dashed lines shaded areas and dashed lines represent 95% confidence intervals and means, respectively, for the group. Small symbols and vertical thin lines show the individual subject means and 95% confidence intervals. All phases of the experiment that underwent statistical analyses contained 30 trials (5 in each direction). A rest period (approximately 1–2 min) occurred during the learning phase after a second series of 240 trials while data collection equipment was reset
After training, the disturbance was unexpectedly removed and the initial direction error shifted significantly, exhibiting a clear after-effect of adaptation (Fig. 2d). Both stroke and healthy subjects showed a marked shift in their initial direction that was opposite to the direction seen when they were initially exposed to the force field (compare Figs. 3 and 4; compare phases 2–6). On average, the stroke survivors’ limb movements were initially perturbed by the same amount (Fig. 3, Phase 2) as the healthy controls (Fig. 4, Phase 2). In contrast, the after-effect of the stroke subjects was significant (Fig. 3, Phase 6) but it was also significantly smaller than the healthy subjects by about 26% (Fig. 4, Phase 6). All of the healthy subjects’ after-effects were significantly shifted from baseline, and while stroke subjects’ shifts were all in the direction one would expect as an after-effect, only 10 of the 18 had shifts that were statistically significant (P<0.05 in individual t tests). Stroke subjects as a group, however, showed a significant shift (P<0.05 in a paired t test of subject averages).
Note that stroke survivors’ errors during baseline appear similar to those of the healthy subjects (compare Figs. 3 and 4, Phase 1) because these figures display the average overall movement directions. Stroke survivors’ larger errors are revealed only by the larger error bars. Stroke subjects were also more variable within movement directions. Trial-to-trial standard deviations of the initial direction error during the baseline phases was nearly three times higher for the stroke subjects (the average of the stroke subject’s standard deviations was 16.2° compared to 5.8° for healthy; P<0.001). It is important to stress that the goal of this study was to merely test for adaptation and not to correct the trajectories with a specially designed force field. Therefore, some after-effects were in the wrong direction (i.e., the errors were amplified), and some were shifted beyond a straight path to the target.
The observed shift in direction from baseline (unperturbed) to after-effects (also unperturbed) is an excellent indication of motor adaptation. Absence of adaptation would result in a zero shift. In contrast, adaptation capacity, as quantified by the amount of this shift, was well above and significantly greater than zero for stroke survivors (in Fig. 5, the wings indicate a 95% confidence interval). Nevertheless, adaptation capacity was slightly but significantly larger for healthy subjects (Fig. 5, wings). We detected no difference in adaptation capacity between the subjects that received clockwise and the subjects that received counterclockwise forces. Stroke survivors’ changes in performance were not as large as that of the healthy subjects for the same amount of practice (compare Figs. 3 and 4), suggesting that longer training might also result in more persistent adaptation in patients.
a Adaptation capacity, in degrees, for the healthy and the stroke survivor groups, determined by calculating each subject’s average change in initial direction error from the baseline phase and the after-effects phase. Wings represent 95% confidence intervals of the group. Stroke survivors show a large capacity to adapt, but not as strong as the healthy group. b Correlation of the adaptation capacity (horizontal axes) with three clinical scores that were measured in this study. Each stroke survivor represents a dot
To further explore the possibility that individuals with stroke might have a decreased learning rate, we fit each subject’s learning phase data to a simple exponential model,
where A is an offset, B the amount of learning (the change of the trajectory errors), C the time constant for the error to decrease (inversely related to rate of learning), and t is the movement number. However, we found no significant differences in either the time constant or the amount of learning between the healthy subjects and individuals with stroke.
Adaptation capacity was not found to correlate well with the clinical measures of Elbow Modified Ashworth (r 2=0.0013876, P>0.8), Chedoke (r 2=2.7943e-006, P>0.9), and the upper extremity portion of the Fugl–Meyer (r 2=0.0040592, P>0.8) (Fig. 5b). Hence, it may be difficult to predict a person’s ability to adapt based on these clinical scores. They also fail to show any evidence that the severely impaired individuals lack the ability to adapt.
While Figs. 3 and 5 indicate a clear and positive answer to the question about whether stroke survivors can adapt, these data do not reveal how the training forces might restore function. In an initial attempt to shed some light on this question, our analysis program identified each subject’s directions of largest error — the directions that could be improved upon the most. During training, these movement errors were either magnified or reduced by the forces (depending on how the force field happened to be pushing for each subject and each direction). Movement directions of largest errors (e.g., movements indicated by the arrows in Fig. 2) were only selected if they had significant error to begin with. For each subject, our software selected and analyzed up to two movement directions. The first was the direction that showed the largest initial error that was reduced by the forces, while the second was the direction that showed the largest initial error that was amplified by the forces. If no significant error was present to begin with, the movement was not considered. To determine the amount of error amplification/reduction, we calculated the dot product between the average training force direction and the average movement error direction (horizontal axis on Fig. 6). Positive values of this dot product indicated that the training forces tended to magnify the error, while negative values indicated that the training forces tended to reduce the error.
Cross plot of the performance improvement versus error magnification caused by the force field for different movement directions on the stroke survivors. Performance improvement was calculated by measuring the reduction initial direction error from the baseline phase to the final phase of the experiment. Positive represents an improvement in performance. Error magnification was determined by calculating the dot product between the average training force direction and the average movement error direction. Positive values of this dot product indicated that the training forces tended to magnify the error, while negative values indicated that the training forces tended to reduce the error. Boxes with horizontal centerlines represent the mean and 95% confidence intervals of the three distinct groups: (1) the group in which the error was magnified during training (right), (2) the control group in which error was unchanged because no forces were applied (center), and (3) the group in which error was reduced during training (left). Vertical whiskers extending from the box plots indicate 2-standard deviations from the mean. The diagonal line represents linear least-squares regression fit of the data shown
By the end of the experiment (five final movements in each direction), after the forces had been turned off for 120 movements (final washout phase), we found that the relationship between error magnification and the improvement was significant (vertical axis on Fig. 6). Significant improvements occurred only when the training forces magnified the original errors, and not when the training forces reduced the errors or they were zero [F(1,13)=4.29, P<0.001]. Because three of the points were further than two standard deviations from the mean and appeared as outliers (Fig. 6, indicated by small horizontal arrows), we reran the analysis without them, but obtained the same results — error-amplification training resulted in significant improvement and error-reduction training resulted in significant detriment by the end of the experiment. In summary, by restricting our attention only to trials that had significant error to begin with, and then by separating movement directions into error-reducing or error-enhancing training, the evidence suggests that the error-enhancing forces may be more effective than the error-reducing forces for correcting the initial movement direction of the hand.
Discussion
This study provides new evidence that stroke survivors retain their ability to adapt their arm movements when they are exposed to an altered mechanical environment (a force field), although at a somewhat diminished level. This is evidenced by the Adaptation Capacity measure (Fig. 5). Moreover, for movement directions that begin with significant errors, significant improvement occurred only when the training forces magnified the original errors (Fig. 6).
With regard to the question of whether stroke survivors can adapt, this study confirms earlier studies conducted by others. Raasch and colleagues (Raasch et al. 1997) performed a preliminary study that provided encouraging evidence for adaptation in stroke survivors. In that study, a force was imposed during hand motion that pushed subjects’ hands away from the desired path with a magnitude proportional to hand speed, a protocol similar to that used in this study. A single-joint study by Dancausea and colleagues (Dancausea et al. 2002) demonstrated the ability of stroke survivors to adapt to a spring-like force. Their results were consistent with ours in that brain-injured individuals needed more trials to diminish errors and their movements were more variable.
Takahashi and Reinkensmeyer have also recently published new results on adaptation after stroke (Takahashi and Reinkensmeyer 2003). Although their study found that the ability to adapt was somewhat diminished in stroke survivors, they found that the ability to adapt was loosely correlated to clinical scores such as the Chedoke Mc-Master score. We found no such correlation in our group of subjects. Several important differences between our study and theirs may account for the discrepancies in our results. (1) Our study investigated mostly shorter movements. Therefore, a larger proportion of the movement we observed was likely controlled by early feedforward (and hence internal-model driven) components of control. Takahashi and Reinkensmeyer allowed the movement lengths to vary from subject to subject. Furthermore, in many cases the movements were close to the edge of the subjects’ achievable workspace. These longer and more distal movements may have been effected to a larger degree by factors such as contractures and spasticity that are unrelated to the presence or absence of internal models. We attempted to minimize these factors in our study by targeting the midregion of the arm’s workspace. (2) While Takahashi and Reinkensmeyer compared movement adaptation between the paretic and the nonparetic limb, we compare adaptation in paretic subjects to adaptation in healthy and age-matched controls. Indeed, individuals who have suffered a stroke do not perform as well with their unimpaired arm as age-matched healthy subjects, but they tend to show the same abilities to learn (Pohl and Winstein 1999). (3) Takahashi and Reinkensmeyer also did not support the arm against gravity. As those authors suggested, the multiaxis force generation constraints that come into play when generating large forces (perhaps even if those large forces are required in only one direction — against gravity), or the inability to produce force at a high enough rate, may limit adaptation in the most weakened subjects. Strength has been shown to be dramatically influenced by elevating the arm against gravity (Beer et al. 1999). The subjects in the present study did not have to overcome this large gravitational force requirement, and thus were operating in a wholly different region in force space. (4)Takahashi and Reinkensmeyer used a maximum force of 3.5 N, while our robotic forces were typically much stronger, reaching amplitudes as high as 15 N. (5) While our study used perturbing forces that depended upon hand velocity, Takahashi and Reinkensmeyer used a time-dependent force with a constant direction (to the side). Nevertheless, it is possible that their forces may have led to a similar adaptation as ours because when subjects adapt to a time-dependent force, they tend to build an internal representation that is not dependent on time (Conditt and Mussa-Ivaldi 1999). (6) Finally, the most provocative difference is that our protocol enabled us to look at how improvement in performance is influenced by the direction in which the training forces are acting. Although this study did not specifically intend to reduce or magnify error, our preliminary evidence suggests that error-magnifying forces may be most effective.
Our study also has some important limitations. First, the apparatus did not allow motion out of the horizontal plane, and the effects of gravity were minimized at all times by an arm support (Fig. 1). A key motor deficit seen in stroke is the inability of the nervous system to counteract gravity while still making targeted movements (Dewald and Beer 2001). An important extension of our approach would be to test adaptation in the context of 3D activities that require supporting the arm against gravity.
A second limitation is that the size and simplicity of the movements may not allow us to extend our conclusions to unconstrained and functionally relevant motions. Larger 3D motions, consistent with the activities of daily living would likely be more relevant to the recovery of the most important motor functions. We expect that these shortcomings will be addressed by using stronger full-dimensional robotic systems combined with more advanced visual display. The present study also uses some subjects as their own controls. While we did not detect any difference in these subjects, their increased exposure to the device might have biased the data. Yet another potential problem was that it was impossible to keep the three groups of subjects fully balanced in all ways. For example, it is possible that the clockwise force group had subjects with slightly higher spasticity (see Table 1).
Another limitation is that we did not control for the resting time. In order to prevent fatigue, intimidation, and attentional loss due to boredom, subjects were free to take rests. It remains to be seen whether rests may play a critical part in the the adaptive process.
An additional limitation to this study may be that the focus was limited to straightness of the hand path. Making straighter and smoother movements need not to be the only goal or the principal goal of a therapy. Optimal functional recovery for these individuals may be something other than healthy-looking movements.
Related to this issue is the breakdown of predictive feedforward control and corrective feedback control. While our measure, initial direction error, does a good job of characterizing the path errors early in each movement (first 25% of the distance to target), it does not directly address the time interval typically associated with human feedforward control and the launching of a movement. The time that the subjects crossed the 25% mark was 0.17±0.04 s (average ± standard deviation), which was significantly different for the healthy subjects at 0.14±0.03 s (two-tailed t test, P<0.05). While these values are still within the range of feedforward control that occurs prior to supraspinal-feedback corrections (0.12 – 0.18 s) (Schmidt 1988), they do not rule out elements of spinal reflex feedback that can be as fast as 0.03 s (Dewhurst 1967). Therefore, initial direction error should not be fully interpreted as a direct measure of feedforward control error.
The most important limitation of this study is that only a proper prolonged clinical research study with separate groups of stroke survivors (one group receiving prolonged error-enhancing forces, one group receiving error-reducing forces and one group receiving no forces) is the only appropriate method for evaluating error-enhancement. What is presented here is only compelling preliminary evidence supporting such a strategy.
An issue that was not addressed by this study is the likely relation between the neural structures damaged by the stroke and the adaptive performance. Lesion site information was not available for all subjects (Table 1), and in this initial study the data are still too sparse to draw conclusions on the location, extent, and severity of the strokes and how they may have influenced each individual’s results. Adaptability may also be influenced by other factors such as lesion hemisphere, the time since stroke, and the type and dosage of rehabilitation that the subjects received. More data are constantly being acquired, but it may take large numbers of subjects before these factors show any effect. Nevertheless, adaptation was statistically significant in 10 of the 18 individuals with stroke that we tested.
While forces that amplified error appeared to benefit, forces that reduced error led to the opposite — after-effects that increased the initial direction error (Fig. 6). Hence, errors can be decreased or increased with the right transient perturbation. It could be that the nervous system is simply less concerned with the movement straightness following stroke. Indeed, Krebs and (Krebs et al. 1999a) colleagues have shown that stroke subjects exhibit jerky and multisegmented movements that tend to coalesce into smoother, straighter movements as recovery progresses.
It is also important to speculate on the mechanisms that might allow a stroke subject to decrease directional errors with this paradigm that cannot decrease by simple practice alone. Sensory feedback systems may need to detect a stimulus with a magnitude that is large enough to trigger the recovery process. Such distorting interventions that trigger recovery have been shown to be promising in individuals with stroke that suffer from hemispatial neglect (Rossetti et al. 1998). Smaller errors may be imperceptible or considered less important than other aspects of the movement such as getting to the target, conserving energy, or minimizing discomfort. Another possibility is that the nervous system is trying to use motor pathways that are no longer intact, and the learning is a way to trick the nervous system into trying a new and nonintuitive pathway that it would otherwise not ever consider. Such questions will require further study using imaging, transcranial magnetic stimulation, or implants.
A final question not answered in this study is whether beneficial after-effects persist beyond the final 120 movements in which the forces were absent. Preliminary studies in our laboratory on stroke survivors have revealed that after-effects persist when these resemble normal, unperturbed movements (Raasch et al. 1997). In fact, after-effects may become permanent if they are perceived by the subject to be an improvement with respect to the initial behavior. However, not all patients may benefit from this type of procedure. Subjects who show poor ability to adapt, such as cerebellar stroke survivors, may have great difficulty dealing with resistive techniques (Weiner et al. 1983; Sanes et al. 1990; Bastian et al. 1996). Moreover, chronic stroke survivors suffering from long-term changes in their muscle systems (i.e., atrophy and tissue shortening) also may not benefit from such techniques.
While our force fields shifted the central tendencies of the subjects, they did nothing to change motor variability, which is known to be larger in stroke survivors (Fisk and Goodale 1988). Our study revealed that stroke survivors were more variable both within trials, from movement to movement, and across subjects. It is logical that the nervous system would naturally reduce its learning rate when sensory or motor inconsistencies and uncertainties caused by stroke make it difficult to form an exact internal model. Such is the case in artificial systems that learn (Rumelhart and McClelland 1986). Nevertheless, several studies suggest that increasing trial-to-trial variability with externally applied forces does not impair motor learning rate in healthy subjects (Scheidt et al. 2001; Takahashi et al. 2001; Reinkensmeyer et al. 2003). It remains to be seen whether stroke subjects tend to learn more slowly because they are more variable or for some other reason.
Nevertheless, several other studies agree with our results that error augmentation leads to enhanced learning. Learning how to counteract a force disturbance in a in a walking study increased by approximately 26% when the field was transiently amplified (Emken and Reinkensmeyer 2005). Artificially giving smaller feedback on force production has caused subjects to apply larger forces to compensate (Brewer et al. 2005). Several studies have shown how the nervous system can be “tricked” by giving altered sensory feedback (Flanagan and Rao 1995; Srinivasan and LaMotte 1995; Robles-De-La-Torre and Hayward 2001; Ernst and Banks 2002; Sainburg et al. 2003; Brewer et al. 2004; Kording and Wolpert 2004a). However, augmented feedback on practice conditions has not always proven therapeutically beneficial in stroke (Winstein et al. 1999). It may be that there are limits to the amount of error augmentation that is useful (Kording and Wolpert 2004b; Wei et al. 2005).
The implications of successful implicit learning are that one can learn at a nearly subconscious level with minimal attention and with less motivation than more explicit types of practice, like pattern tracing. Training typically requires a balance of repetitive practice, strengthening, expert guidance, and appropriate feedback. We believe that the type of implicit learning demonstrated in this study — one that augments errors — may provide an excellent rehabilitation tool to enhance performance. In fact, explicit information has been shown to disrupt the acquisition of motor skills in participants with stroke but not in healthy controls (Boyd and Winstein 2003; Boyd and Winstein 2004).
One might suggest that the changes seen in stroke patients are due to a reduction in tone (spasticity). Evidence has suggested that repeated muscle stretches may temporarily reduce spastic hypertonia (Schmit et al. 2000) (although we are not aware of this being shown during voluntary movement). Even though a reduction in tone cannot directly explain a benefit from error-augmenting forces (they would reduce the amount of stretch to any muscle, not increase it), one possibility exists where the result of Schmit and colleagues might apply. Since spasticity is velocity-dependent and higher velocities occur well after a movement has been launched, anticipating a spastic muscle action might cause a person to learn to precompensate with a shift early in the movement so that the spastic response carries the limb to the target. For example, a person might launch their outward movements more laterally in anticipation of a later biceps spasm. In this case, exposure to error-augmenting forces would further stretch and increase the excitation of the muscle. Repeating such activity might reduce the spastic hypertonia as was seen in isometric elbow flexors (Schmit et al. 2000) once the forces stop, making it easier for the subject to aim more directly at the target. However, this explanation only works for some of the outcomes of our study. Subjects that deviate in the opposite direction to this example should have had increased initial direction error as an after-effect, which was not the case. Furthermore, errors during planar movements after stroke are believed to be consistent with a lack of feedforward compensation for interaction torques, not an expression of spastic stretch reflexes (Beer et al. 2000). Hence a “stretching explanation” appears to be unlikely for our results, although it remains to be seen whether spastic reflexes can be reduced for muscle stretches that take place during repeated voluntary movements.
The results of this study have possible implications in rehabilitation because a properly induced adaptive process might be exploited to assist in the restoration of function. Robotic devices, combined with sophisticated and precise computer programs, provide new possibilities for improving and accelerating recovery. One can envision the possibility to custom-designed subject-specific force fields generated by a model of the patient’s biomechanics and motor impairments (Mussa-Ivaldi and Patton 2000; Patton and Mussa-Ivaldi 2001; Patton and Mussa-Ivaldi 2003). Preliminary testing of this approach has proved successful in some stroke survivors (Patton et al. 2001a). Other objectives such as extending the range of motion would require other approaches. What is clear is that opportunities for recovery after stroke are possible by extending intensive therapy beyond present inpatient rehabilitation stays, and robotic therapy may be one way to economically accomplish this (Fasoli et al. 2004). While error-enhancing forces are useful for inducing adaptive responses, they are likely to be most effective if combined with other rehabilitation strategies.
Studies using robotics for rehabilitation, assessment, and training have had some success (Krebs et al. 1998a; Lum et al. 1999). The many paradigms associated with adaptive training may add to the repertoire of possible strategies for rehabilitation. In the search for the most optimal method of training among the many possibilities, this preliminary encouraging evidence on error-augmentation points to future studies that exploit the natural adaptive tendencies in the nervous system for restoring function.
References
Bastian AJ, Martin TA, Keating JG, Thach WT (1996) Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol 76:492–509
Beer RF, Dewald JPA, Rymer WZ (2000) Deficits in the coordination of multijoint arm movements in patients with hemiparesis. Exp Brain Res 131:305–319
Beer RF, Given JD, Dewald JPA (1999) Task-dependent weakness at the elbow in patients with hemiparesis. Arch Phys Med Rehabil 80:766–772
Bhushan N, Shadmehr R (1999) Computational nature of human adaptive control during learning of reaching movements in force fields. Biol Cybern 81:39–60
Bobath B (1978) Adult hemiplegia: evaluation and treatment. Heinemann, London
Bock O (1990) Load compensation in human goal-directed arm movements. Behav Brain Res 41:167–177
Boyd LA, Winstein CJ (2001) Implicit motor-sequence learning in humans following unilateral stroke: the impact of practice and explicit knowledge. Neurosci Lett 298:65–69
Boyd LA, Winstein CJ (2003) Impact of explicit information on implicit motor-sequence learning following middle cerebral artery stroke. Phys Ther 83:976–989
Boyd LA, Winstein CJ (2004) Providing explicit information disrupts implicit motor learning after basal ganglia stroke. Learn Mem 11:388–396
Brewer B, Klatky R, Matsuoka Y (2005) Perceptual limits for a robotic rehabilitation environment using visual feedback distortion. IEEE Trans Neural Sys Rehabil Eng in press
Brewer BR, Klatzky R, Matsuoka Y (2004) Effects of visual feedback distortion for the elderly and the motor-impaired in a robotic rehabilitation environment. In: IEEE International conference on robotics and automation (ICRA), New Orleans
Burgar C, Lum P, Shor P, Van der Loos H (2000) Development of robots for rehabilitation therapy: the Palo Alto VA/Stanford experience. J Rehabil Res Dev 376:663–673
Conditt MA, Gandolfo F, Mussa-Ivaldi FA (1997) The motor system does not learn the dynamics of the arm by rote memorization of past experience. J Neurophysiol 78:554–560
Conditt MA, Mussa-Ivaldi FA (1999) Central representation of time during motor learning. Proc Nat Acad Sci 96:11625–11630
Dancausea N, Ptitob A, Levin MF (2002) Error correction strategies for motor behavior after unilateral brain damage: short-term motor learning processes. Neuropsychologia 40:1313–1323
Delisa JA, Gans BM (eds) (1993) Rehabilitation medicine. J. B. Lippincott Company, Philadelphia
Dewald J, Beer R (2001) Evidence for abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve 24:273–283
Dewhurst DJ (1967) Neuromuscular control system. IEEE Trans Biomed Eng 14:167–171
Emken JL, Reinkensmeyer DJ (2005) Robot-enhanced motor learning: accelerating internal model formation during locomotion by transient dynamic amplification. IEEE Trans Neural Syst Rehabil Eng in press
Ernst M, Banks M (2002) Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415:429–433
Fasoli SE, Krebs HI, Ferraro M, Hogan N, Volpe BT (2004) Does shorter rehabilitation limit potential recovery poststroke? Neurorehabil Neural Repair 18:88–94
Fisk JD, Goodale MA (1988) The effects of unilateral brain damage on visually guided reaching: hemispheric differences in the nature of the deficit. Exp Brain Res 72:425–435
Fitts PM (1964) Perceptual-motor skill learning. In: MeHou AW (ed) Categories of human learning. Academic, New York, pp 243–245
Flanagan JR, Rao AK (1995) Trajectory adaptation to a nonlinear visuomotor transformation: evidence of motion planning in visually perceived space. J Neurophysiol 74:2174–2178
Flash T, Gurevitch F (1992) Arm movement and stiffness adaptation to external loads. In: Proceedings of the 13th IEEE engineering in medicine and biology conference, vol 13. Orlando, pp 885–886
Gandolfo F, Mussa-Ivaldi FA, Bizzi E (1996) Motor learning by field approximation. Proc Nat Acad Sci USA 93:3843–3846
Ghez (1991) The control of movement. In: Kandel ER, Scwartz JH, Jessel TM (eds) Principles of neural science. Appleton & Lange, Nor wolk, pp 533–547
Gomez Beldarrain M, Grafman J, Pascual-Leone A, Garcia-Monco JC (1999) Procedural learning is impaired in patients with prefrontal lesions. Neurology 52:1853–1860
Held R, Freedman SJ (1963) Plasticity in Human Sensorimotor Control. Science 142:455–461
Hemami H, Stokes BT (1982) Qualitative discussion of mechanisms of feedback and feedforward control of locomotion. IEEE Trans Biomed Eng 30:163–189
Kahn LE, Zygman ML, Rymer WZ, Reinkensmeyer DJ (2001) Effect of robot-assisted and enassisted exercise on functional reaching in chronic hemiparesis. In: IEEE engineering in medicine and biology society (EMBS) conference. IEEE, Istanbul, pp 1344–1347
Kawato M (1990) Feedback-error-learning neural network for supervised learning. In: Eckmiller R (ed) Advanced neural computers. North-Holland, Amsterdam, pp 365–372
Kawato M, Wolpert D (1998) Internal models for motor control. Novartis Foundation Symposium 218:291–304
Kording KP, Wolpert DM (2004a) Bayesian integration in sensorimotor learning. Nature 427:244–247
Kording KP, Wolpert DM (2004b) The loss function of sensorimotor learning. Proc Natl Acad Sci USA 101:9839–9842
Krakauer JW, Ghilardi MF, Ghez C (1999) Independent learning of internal models for kinematic and dynamic control of reaching. Nat Neurosci 2:1026–1031
Krebs HI, Aisen ML, Volpe BT, Hogan N (1999a) Quantization of continuous arm movements in humans with brain injury. Proc Nat Acad Sci USA 96:4645–4649
Krebs HI, Brashers-Krug T, Rauch SL, Savage CR, Hogan N, Rubin RH, Fischman AJ, Alpert NM (1998a) Robot-aided functional imaging: application to a motor learning study. Hum Brain Mapp 6:59–72
Krebs HI, Hogan N, Aisen ML, Volpe BT (1998b) Robot-aided neurorehabilitation. IEEE Trans Rehabil Eng 6:75–87
Krebs HI, Hogan N, Hening W, Adamovich SV, Poizner H (2001) Procedural motor learning in Parkinson’s disease. Exp Brain Res 141:425–437
Krebs HI, Hogan N, Volpe BT, Aisen ML, Edelstein L, Diels C (1999b) Robot-aided Neuro-rehabilitation in Stroke: Three Year follow-up. In: Sixth international conference of rehabilitation robotics, Stanford
Krebs HI, Volpe BT, Aisen ML, Hogan N (2000) Increasing productivity and quality of care: robot-aided neuro-rehabilitation. J Rehabil Res Dev 37:639–652
Lackner JR, DiZio P (1994) Rapid adaptation to Coriolis force perturbations of arm trajectories. J Neurophysiol 72:299–313
Lisberger S (1988) The neural basis for the learning of simple motor skills. Science 242:728–735
Lum P, Burgar C, Shor P, Majmundar M, Van der Loos M (2002) Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper limb motor function following stroke. Arch Phys Med Rehabil 83:952–959
Lum PS, Burgar CG, Kenney DE, Van der Loos HF (1999) Quantification of force abnormalities during passive and active-assisted upper-limb reaching movements in post-stroke hemiparesis. IEEE Trans Biomed Eng 46:652–662
Miall RC, Weir DJ, Wolpert DM, Stein JF (1993) Is the cerebellum a Smith Predictor? J Motor Behav 25:203–216
Mussa-Ivaldi FA, Patton JL (2000) Robots can teach people how to move their arm. In: IEEE international conference on robotics and automation (ICRA), San Francisco
Patton J, Mussa-Ivaldi F (eds) (2001) Robotic teaching by exploiting the nervous system’s adaptive mechanisms. IOS, Amsterdam
Patton J, Mussa-Ivaldi F, Rymer W (2001a) Altering Movement Patterns in Healthy and Brain-Injured Subjects Via Custom Designed Robotic Forces. In: IEEE-EMBC2001, the 23rd annual international conference of the ieee engineering in medicine and biology society (EMBS), Istanbul
Patton J, Mussa-Ivaldi F, Rymer W (2001b) Robotic-induced improvement of movements in hemiparetics via an implicit learning technique. Society for Neuroscience, San Diego
Patton JL, Mussa-Ivaldi FA (2003) Robot-assisted adaptive training: custom force fields for teaching movement patterns. IEEE Trans Biomed Eng (in press)
Pine ZM, Krakauer JW, Gordon J, Ghez C (1996) Learning of scaling factors and reference axes for reaching movements. Neuroreport 7:2357–2361
Pohl PS, Winstein CJ (1999) Practice effects on the less-affected upper extremity after stroke. Arch Phys Med Rehabil 80:668–675
Raasch CC, Mussa-Ivaldi FA, Rymer WZ (1997) Motor Learning in reaching movements by hemiparetic subjects. Soc Neurosci Abstr 924.17:2374
Reinkensmeyer DJ, Iobbi MG, Kahn LE, Kamper DG, Takahashi CD (2003) Modeling reaching impairment after stroke using a population vector model of movement control that incorporates neural firing-rate variability. Neural Comput 15:2619–2642
Robles-De-La-Torre G, Hayward V (2001) Force can overcome object geometry in the perception of shape through active touch. Nature 412:445–448
Rossetti Y, Rode G, Pisella L, Farne A, Li L, Boisson D, Perenin MT (1998) Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature 395:166–169
Rumelhart DE, Hinton GE, Williams RJ (1986) Learning representations by back-propagating errors. Nature (London) 323:533–536
Rumelhart DE, McClelland JL (eds) (1986) Parallel distributed processing: explorations in the microstructure of cognition. MIT, Cambridge
Sainburg RL, Lateiner JE, Latash ML, Bagesteiro LB (2003) Effects of altering initial position on movement direction and extent. J Neurophysiol 89:401–415
Sanes JN, Dimitrov B, Hallett M (1990) Motor learning in patients with cerebellar dysfunction. Brain 113:103–120
Scheidt RA, Dingwell JB, Mussa-Ivaldi FA (2001) Learning to move amid uncertainty. J Neurophysiol 86:971–985
Scheidt RA, Reinkensmeyer DJ, Conditt MA, Rymer WZ, Mussa-Ivaldi FA (2000) Persistence of motor adaptation during constrained, multi-joint, arm movements. J Neurophysiol 84:853–862
Scheidt RA, Rymer WZ (2000) Control Strategies for the Transition From Multijoint to Single-Joint Arm Movements Studied Using a Simple Mechanical Constraint. J Neurophysiol 83:1–12
Schmidt RA (1988) Motor control and learning. Human kinetics Publishers, Champaign
Schmit BD, Dewald JP, Rymer WZ (2000) Stretch reflex adaptation in elbow flexors during repeated passive movements in unilateral brain-injured patients. Arch Phys Med Rehabil 81:269–278
Seidler RD, Purushotham A, Kim SG, Ugurbil K, Willingham D, Ashe J (2002) Cerebellum activation associated with performance change but not motor learning.[see comment]. Science 296:2043–2046
Shadmehr R, Mussa-Ivaldi FA (1994) Adaptive representation of dynamics during learning of a motor task. J Neurosci 14:3208–3224
Squire LR (1986) Mechanisms of memory. Science 232:1612–1619
Srinivasan MA, LaMotte RH (1995) Tactual discrimination of softness. In: J Neurophysiol, vol 73, pp 88–101
Stein J, Krebs HI, Frontera WR, Fasoli SE, Hughes R, Hogan N (2004) Comparison of two techniques of robot-aided upper limb exercise training after stroke. Am J Phys Med Rehabil 83:720–728
Takahashi CD, Scheidt RA, Reinkensmeyer DJ (2001) Impedance control and internal model formation when reaching in a randomly varying dynamical environment. J Neurophysiol 86:1047–1051
Takahashi CG, Reinkensmeyer DJ (2003) Hemiparetic stroke impairs anticipatory control of arm movement. Exp Brain Res 149:131–140
Taub E (2000) Constraint-induced movement therapy and massed practice [letter; comment]. Stroke 31:986–988
Taub E, Miller N, Novack T, Cook Ed, Fleming W, Nepomuceno C, Connell J, Crago J (1993) Technique to improve chronic motor deficit after stroke. Arch phys med rehabil 74:347–354
Taub E, Uswatte G, Pidikiti R (1999) Constraint-Induced Movement Therapy: a new family of techniques with broad application to physical rehabilitation–a clinical review. [see comments]. J Rehabil Res Dev 36:237–251
Thoroughman KA, Shadmehr R (2000) Learning of action through adaptive combination of motor primitives. [see comments]. Nature 407:742–747
Volpe B, Krebs H, Hogan N, Edelstein L, Diels M, Aisen M (2000) A novel approach to stroke rehabilitation; robot-aided sensorimotor stimulation. Neurology 54:1938–1944
Volpe BT, Krebs HI, Hogan N (2001) Is robot-aided sensorimotor training in stroke rehabilitation a realistic option? Curr Opin Neurol 14:745–752
Volpe BT, Krebs HI, Hogan N, Edelsteinn L, Diels CM, Aisen ML (1999) Robot training enhanced motor outcome in patients with stroke maintained over 3 years. Neurology 53:1874–1876
Voss DR, Ionta MK, Myers BJ (1985) Proprioceptive neuromuscular facilitation. Harper and Row, Philadelphia
Wei Y, Bajaj P, Scheidt RA, Patton JL (2005) A real-time haptic/graphic demonstration of how error augmentation can enhance learning. In: IEEE international conference on robotics and automation (ICRA), Barcelona
Weiner MJ, Hallett M, Funkenstein HH (1983) Adaptation to lateral displacement of vision in patients with lesions of the central nervous system. Neurology 33:766–772
Winstein CJ, Merians AS, Sullivan KJ (1999) Motor learning after unilateral brain damage. Neuropsychologia 37:975–987
Wolf SL, Lecraw DE, Barton LA, Jann BB (1989) Forced use of hemiplegic upper extremities to reverse the effect of learned nonuse among stroke and head-injured patients. Exp Neurol 104:125–132
Acknowledgements
Supported by AHA 0330411Z, NIH 1 R24 HD39627-01, NINDS R01 NS35673.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patton, J.L., Stoykov, M.E., Kovic, M. et al. Evaluation of robotic training forces that either enhance or reduce error in chronic hemiparetic stroke survivors. Exp Brain Res 168, 368–383 (2006). https://doi.org/10.1007/s00221-005-0097-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0097-8