Abstract
The goal of the present study was to compare prehension movements of the dominant and the non-dominant hand. Twenty right-handed volunteers (age 20–30 years) reached forward to grasp a cylindrical object, which was lifted and then placed into a target position in a retraction–insertion movement. The movements were performed at three different velocities (normal, deliberately fast, or slowly) both, under visual control, and in a no-vision condition. Analysis of the kinematic data revealed that the speed of hand transport influenced pre-shaping of both hands in a similar way. In the visual condition, the grip aperture increased about linearly with peak transport velocity, while it increased non-linearly with shorter movement duration. Comparison of the regression parameters showed that these relationships were nearly identical for both hands. The dominant hand was faster in inserting the object into the target position. Otherwise, no significant inter-manual differences were found. During prehension without visual control, the fingers opened more and movement duration was prolonged. Except for a larger grip aperture of the dominant hand at the end of the acceleration phase, the kinematic data of both hands were again comparable. This invariance was in contrast to performance in fine motor skills such as a pegboard test and drawing movements, where there was a clear advantage of the dominant hand. The similar pre-shaping of both hands during prehension is discussed with regard to a common motor representation of grasping.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reaching out to grasp an object includes two different motor components, hand transport and grip formation. The transport component (reaching), which brings the hand to the target, involves contractions of mainly proximal muscles acting on the shoulder and elbow joints. Grip formation (pre-shaping) adapts the fingers‘ configuration according to the size and shape of the target object which the hand is approaching. It has been hypothesized that both components, transport and grip formation, are controlled via distinct visuomotor channels, whose motor commands are coordinated to ensure efficient prehension (Jeannerod 1986; Jeannerod et al. 1995). Experiments in monkeys have demonstrated that the visuomotor transformation of an object’s size, shape and orientation into a specific configuration of the fingers is accomplished by a neuronal circuit that is formed by the inferior parietal lobule and the ventral premotor cortex, which projects to the primary motor cortex that steers the differentiated finger movements via cortico-motoneuronal fibers (Lemon 1993; Rizzolatti and Luppino 2001). Brain imaging research suggests that the neuronal organization in humans is similar (Binkofski et al. 1998). Kinematic studies of prehension have furthermore shown that the pre-shaping of the hand is not only influenced by the object’s intrinsic properties, but also by other factors such as the presence or absence of visual feedback, the speed of hand transport, and possibly also object distance and position (Chieffi and Gentilucci 1993; Paulignan et al. 1997). The aperture of the grasping hand is higher in conditions which impede the accuracy of reaching, such as fast movements (Wing et al. 1986; Wallace and Weeks 1988), and movements in the dark (Jakobson and Goodale 1991; Churchill et al. 2000; Schettino et al. 2003). Developmental studies have demonstrated that young children open their hands relatively wider than older ones (Kuhtz-Buschbeck et al. 1998, Smyth et al. 2004). Also anesthesia of the fingers leads to a wider grip aperture during prehension (Gentilucci et al. 1997).
Most of these neuroimaging and kinematic studies have so far been focused on reach-to-grasp movements of the dominant hand. Yet, handedness is a basic feature of the human motor behavior. Approximately 90% of humans are right-handers, who usually achieve better results with their dominant than with their left hand (Corballis 1997). The superior performance of the dominant hand becomes most evident in precision motor tasks such as handwriting or dextrous manipulation (Annett 1992). In many bi-manual activities of daily life, the non-dominant hand has a holding or stabilizing function while the dominant hand manipulates the object (e.g. when picking coins out of a purse). When small objects are lifted and held in a precision grip, the grip force of the non-dominant hand is higher than the precision grip force of the dominant hand, which is scaled with a narrower safety margin against slip (Kuhtz-Buschbeck et al. 2000, their Fig. 2).
In several recent studies, Sainburg and co-workers compared reaching movements of the dominant and non-dominant arm (Sainburg and Kalakanis 2000; Sainburg 2002; Bagesteiro and Sainburg 2002; Sainburg and Schaefer 2004). They found considerable interlimb differences in the coordination of muscle and intersegmental torques and concluded that main factor distinguishing dominant from non-dominant arm performance the facility governing the control of limb dynamics (Sainburg 2002). However, few studies have compared grasping kinematics of both hands during prehension movements. Castiello et al (1993, 1997) reported that right (RH) and left hand (LH) prehension movements have a similar temporal structure. Smeets and Brenner (2001) found somewhat more variable movements of the non-dominant hand in seven subjects, who reached out to grasp disks with a rather unusual hand posture. We are not aware of a kinematic study which compared grip formation of the LH and RH under different temporal constraints. One could expect that a less dextrous performance of the LH would manifest itself with a higher grip aperture, especially during fast movements (speed-accuracy trade-off). In the present study, right-handed volunteers reached out with three different velocities to grasp cylindrical objects: moving with natural speed, deliberately fast, or slowly. The objects were inserted into a target position. Prehension trials were performed with visual control and in a no-vision condition. The results, however, did not reveal significant differences of pre-shaping between the LH and RH in the visual condition. In the no-vision condition, the RH grip aperture was somewhat larger than the LH aperture at the end of the acceleration phase. Otherwise, all kinematic parameters of the reach-to-grasp were comparable. The uniform kinematic pattern is discussed in relation to an effector-independent level of the motor representation of grasping.
Methods
Twenty healthy volunteers (10 women, 10 men) aged 20–30 years (mean age, 23.7 years) took part in the present study. All were right-handed, and none of them had a previous history of any neurological disorder. All gave informed consent before the experiments, which had been approved by the Ethics Committee of the Medical Faculty of Kiel University (Germany). Three tests were used to describe the handedness of the subjects. Firstly, we used a questionnaire (Annett 1970) with 15 questions about hand preference (e.g. writing, throwing, striking a match). Secondly, dexterity of both hands was examined with the Purdue Pegboard (Lafayette Co, Lafayette, IN, USA). The subjects placed small pegs (diameter 3 mm, length 25 mm) into board holes in three 30 s test sessions per hand as fast as possible, and the number of pegs was averaged from these trials. Thirdly, a “square marking” test was applied. As one of the paper tests described by Annett (1992), this procedure is known to show clear intermanual differences. Following a short practice session, the squares (6.3 mm edge length) of a grid, printed on paper, were marked with crosses as fast as possible with a pencil for 30 s. The laterality index I was calculated from the numbers of crosses or pegs reached by either hand (R, right; L, left) with the formula I = [(R-L)/(R+L)] ·100.
Experimental procedure
The left and right arm lengths (distance between acromion and styloid process of the radius) were measured to adjust the experimental set-up for each subject. We also measured the maximum finger span as the distance between the pads of the thumb and index finger of the spread hand. The arm lengths (55.5±3.3 cm) and finger spans (12.5±1.4 cm) of both upper limbs were alike. The volunteers sat in an adjustable chair, facing a dark table surface (100×70 cm). At the onset of each trial, the hand and half of the forearm rested on the table in front of the trunk in a semiprone position (Fig. 1a). Thumb and index finger lightly touched a small knob which marked the starting point on the table. The object was a white upright plastic cylinder (ø 15 mm, height 40 mm, weight 12 g) with a small footplate (ø 25 mm, 4 mm high) standing in a holder. The distance between starting point and object was 60% of the arm length. Upon a short acoustic start signal (beep), the subjects reached out to grasp the object in a forward prehension movement (Fig. 1b–d). They lifted it from the holder and carried it to a second holder in a backward retraction movement (Fig. 1e–g). There they inserted the object’s footplate into the circular opening (diameter 25.5 mm, depth 4 mm) of the second holder, and released the cylinder (Fig. 1h, i). No effort was needed for the insertion. The distance between the first and the second holder was 40% of the arm length (=distance of retraction movement).
Prehension and retraction–insertion movements. The subject reaches forward to grasp and lift a cylindrical object out of a holder (a–d). The cylinder is carried to a second holder in a retraction movement (e–g) and its footplate is inserted into the holder’s circular opening (g–h). The inset in G shows the object at this opening with higher magnification
Within each subject, we varied the three factors hand (LH/RH), visual condition (with/ without vision), and movement velocity (slow, normal, fast). LH and RH movements were examined in two consecutive sessions. Each session consisted of two blocks; one block for the visual condition and another block for the no-vision condition. In the visual condition, the hand, object, and set-up were visible all the time. In the no-vision condition, the light was extinguished simultaneously with the start signal, so that the forward prehension and the retraction–insertion movements had to be performed in complete darkness. Each block consisted of three runs (12–14 trials per run), each of which was performed according to one instruction concerning movement velocity. In the normal runs, the volunteers were allowed to reach and grasp with their self-chosen natural speed. They should deliberately move faster or, respectively, more slowly, in the other runs. Prior to each session, a pre-training was performed for the slow and fast movements: Sounds of appropriate length, generated by a pulse generator (Master-8; AMPI, Jerusalem, Israel), indicated the desired movement time of the reach-to-grasp (fast trials: 500 ms; slow trials: 1500 ms). These time intervals were chosen according to previously published data (Kuhtz-Buschbeck et al. 1998). The subjects practised about six trials per velocity with these sounds as a guideline, which, however, were not audible during the actual experiments. The order of the different conditions (hand, vision, velocity) were counterbalanced across subjects. Yet, within each subject, the order was the same for the LH and RH.
Kinematic data recording and analysis
An optoelectronic motion analysis system (MacReflex; Qualisys, Goeteborg, Sweden) with a sampling frequency of 50 Hz was used, consisting of three cameras equipped with infra-red light emitting diodes (IRED) and videoprocessors. The light was reflected by three light-weight half-spherical markers (diameter 7 mm), which were attached to the wrist above the styloid process of the radius, and onto the nails of the thumb and index finger. The three-dimensional coordinates of the marker centroids were recorded and transferred to a PC for the interactive evaluation and calculation of kinematic parameters. Two other IREDs were connected to the pulse generator and to two photosensors which were concealed in the holders. These IREDs indicated: the start signal; the end of the reach-to-grasp, when the object was lifted by more than 0.5 mm from the first holder (Fig. 1d); and the end of the retraction–insertion, when its footplate was placed into the opening of the second holder (Fig. 1h). Methodical details have been published previously (Kuhtz-Buschbeck et al. 1998).
Reach-to-grasp movement
The reaction time was defined from the start signal until movement onset (Fig. 2, line 2), when the velocity of the wrist exceeded 2 cm/s to increase thereafter. Movement duration of the reach-to-grasp started from this moment and finished when the object was lifted (Fig. 2, line 3). Transport parameters extracted from the wrist position data comprised: mean and peak (V max) hand velocity, the absolute and relative (% of movement duration) times of V max and of peak deceleration, the length of the movement trajectory, and the maximum height of the wrist above baseline (=starting) level. Grip formation was studied by measuring the distance between the thumb and index finger markers. The early grip aperture (c in Fig. 2) was determined at V max, which is after about 40% of the movement duration. The maximum grip aperture (d in Fig. 2) was attained later, during the deceleration phase. We also calculated the absolute and relative timing of this event.
Hand velocity and grip aperture profiles. Vertical lines indicate the start signal (1), movement onset (2), the end of the reach-to-grasp (3) when the object is lifted, the end of the retraction phase (4), the insertion of the object into target position (5). RT, reaction time; MD, movement duration; RP, retraction phase; IP, insertion phase. Points a–e denote peak hand velocity V max (a), maximum deceleration (b), early grip aperture (c) at V max, maximum grip aperture (d), and peak velocity of hand retraction rV max (e). Inserts show original hand velocity (VEL) and grip aperture (GA) curves of one run of fast trials to illustrate the intra-individual variability.
Retraction-insertion movement
This movement was composed of two phases, the retraction and the insertion phase. The retraction phase started when the object was lifted from the first holder, and comprised the first movement unit of hand retraction, i.e. the first acceleration–deceleration sequence which brought the object to the second holder. Peak retraction velocity (rV max), the timing of rV max, and the height of the curved wrist trajectory were calculated. The end of the retraction phase was defined when the velocity of the wrist fell below a threshold of 10 cm/s (Fig. 2, line 4). During the subsequent insertion phase, the object was placed into the circular aperture of the second holder. This phase ended when the object reached the goal position (Fig. 2, line 5). We also calculated the search path as the length of the trajectory covered by the wrist marker while the object was manipulated into the holder’s opening during the insertion phase.
For each person 9–10 movement trials were evaluated for each combination of factors. Only those trials were included where all markers were sufficiently visible. The dropout rate was about 10% (invisible markers, reflection artifacts, camera failure). From the usable trials, mean values of each kinematic parameter were calculated for each individual. Separately for the visual and the no-vision conditions, these mean results were entered into 2×3 (hand×velocity) repeated measures analyses of variance (ANOVAs). If the effect of the factor hand was significant, post-hoc contrasts (LH vs. RH) were carried out with paired t-tests. A higher grip aperture of the non-dominant hand compared to the dominant hand was expected. We did not plan contrasts between different velocity levels or between visual conditions, since the influence of these factors is well-known (Wing et al. 1986; Jakobson and Goodale 1991; Churchill et al. 2000).
To illustrate the speed-accuracy trade-off for each hand, the grip aperture data were plotted against V max, and against movement duration. Linear and non-linear regressions (equations according to Nelson, 1983) and Pearson’s correlation coefficients (r) were calculated with the SPSS application package (Version 10; SPSS Inc, Chicago, USA). We also fitted regression lines to the data of each individual subject, and compared the slopes and intercepts of the LH and RH lines with paired t-tests. Significance level was set at P<0.05.
Results
The tests of handedness confirmed the significantly better performance of the right dominant hand (Fig. 3). The laterality index was 22±5 (mean ± SD) for the square marking test, and 4±3 for the Purdue Pegboard. The results of women and men were comparable.
Handedness. The number of squares that were marked in the paper-test, and the number of pegs inserted into pegboard holes are indicated (means ± SD of the 20 right-handed subjects). Open symbols: left hand (LH); black symbols: right hand (RH). Significant inter-manual differences are marked with asterisks (paired t-test, * P<0.05; ** P<0.01)
Visual condition
Scaled according to the arm length, the object distance was 33.3±2 cm for trials performed with either hand. For the visual condition, kinematic data of the LH and RH are presented in Table 1. The relationships between grip formation and reaching velocity of the RH and LH are illustrated in Fig. 4. Each data point represents one run, i.e. the mean of ∼10 trials of one hand in one subject at a certain velocity level. The early grip aperture increased with peak hand velocity, and the regression lines of the LH and RH are almost the same (Fig. 4a). The slopes of the regression lines of the maximum aperture are steeper (Fig. 4b). Again, the lines of the LH and RH are nearly identical. Furthermore, calculation of the regression lines in each individual subject, and subsequent comparison of the LH and RH slopes and intercepts (Table 2) revealed no significant differences between hands. Figure 4c,d illustrate that the early and maximum grip aperture both increased in a non-linear fashion when the movement duration of the reach-to-grasp shortened. The functions that characterize this relation for the LH and RH are almost congruous. All correlations illustrated in Fig. 4a–d were significant (P<0.05). The correlation between pre-shaping and V max was characterised by positive coefficients r for the early (LH: 0.51; RH: 0.58) and the maximum grip aperture (LH: 0.8; RH: 0.79). Negative coefficients r specified the relations between movement duration and early grip aperture (LH: −0.41; RH: −0.47), or, respectively, maximum aperture (LH: −0.73; RH: −0.69).
Pre-shaping in the visual condition. The relationships between peak hand transport velocity and early grip aperture (a), and between peak hand velocity and maximum grip aperture (b) are shown with data from all 20 subjects. (c, d) Relations between early (respectively, maximum) grip aperture and movement duration. Each symbol indicates mean data of one run (10 trials) at one velocity level (slow, normal, or fast) in one subject. Open symbols and broken lines: left hand (LH). Black symbols: right hand (RH). The equations of the regression lines and curves are given.
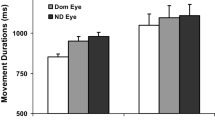
No significant main effects of the factor “hand” were found for any of the reach-to-grasp variables in the visual condition (Table 1). However, an interaction between hand and speed (F2,38= 4.0, P<0.05) existed for the mean transport velocity, as the RH was inclined to move faster than the LH during speeded prehension (post-hoc t-test, P=0.061). Another interaction was found (F2,38= 6.3, P<0.01) for the relative time to V max. In the slow condition, the RH reached V max a little earlier (at 31% of the movement duration, P<0.05) than the LH (33%). However, averaged across velocities, the relative times of V max (LH, 38%; RH, 37% of movement duration), peak deceleration (LH, 64%; RH, 65%), and maximum grip aperture (LH, RH: both 78%) were comparable for both hands, as were the absolute time intervals (Table 1). Hence, in the visual condition, the forward prehension movement and also the retraction phase were performed nearly in the same manner by either hand (Table 1). Significant differences between hands became evident when the object was inserted into the second holder. The insertion time (F1,19= 10.7, P<0.01) and search path (F1,19= 14.4, P<0.01) of the RH were shorter compared to the LH, so the dominant hand was more efficient in manipulating the object into the goal position (see Fig. 1g, h). Post-hoc tests confirmed these differences between hands (see asterisks in Table 1).
No-vision condition
Kinematic data of the prehension and retraction movements performed in darkness are listed in Table 3. As expected, the grasping hands opened more than in the visual condition. The grip aperture values of the LH and RH are depicted in Fig. 5. Both, the early grip aperture (Fig. 5a) and the maximum finger distance (Fig. 5b) increased with peak transport velocity. Both correlations were significant (P<0.01), with similar coefficients r of both hands for the early (LH: 0.58; RH: 0.57) and the maximum finger aperture (LH: 0.55; RH: 0.58). The regression lines of both hands are nearly parallel, but the LH line in Fig. 5a is shifted downward by ∼3 mm towards a narrower early grip aperture (Fig. 5a). This difference between hands did not reach significance when the slopes and intercepts of the individual regression lines were compared with paired t-tests (Table 2). Figure 5c, d demonstrate the relation between movement duration and grip apertures. Despite the scatter of the data, also the coefficients r of these correlations were significant (P<0.05) for the early (LH: −0.50; RH: −0.53) and the maximum aperture (LH, −0.55; RH, −0.52). Again, the regression curves of both hands are approximately parallel, with a tendency towards a narrower LH grip aperture (see Fig. 5c).
Pre-shaping in the no-vision condition. Relations between peak hand velocity and early grip aperture (a), and between peak velocity and maximum grip aperture (b) are shown. Below: relations between early (c) or maximum (d) grip apertures and movement duration. Open symbols and broken lines: left hand (LH). Black symbols: right hand (RH). The equations of the regression lines are given. Otherwise as in Fig. 4.
Most kinematic variables of RH and LH movements were comparable in the no-vision condition (Table 3). The only significant main effect of “hand” (F1,19= 8.2, P<0.01) was found for the early grip aperture, but it was opposite of the predicted effect. In the normal and in the fast conditions, the RH was opened wider than the contralateral hand at maximum transport velocity (early grip aperture, post-hoc t-tests, P<0.05). Moreover, the maximum grip aperture of the RH tended to be larger than the one of the LH during the fast no-vision trials (F1,19= 3.7; post hoc t-test P<0.07). The absolute time intervals of prehension, and also the relative timings of V max (LH, RH: both 31% of movement duration), peak deceleration (LH: 51%; RH: 50%), and maximum grip aperture (LH: 62%, RH: 61%) were similar between hands. No significant effects of handedness were found for the retraction–insertion without vision either.
Effects of velocity and modality
In the visual condition, all kinematic variables listed in Table 1 were significantly influenced by the speed of the movement. The main effects (ANOVA, P<0.05) of an increase in velocity (slow→normal→fast) included a shortening of all time intervals, a decrease of trajectory length and wrist height, an enlarged grip aperture, a shift of the relative time of V max towards the end of the movement, and a shorter search path. In the no-vision condition, analogous main effects of velocity were found for all but three variables: the trajectory length of the reaching hand, the wrist height, and the search path did not change significantly with speed in the no-vision trials. Significant main effects of the visual condition (P<0.05; three way ANOVA vision×hand×velocity) upon the reach-to-grasp were found for all variables except V max. Compared to the visual condition, the no-vision trials showed increased durations of the prehension, retraction, and insertion phases, a longer reaching trajectory and wider grip apertures. The reaction time was shorter, and the relative times of V max, peak deceleration and maximum grip aperture were shifted towards the onset of the reach-to-grasp when visual control was absent. Figure 6 illustrates the relation between the grip aperture and its relative timing. In the visual condition, the maximum aperture was reached significantly later (at ∼78% of the movement duration) than in the no-vision condition, where it occurred after about 62% of the movement duration (P<0.05). However, within each modality, we found no correlation (coefficients r between 0 and −0.3; n.s.) between peak aperture values and their timing (Fig. 6). Significant main effects of the modality were also found for the retraction-insertion movement: in the no-vision condition, rV max was higher and occurred earlier than in the visual trials, whereas the search path and insertion phase were prolonged.
Timing of the maximum grip aperture. Peak grip aperture values are plotted against their relative timing during the reach-to-grasp (expressed as a fraction of the movement duration). Open symbols, dotted lines: left hand (LH). Black symbols, solid lines: right hand (RH). The straight thin regression lines are drawn to show trends, but the correlation coefficients r were not significant. The thicker broken-line curves show the predicted relationship according to Smeets and Brenner (1999).
Discussion
At the onset of this study, we had expected to find differences between reach-to-grasp movements of the dominant and non-dominant hand, which may not be amply visible, but should have been detected by the quantitative kinematic analyses. We had assumed that the less dextrous non-dominant LH would grasp with a wider safety margin, i.e. with a larger grip aperture than the RH. Developmental studies of prehension have shown that, in parallel with the refinement of other hand motor skills, the grip aperture becomes relatively smaller during the first decade of life (Kuhtz-Buschbeck et al. 1998; Smyth et al. 2004). A wider opening of the grasp can compensate for inaccuracies of hand transport, e.g. when an increased speed results in a higher variability of the movement endpoint. This enlargement of the maximum aperture with increasing transport velocity, previously described by others (Wing et al. 1986; Bootsma et al. 1994; Mason and Carnahan 1999) for the dominant hand, was confirmed here for both hands. Also the consequences of a change in the visual condition, which are known from the literature (Churchill et al. 2000; Schettino et al. 2003; Winges et al. 2003), were reproduced in this study. Smeets and Brenner (1999) predicted that in prehension trials with a large “extra grip” like those of the no-vision condition, the maximum aperture would be reached earlier than during trials with a smaller “extra grip” (their Fig. 8). The “extra grip” is the difference between peak grip aperture and object size. Our results confirm this prediction concerning the relative timing when no-vision and the visual trials are compared, but within each visual condition, significant correlations between peak aperture and its timing were lacking (see Fig. 6), presumably because the aperture differences of about 2 cm between slow and fast trials were too small to detect a relationship.
The effects of the velocity and visual condition were comparable for the LH and RH, so nearly all kinematic data were equivalent. The non-dominant LH exhibited neither a pre-shaping with a larger aperture nor a different movement duration and/or transport velocity. Statistical comparison of the slopes and intercepts of the individual regression lines did not reveal clear-cut disparities of pre-shaping between hands (see Table 2). In contrast to our initial prediction, the grip aperture of the RH tended to be larger than the LH aperture in the no-vision condition (see Fig. 5). This difference reached significance for the early grip aperture, which suggests that pre-shaping is initiated earlier during RH than LH movements. Yet, some significant results of the post-hoc comparisons may have occurred due to the multiple testing of the various parameters. We did not apply a Bonferroni correction, which would have nullified any difference between hands. All in all, our data therefore indicate that RH and LH grasped in nearly the same manner. Of course, the negative statistical result of this study cannot not rule out that notable differences would have emerged if a larger number of subjects had been tested.
Movements of the digits in grasping disks with the RH and LH, and in bi-manual grasping, were recently analyzed in seven subjects by Smeets and Brenner (2001). In contrast to the present study, the temporal constraints and visual conditions were not varied. Furthermore, the orientations of the surfaces, where the fingerpads should contact the disks, were perpendicular to the movement direction, which resulted in a rather unusual grasping posture. Despite these methodical differences, Smeets and Brenner came to a similar conclusion in stating that grasping with the dominant and non-dominant hand is remarkably similar. The movements of the non-dominant hand’s digits were slightly more variable (their Fig. 5), but no significant differences were reported.
This similitude is in contrast to tests of handedness, which require fast and accurate object (e.g. pencil, pegs) manipulations. In accordance with previously published data (Annett 1992), we found conspicuous differences between the LH and RH for the rapid drawing movements of the square-marking test (Fig. 3). The superiority of the dominant RH became also evident in the Purdue Pegboard task, where, in rapid succession, pegs had to be grasped and placed into holes as fast as possible. Small precise translational and tilting movements were necessary to insert these objects. A similar, although less difficult manipulation of the cylinder took place at the end of the retraction–insertion movement (see Fig. 1g, h). Also this task was mastered more efficiently by the RH than by the LH, as evident from the shorter insertion time and search path. All these manipulations required dextrous finger movements, which are known to be controlled via crossed cortico-motoneuronal projections emerging from the primary motor cortex (Lemon 1993). Activities that require high precision in interjoint coordination and trajectory formation (e.g. drawing, fine manipulation, but also targeted ball throwing) are performed better with the dominant arm and hand (Healey et al. 1986).
Differences between goal-directed movements of both arms become evident when limb dynamics are analyzed. In a series of studies, Sainburg and coworkers examined such interlimb differences of reaching in right-handed- subjects with a particular experimental setup. The arm was supported over a horizontal surface by an air-jet system, so that the effects of gravity and friction were minimized, and the reaching movements were carried out in a horizontal plane (Sainburg and Kalakanis 2000). The hand was moved to different targets while vision of the arm and hand was blocked. Only shoulder and elbow joint angles changed, whereas all joints distal to the elbow were immobilized and the trunk was restrained. The joint coordination patterns differed systematically between the dominant and non-dominant arm. Inverse dynamic analyses indicated that dominant arm movements were characterized by a more skilful coordination of muscle action with intersegmental dynamics. Despite the dominant arm advantage in dynamic control, however, the targets were reached with similar accuracy by both hands (Sainburg and Kalakanis 2000; Sainburg 2002). This finding is in accordance with our data of pre-shaping, although the paradigm and experimental setup of Sainburg and coworkers are different: With a similar final position accuracy of both hands, it is not surprising that the grip apertures of the DH and NDH are comparable. If the dominant arm advantage in dynamic adaptation and control of limb segment inertial interaction (Sainburg 2002) does not result in smaller position errors at the end of the movement, the dominant hand cannot “afford” a smaller grip aperture.
For some target positions, Sainburg and Kalakanis (2000) even found that the non-dominant hand reached the goal with slightly smaller final position errors than the dominant hand (difference about 3 mm; their Fig. 4). This surprising difference was reproduced in a further study of reaching without visual feedback (Bagesteiro and Sainburg 2002, their Fig. 3D) and was ascribed to a preferential use of closed-loop control mechanisms, using proprioceptive feedback, in the deceleration phase of non-dominant arm reaching movements. It is possible that the rather small LH peak grip aperture in the no-vision condition (see Fig. 5, broken lines) reflects a better final position accuracy of the non-dominant arm as reported by Sainburg and Kalakanis (2000). Since the inter-manual difference was not significant, however, this inference remains speculative.
In keeping with our data, Sainburg and Schaefer (2004) found similar peak velocities of both hands during reaching movements towards targets that were located at different distances. With increasing movement amplitude, however, time to peak velocity increased much more during non-dominant than dominant arm movements, and was generally longer in the non-dominant limb (their Fig. 4). A pulse width control (non-dominant side) and a pulse height control strategy (dominant side) were inferred from these results. By contrast, time to peak velocity was similar for RH and LH prehension movements in our study (see tables 1, 3). Differences between the paradigms (horizontal reaching under reduced gravity conditions vs. quasi-natural prehension) may account for this. Furthermore, we did not vary object distance systematically.
The similarity of the LH and RH grasping movements may suggest that they share a common level of representation in motor programming. Also previous studies reported invariant kinematic patterns for different types of prehension. Tresilian and Stelmach (1997) compared the characteristics of uni-manual prehension with a bi-manual task, where an object was grasped between the pads of the two index fingers. They found a very similar temporal evolution of the aperture and transport components and analogous adaptations to different task constraints regardless of the effector. Gentilucci et al. (2004) reported that grasping an object with a tool showed some kinematic features that were very similar to those of the natural grasp, despite the different biomechanical properties of the grasping effectors. A unique planning may also underlie the act of human grasping with hand and mouth, with the same premotor neurons being involved in the generation of appropriate grasp motor commands to the different effectors (Gentilucci et al. 2001).
Although it is speculative to infer a common motor representation from the similar kinematic data of LH and RH movements, there is at least some evidence from brain imaging studies in humans supporting that movements of either hand share some cortical representations. In right-handed volunteers, the cortex lining the left intraparietal sulcus was active during the execution of simple and complex finger movements by the LH and RH (Kuhtz-Buschbeck et al. 2003). Bilateral activity of dorsal premotor areas was demonstrated during complex movements of either hand by Kawashima et al (1998). A recent fMRI study found nearly symmetrical bilateral activation of the anterior intraparietal cortex during grasping movements performed with the dominant RH (Culham et al. 2003; their Table 2). Since these left and right parietal areas were more strongly activated by grasping than by reaching, they seemed to be specifically involved in the control of pre-shaping of the right fingers. Also other imaging studies demonstrated bilateral activation of the anterior intraparietal and premotor cortex during RH grasping (Matsumura et al. 1996; Binkofski et al. 1998). The parietal-premotor circuit is known to be involved in the transformation of an object’s intrinsic properties into specific grips (Sakata and Taira 1994; Rizzolatti and Luppino 2001). A conjunction analysis, which could detect common cortical activations during LH and RH prehension movements, has, to our knowledge, not yet been published. Still, the bilateral posterior parietal activity associated with RH grasping renders it likely that some overlapping regions would also be active during LH prehension movements.
References
Annett M (1970) A classification of hand preference by association analysis. Br J Psychol 61:303–321
Annett M (1992) Five tests of hand skill. Cortex 28:583–600
Bagesteiro LB, Sainburg RL (2002) Handedness: dominant arm advantages in control of limb dynamics. J Neurophysiol 88:2408–2421
Binkofski F, Dohle C, Posse S, Stephan KM, Hefter H, Seitz RJ, Freund HJ (1998) Human anterior intraparietal area subserves prehension: a combined lesion and functional MRI activation study. Neurology 50:1253–1259
Bootsma RJ, Marteniuk RG, MacKenzie CL, Zaal FT (1994) The speed-accuracy trade-off in manual prehension: effects of movement amplitude, object size and object width on kinematic characteristics. Exp Brain Res 98:535–541
Castiello U, Bennett KM (1997) The bilateral reach-to-grasp movement of Parkinson’s disease subjects. Brain 120:593–604
Castiello U, Bennett KM, Stelmach GE (1993) The bilateral reach to grasp movement. Behav Brain Res 56:43–57
Chieffi S, Gentilucci M (1993) Coordination between the transport and the grasp components during prehension movements. Exp Brain Res 94:471–477
Churchill A, Hopkins B, Rönnqvist L, Vogt S (2000) Vision of the hand and environmental context in human prehension. Exp Brain Res 134:81–89
Corballis MC (1997) The genetics and evolution of handedness. Psychol Rev 104:714–727
Culham JC, Danckert SL, DeSouza JF, Gati JS, Menon RS, Goodale MA (2003) Visually guided grasping produces fMRI activation in dorsal but not ventral stream brain areas. Exp Brain Res 153:180–189
Gentilucci M, Toni I, Daprati E, Gangitano M (1997) Tactile input of the hand and the control of reaching to grasp movements. Exp Brain Res 114:130–137
Gentilucci M, Benuzzi F, Gangitano M, Grimaldi S (2001) Grasp with hand and mouth: a kinematic study on healthy subjects. J Neurophysiol 86:1685–1699
Gentilucci M, Roy AC, Stefanini S (2004) Grasping an object naturally or with a tool: are these tasks guided by a common motor representation ? Exp Brain Res 157:496–506
Healey JM, Liederman J, Geschwind N (1986) Handedness is not an unidimensional trait. Cortex 22:33–53
Jakobson LS, Goodale MA (1991) Factors affecting higher-order movement planning: a kinematic analysis of human prehension. Exp Brain Res 86:199–208
Jeannerod M (1986) The formation of finger grip during prehension. A cortically mediated visuomotor pattern. Behav Brain Res 19:99–116
Jeannerod M, Arbib MA, Rizzolatti G, Sakata H (1995) Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci 18:314–320
Kawashima R, Matsumura M, Sadato N, Naito E, Waki A, Nakamura S, Matsunami K, Fukuda H, Yonekura Y (1998) Regional cerebral blood flow changes in human brain related to ipsilateral and contralateral complex hand movements – a PET study. Eur J Neurosci 10:2254–2260
Kuhtz-Buschbeck JP, Stolze H, Jöhnk K, Boczek-Funcke A, Illert M (1998) Development of prehension movements in children: a kinematic study. Exp Brain Res 122:424–432
Kuhtz-Buschbeck JP, Sundholm LK, Eliasson AC, Forssberg H (2000) Quantitative assessment of mirror movements in children and adolescents with hemiplegic cerebral palsy. Dev Med Child Neurol 42:728–736
Kuhtz-Buschbeck JP, Mahnkopf C, Holzknecht C, Siebner H, Ulmer S, Jansen O (2003) Effector-independent representations of simple and complex imagined finger movements: a combined fMRI and TMS study. Eur J Neurosci 18:3375–3387
Lemon RN (1993) The G. L. Brown Prize Lecture. Cortical control of the primate hand. Exp Physiol 78: 263–301
Mason AH, Carnahan H (1999) Target viewing time and velocity effects on prehension. Exp Brain Res 127:83–94
Matsumura M, Kawashima R, Naito E, Satoh K, Takahashi T, Yanagisawa T, Fukuda H (1996) Changes in rCBF during grasping in humans examined by PET. Neuroreport 7:749–752
Nelson WL (1983) Physical principles for economies of skilled movements. Biol Cybern 46:135–147
Paulignan Y, Frak VG, Toni I, Jeannerod M (1997) Influence of object position and size on human prehension movements. Exp Brain Res 114:226–234
Rizzolatti G, Luppino G (2001) The cortical motor system. Neuron 31:889–901
Sainburg RL (2002) Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res 142:241–258
Sainburg RL, Kalakanis D (2000) Differences in control of limb dynamics during dominant and nondominant arm reaching. J Neurophysiol 83:2661–2675
Sainburg RL, Schaefer SY (2004) Interlimb differences in the control of movement extent. J Neurophysiol 92:1374–1383
Sakata H, Taira M (1994) Parietal control of hand action. Curr Opin Neurobiol 4:847–856
Schettino LF, Adamovich SV, Poizner H (2003) Effects of object shape and visual feedback on hand configuration during grasping. Exp Brain Res 151:158–166
Smeets JB, Brenner E (1999) A new view on grasping. Motor Control 3:237–271
Smeets JB, Brenner E (2001) Independent movements of the digits in grasping. Exp Brain Res 139:92–100
Smyth MM, Peacock KA, Katamba J (2004) The role of sight of the hand in the development of prehension in childhood. Q J Exp Psychol A 57:269–296
Tresilian JR, Stelmach GE (1997) Common organization for unimanual and bimanual reach-to-grasp tasks. Exp Brain Res 115:283–299
Wallace SA, Weeks DL (1988) Temporal constraints in the control of prehensile movement. J Mot Behav 20:81–105
Wing AM, Turton A, Fraser C (1986) Grasp size and accuracy of approach in reaching. J Mot Behav 18:245–260
Winges SA, Weber DJ, Santello M (2003) The role of vision on hand preshaping during reach to grasp. Exp Brain Res 152:489–498
Acknowledgements
We thank Dr. I. Schröder and Professor M. Illert for their help. This work was financially supported by the Hensel-Stiftung.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grosskopf, A., Kuhtz-Buschbeck, J.P. Grasping with the left and right hand: a kinematic study. Exp Brain Res 168, 230–240 (2006). https://doi.org/10.1007/s00221-005-0083-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0083-1