Abstract
Previous studies in our laboratory examining pointing and reach-to-grasp movements of Parkinson’s disease patients (PDPs) have found that PDPs exhibit specific deficits in movement coordination and in the sensorimotor transformations required to accurately guide movements. We have identified a particular difficulty in matching unseen limb position, sensed by proprioception, with a visible target. In the present work, we further explored aspects of complex sensorimotor transformation and motor coordination using a reach-to-grasp task in which object shape, visual feedback, and dopaminergic medication were varied. Normal performance in this task requires coordinated generation of appropriate reach, to bring the hand to the target, and differentiated grasp, to preshape the hand congruent with object form. In Experiment 1, we tested PDPs in the off-medication state. To examine the dependence of subjects on visual feedback and their ability to implement intermodal sensory integration, we required them to reach and grasp the target objects in three conditions: (1) Full Vision, (2) Object Vision with only the target object visible and, (3) No Vision with neither the moving arm nor the target object visible. PDPs exhibited two types of deficits. First, in all conditions, they demonstrated a generalized slowing of movement or bradykinesia. We consider this an intensive deficit, since it involves largely a modulation of the gain of specific task parameters: in this case, velocity of movement. Second, they were less able than controls to extract critical proprioceptive information and integrate it with vision in order to coordinate the reach and grasp components of movement. These deficits which involve the coordination of different inputs and motor components, we classify as coordinative deficits. As in our previous work, the PDPs’ deficits were most marked when they were required to use proprioception to guide their hand to a visible target (Object Vision condition). But even in the full-vision condition, their performance only became fully accurate when both the target and effector (hand) were simultaneously visible. In Experiment 2, PDPs were tested on their dopaminergic replacement therapy. Dopaminergic treatment significantly ameliorated the bradykinesia of the PDPs, but produced no changes in the hand preshaping deficiencies of PDPs. These results suggest that adequate treatment of the PDPs may more readily compensate for intensive, than coordinative deficits, since the latter are likely to depend on specific and time-dependent neural interdependencies that are unlikely to be remediated simply by increasing the gain of a pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A number of specific sensorimotor processing deficits have been suggested to characterize impairment of voluntary movement in Parkinson’s disease. In brief, these include: (1) a generalized slowing of movements (Godaux et al. 1992; Brown and Marsden 1999; Berardelli et al. 2001), (2) a difficulty in assembling complex movements (Benecke et al. 1987a; Agostino et al. 1998; Gentilucci and Negrotti 1999a, b; Alberts et al. 2000), (3) a reliance on sensory input, especially visual, to guide and correct movement (Flowers et al. 1976; Beuter et al. 1990; Georgiou et al. 1993; Poizner et al. 2000; Adamovich et al. 2001) and (4) a particular deficiency in processing proprioceptive input (Chong et al. 2000; Klockgether et al. 1994, 1995).
The first deficit—the generalized slowing of movement—is reflected in the well-known clinical characteristics of bradykinesia and akinesia. Since it has been shown that PD patients (PDPs) are capable of modulating their speed of movement, it is often a relative, rather than absolute slowness which is at issue. Like force, speed can be considered a unidimensional or intensive aspect of movement and there is considerable evidence that, in many situations, such intensive dimensions of performance can be altered by scaling an internal metric of movement (Pine et al. 1996). The latter three deficits, however, involve more coordinative aspects of movement: either the coordination of different movement components or the relation of sensory to motor coordinates. In some cases, they require the translation of one specific coordinate system (e.g. visual space) into another (a motor map) or the integration of two different coordinate systems (e.g. those centered upon the shoulder and finger joints) which must be integrated to effectuate multi-joint movements.
In our laboratory, we have been studying the coordinative aspects of movements in PDPs compared to age-matched controls. Our plan has been to determine exactly which aspects of a sensorimotor coordination are deficient. We have been able to show that PDPs can use either a present or remembered visual map to guide pointing movements, but show particular deficits when they must coordinate proprioceptive information from their unseen arm with a visual target (Adamovich et al. 2001). Similarly, we have shown that coordination of arm and trunk movements when both are used in pointing become impaired until vision permits the simultaneous view of both finger and target (Poizner et al. 2000). Vision thus functions not only to assure accurate targeting, but also in the integration of different movement components.
More recently, we have expanded our study to examine the reach and grasp movement. This movement consists of two distinct, but integrated components (Jeannerod 1981, 1984) and has been extensively studied. Earlier prehension experiments with PD subjects have generally shown accurate grasps despite some coordination deficits (Castiello et al. 1994; Ingvarsson et al. 1997; Tresilian et al. 1997; Alberts et al. 1998). Unfortunately, only a few studies have been conducted in PDPs in the absence of L-dopa medication (Gentilucci and Negrotti 1999a, b; Castiello et al. 2000). In these studies, a major difficulty has been a deficiency in reaching normal speed (failure in control of intensive aspect of movement). In a preliminary study, we compared the performance of PDPs in the off-medication state to controls in grasping different shaped objects that require different coordination of individual fingers (Schettino et al. 2003b). We found that PDPs were not only slower than controls, but showed deficits in integrating their grasp with their reach, primarily evident as a delayed differentiation between the required grasps for different shaped targets. This failure in coordinating the two motor components and matching the motor action to the target was present even with full vision of both arm and target. As in our previous study of arm and trunk coordination (Poizner et al. 2000), fully integrated behavior was delayed until the hand approached the target and both could be simultaneously visualized.
In the present article, we report on two studies involving hand preshaping during the reach-to-grasp movement of PDPs. In the first one we asked which sensory inputs were most required to facilitate the accuracy of grasp and integration of reach and grasp in PDPs. In order to do this, we exposed PDPs in an off-state and age matched controls to targets presented in three feedback conditions: Full Vision—both hand and target visible; Object Vision—only target visible; and No Vision—neither the hand nor the target object visible. Our prediction, based on our previous work, was that an intensive deficit of slowed movement would be present under all conditions, but that PDPs would be increasingly impaired in coordinative aspects of movement as visual feedback was withdrawn. We predicted that the Object Vision condition and No Vision conditions would be specifically problematic for them since these conditions require matching proprioceptive and visual coordinates.
Our second study was designed to test whether currently available therapeutic approaches to PD are able to resolve the coordinative as well as the intensive deficits observed in PDPs. The most common finding in studies comparing the performance of PD patients on versus off DA therapy is an increase in movement speed and/or in the stability of performance of relatively simple tasks after levodopa (L-dopa) administration, that is, an improvement in the intensive properties of motor performance (Baroni et al. 1984; Benecke et al. 1987b; Johnson et al. 1994; Hocherman et al. 1998; Castiello et al. 2000; Kelly et al. 2002). However, benefits to coordinative aspects of motor performance have been much less consistent and are often absent. Benecke et al. (1987b) reported an improvement in the performance of complex movements by PD subjects following L-dopa ingestion. In contrast, a number of studies have found little evidence of L-dopa derived amelioration in a variety of measurements (Blin et al. 1991; Caligiuri et al. 1992; Johnson et al. 1994, 1996; Horak et al. 1996; Chong et al. 2000; Feigin et al. 2002), and it has even been reported that proprioception is acutely depressed in Parkinson’s disease by dopaminergic medications (O’Suilleabhain et al. 2001). Feigin et al. (2002), for example, found that L-dopa infusion significantly improved OFF state clinical motor ratings and movement time in a task in which PD patients performed a paced sequence of reaching movements. However, they also found that L-dopa infusion significantly worsened the spatial errors of these movements. Likewise, Johnson et al. (1994) report that L-Dopa administration can improve movement velocity, but does not necessarily improve movement accuracy. We thus predicted that the intensive kind of deficit would be more amenable to the repletion of dopaminergic drive through PD treatment while the coordinative kind of deficit would remain impervious to treatment
Experiment 1: Effects of visual feedback
The major focus of the first experiment was to determine, by altering the feedback informational available, whether PDPs would be selectively impaired in performance under specific feedback conditions. Our previous study had led us to consider that, while both PDPs and controls have difficulty performing in the absence of any visual feedback, PDPs would be relatively more impaired when moving with vision of the target alone –a condition that stresses the need for coordinating visual and proprioceptive information.
Methods
Subjects
9 PD patients and 9 age-matched normal older adults served as subjects (Mean age: PD patients, 70.8 years; controls, 68.22 years, t test for means: t=0.79, ns). The PD patients were clinically evaluated by a trained movement disorders specialist at the time of testing and were found to have mild to moderate Parkinson’s disease (Hoehn and Yahr stages 1.5 to 3). They had experienced symptoms of PD for between 5 and 16 years (mean 8.8 years, SD 3.98). All patients had clinically typical PD and their motor disabilities were responsive to anti-Parkinsonian medications. Any patient having additional deficits in other neural systems (Parkinson plus condition) was excluded. Moreover, no patient had any off-state tremor or peak dose dyskinesias of more than minimal amplitude. Patient clinical features are given in Table 1. All subjects were right handed and both subject groups were screened with the Mini-Mental State Examination (excluded if MMSE, score <25/30) and the Beck depression inventory (excluded if score >10) to exclude patients with depression or dementia. All patients were affected on their right side. There were no Parkinson’s patients whose left side was markedly more affected that their right side. All subjects were free of significant upper limb or trunk arthritis or pain and also free of any significant neurological disease, except for the Parkinson’s disease in the PD patients. All subjects had normal vision or vision corrected to normal. The same subjects participated in Experiment 1 and Experiment 2. The two experiments, separated in time by approximately 1 week, were administered in counterbalanced order across subjects. In Experiment 1, the PD patients were tested in the morning before their first medication dose of the day, having been off their anti-Parkinsonian medications for at least 12 h. All subjects were informed about the nature of the study and signed institutionally approved consent forms.
Apparatus
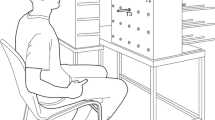
The apparatus and general procedure have been described elsewhere (Schettino et al. 2003a, b). Briefly, subjects sat in front of a flat table and were presented objects of three different shapes. The objects were positioned on a presentation platform that visually isolated the objects at a height of 15 cm from the surface of the table allowing a comfortable grasping position. In order to normalize for differences in subjects’ arm lengths, objects were placed at a distance from the shoulder equal to the length of the subject’s extended arm from shoulder to wrist. Objects were positioned directly in front of the sternum. All objects were approximately the same size. Fig. 1 shows the shapes and the mean angles elicited by each object at contact in the flexion/extension angles of the middle and ring fingers and in the abduction angle between the ring and middle fingers. The objects will be referred to as CC (Concave), CX (Convex) and SQ (Square). During the experiment, both the CC and CX objects were presented with the square edge on the left and the defining indentation or protrusion on the right side. The objects were painted with a light-green fluorescent paint (Seal-Krete) in order to be clearly visible in complete darkness. The background of the apparatus was uniform and dark-colored to reduce visual noise.
a Object shapes used in the experiment. The abscissa represents the angle of flexion/extension of the middle and ring fingers for each object grasp for one subject. The ordinate represents the angle of abduction between the index and middle fingers. Note that objects SQ and CC elicit only minimal abduction during a grasp. b Diagram of the experimental setup
Data capture
The positions of the major joints of the arm were recorded in 3D space using four electromagnetic sensors (MiniBird system, Ascension Technologies, Inc.) attached with adhesive tape to the subject’s arm segments at positions that were referenced to the following body landmarks: shoulder, elbow, wrist and midpoint of the dorsum of the hand. In this study, only the displacement of the wrist was analyzed. Finger joint flexion and extension were obtained via resistive bend sensors embedded in a glove (CyberGlove, Immersion, Inc.) worn on the right hand. The flexion/extension of the metacarpophalangial (MCP) and proximal interphalangial (PIP) joints of the index, middle, ring and little fingers were included in the analysis as well as the abduction/adduction joints of the index-middle and middle-ring fingers (ABD). All the devices were individually connected to RS-232 serial ports of a SGI Octane/SSE workstation (SGI, Inc.) Multi-threaded, high performance drivers for both the MiniBird and CyberGlove were developed that allowed all the devices to stream data to the disk at a rate of 100 Hz.
Procedure and analysis
Each trial started with the subject’s hand in a preset initial position (fingertips and thumb touching one another and held against the sternum). All experimental sessions were videotaped for offline analysis of error patterns. An infrared sensitive camera (SONY CCD-TR416) was used when the experimental conditions required a darkened room. Subjects were instructed to maintain the initial position until they heard a tone signaling the start of the trial. Once the tone sounded, the subjects were to reach to and grasp the object at a comfortable speed. A successful grasp was defined as the thumb being positioned on the left vertical surface opposing the other four fingers. This type of grasp, referred to as finger prehension (Rizzolatti et al. 1988), was chosen due to its production of a well-defined hand configuration. The subjects were further instructed to lift the object vertically and to release it on the table surface next to the presentation platform. Total reaches per experiment were 90 (3 shapes×3 feedback conditions×10 trials). If the subject did not grasp an object successfully, another trial was run in its place. In order to ensure that subjects understood the task and could perform the required motor acts, subjects practiced grasping all objects before the experiment for 5 to 10 trials. Objects were presented in a pseudo-randomized fashion. In the full vision condition, subjects could see both their hands and the target throughout each trial.
Reduced visual feedback conditions
The grasping procedure was essentially the same in the reduced visual feedback conditions as in the Full Vision condition. The main differences were as follows: in the Object Vision condition, the overhead lights were turned off and the experimental room fully darkened so that only the fluorescent objects were visible. This manipulation eliminated visual feedback of the subject’s moving arm during the experiment. After each trial, the lights were switched on for approximately 1–2 s in order to prevent dark adaptation.
Finally, in the No Vision condition subjects were asked to close their eyes upon hearing the tone indicating the start of the trial and to immediately initiate the reach-to-grasp movement. The interval between eye closure and movement initiation was less than 1 s. An experimenter sitting in front of the subject ensured that the subjects complied as required.
These manipulations eliminated visual feedback of environmental cues as well as that of the hand or the hand and object, depending on condition. Earlier work has indicated that the removal of such information may have an effect on kinematic parameters (Connolly and Goodale, 1991; Churchill et al. 2000). However, the results of Churchill et al. (2000), together with those of the majority of previous studies (Wing et al. 1986; Jakobson and Goodale 1991; Chieffi and Gentilucci 1993; Berthier et al. 1996) indicate that the removal of environmental cues does not affect kinematic parameters in an unpredictable fashion. Rather, the elimination of environmental cues appears to lie in a continuum of the amount of visual feedback available resulting in longer movement times (MT), earlier times to peak aperture (TPA) and larger peak apertures (PA). Therefore, we expected our results on kinematic parameters to reflect a compounded effect of the absence of visual cues plus that of the manipulated variable, namely object shape.
Analysis
All glove sensor data were transformed into degrees for analysis. Wrist trajectories were inspected visually using a computerized system for 3D graphic analysis of human multiple-joint movements that permits the precise localization of onset and offset points in the movement as a function of speed and acceleration. All trajectories could be rotated, translated, and scaled in real-time (Poizner et al. 1986, 1990; Kothari et al. 1992). Movement onset was defined as the point in which the wrist sensor reached 5% of the peak velocity during the initial acceleration phase. The offset was defined as the point in which wrist movement shifted direction during the grasping movement, corresponding to the point in time at which the object was lifted.
Wrist kinematics
We analyzed the following kinematic parameters: Peak velocity (PV) and movement time (MT) defined as the time between the onset and the offset as described above.
Discriminant analysis
Two different types of discriminant analysis were used to analyze successfully completed grasps: stepwise discriminant analysis (SDA) and canonical discriminant analysis (CDA). An SDA was performed on the ten joint angles at each of the selected time points (5–95% at 10% intervals) in order to find the best discriminating joints between the hand configurations for each object at each time point. Once a subset of the initial joints was selected (in the absence of statistically significant variables, all joints were included) CDA was performed on the selected joint angles. The relative efficacy of the discriminant analysis can be determined by the percentage of patterns whose group membership is correctly predicted, or conversely, the percentage of patterns that were incorrectly predicted (classification error) at a given time point. CDA thus provides a measurement of the error of hand preshaping during the transport phase of the movement and an estimate of the predictive value of this measure for the final hand shape per object type.
Relationship between object shape and hand configuration dimensions
Earlier work (Santello and Soechting 1998; Schettino et al. 2003a) has shown that the three object shapes included in our study elicit in normal subjects markedly different handshapes. Furthermore, the classification errors between each Object Comparison (the comparison between each pair of shapes CC-CX, CX-SQ, SQ-CC) follow different time courses during reach-to-grasp movements (Schettino et al. 2003a).
Statistical analyses
Effects of Parkinson’s disease
Kinematic measures
Repeated-measures ANOVAs were conducted on the kinematic measures in order to assess the effects of Object shape (CC, CX, SQ) and Condition (Full Vision, Object Vision, No Vision) (within factors) and Group (Controls, PD-OFF) (between factor).
Hand preshaping classification errors
In order to evaluate hand preshaping during the grasping movement, a repeated-measures ANOVA was run on the individual classification error percentages with Condition (vision, object vision, no vision), Object Comparison (CC-CX, CX-SQ, SQ-CC) and Time (5–95%) as within factors and Group (Controls, PD) as a between factor.
Post-hoc tests (Tukey HSD) were employed to test for significant differences between factor means in all significant main effects and second order interactions. Subsequent repeated measures ANOVAs were conducted on factors exhibiting statistically significant effects.
Results
PD subjects made significantly more incorrect and failed grasps than control subjects in the Object-Vision and No-Vision Conditions.
Incorrect and Failed Grasps
As defined in the Methods section, finger position was well-defined in this reach-to-grasp task. Due to this, subjects occasionally made incorrect or failed grasps (wrong finger position, failure to pick up object). In the case of PD subjects, errors would sometimes consist of grasping the object from the side, apparently due to a reduced hand aperture. These incorrect or failed grasps occurred in a small percentage of the total number of trials (average per group for the No Vision condition: Controls 0.73%, PD 4.31%). An ANOVA on the error totals for the Control and PD groups with Condition as within factor and Group as between factor revealed a significant effect of Group (F 1,16 =7.46, P=0.014) and Condition (F 2,2= 8.43, P=0.001), as well as a significant interaction of Group×Condition (F 2,32=4.3, P=0.022). Subsequent post hoc tests revealed that while control subjects did not make more mistakes under reduced visual feedback conditions, PD subjects exhibited larger number of errors as feedback was reduced (Fig. 2). Furthermore, pair-wise comparisons indicated that while PD subjects were significantly less accurate than controls in the Object Vision and No Vision conditions, they did not differ in the Full Vision condition. This result supports the notion that while PD subjects are able to successfully grasp an object as well as elderly controls when they have vision of their hand, they have more difficulty executing accurate movements in the absence of visual feedback of their moving limbs, regardless of whether the target of their movement is visible. Previous studies from our laboratory have also reached the same conclusion in the case of pointing movements. Adamovich et al. (2001) suggested that this deficit may be due to an impairment in the integration of visual and somatosensory inputs in PD. Nevertheless, in spite of their apparent impairment, PD subjects were able to grasp the objects in the prescribed fashion even in the total absence of visual feedback in most trials. Earlier studies have reported deficits in the trajectory of PD subjects during grasping relative to age-matched controls (Castiello et al. 2000). However, PD subjects’ errors did not prevent them from finishing the task successfully. This suggests that PD subjects are able to determine movement direction sufficiently well under no vision conditions to accomplish a reach-to-grasp movement, even though the increased number of errors in the task suggests that their grasping efficiency is reduced.
Effects of PD on kinematic parameters of the reach
PD subjects were significantly slower than control subjects, independently of visual feedback condition.
Peak velocity
Figure 3 shows the means and standard errors (SEM) for peak velocity (PV) for the controls and PD groups to each object shape for all visual feedback conditions. An ANOVA revealed significant main effects of Group (F F1,16=20.91, P=0.0003) and Object (F 2,2=7.28, P=0.0025) as well as a significant interaction of Group×Object (F 2,32=3.47, P=0.048). No other main effects or interactions were statistically significant. Overall, PD subjects were significantly slower than age-matched controls and this effect did not depend upon visual feedback condition. Subsequent post hoc tests revealed that during trials towards the CC object, PV was higher than that for the CX and SQ objects. An ANOVA for PV performed separately for each group followed by post hoc tests indicated that the Object effect was due only to the control group’s performance. This is an interesting result because it indicates that controls subjects modulated their PV depending on the object shape they were about to grasp, while PD subjects did not.
PD subjects had significantly longer movement times than control subjects, independently of visual feedback condition.
Movement duration
Figure 4 shows the means and SEM for movement time (MT) for the controls and PD groups to each object shape for all visual feedback conditions. An ANOVA revealed significant main effects of Group (F 1,16=13.8, P=0.0019), Condition (F 2,2=20.17, P<0.0001) and Object (F 2,2=27.47, P<0.0001). A significant interaction of Object×Group (F 2,32=5.92, P=0.0065) was also found. Subsequent post hoc tests revealed MT differences between all visual feedback conditions, with Full Vision producing the shortest duration, followed by Object Vision and No Vision. Separate ANOVAs for MT per group followed by post hoc tests revealed that the Condition and Object shape effects were present in both group. Post hoc pair-wise comparisons for Object revealed that for both groups, grasping trials directed towards the CC object took longer than those for the other shapes. This is interesting especially in the case of Controls given that it was precisely during these trials that they achieved the fastest speed. Post hoc tests for Condition revealed that the No Vision condition resulted in longer trial durations than the other two conditions.
Effects of PD on the kinematic parameters of prehension
PD subjects showed delayed specification of handshape during the reach across visual feedback conditions, but were particularly impaired in the Object-Vision condition.
Hand preshaping
The temporal pattern of handshape specification during a reach-to-grasp movement depends largely on the shape of the target object (Santello and Soechting 1998; Schettino et al. 2003a, b). We have previously shown that our methodology allows us to probe the handshaping process at different temporal stages based on specific target shape comparisons (Schettino et al. 2003a, b). In order to assess the existence of differences between PD subjects and elderly controls in the evolution of hand preshaping, a repeated measures ANOVA was conducted on the percent classification errors per subject with Object Comparison, Condition and Time as within factors, and Group as between factor. Statistically significant main effects of Group (F (1,16)=12.61, P= 0.0027) and Comparison (F (2, 32)=35.27, P<0.0001) and Time (F (9, 144)=225.31, P<0.0001) were obtained. Post hoc tests indicated that there were significant differences between the Group levels (Control, PD) in Object Comparisons CC-CX and SQ-CC. The Time main effect is an expected occurrence in all hand preshaping ANOVAs given that the classification error consistently decreases as a function of time.
Subsequent repeated measures ANOVAs were conducted on the Object Comparison data exhibiting significant differences.
CC-CX Comparison
The CC-CX comparison involves not only the largest distance in the finger flexion/extension dimension between our objects (see Fig. 1), but also requires a modulation of the abduction dimension. In our previous work (Schettino et al. 2003a), we have determined that early changes occur along the abduction dimension, suggesting that the sudden drop in classification error values are a result of internally-guided processes. A repeated measures ANOVA for the CC-CX comparison revealed significant main effects of Group (F F1,16=6.095, P=0.025), Condition (F 2,2=7.7, P=0.0019) and Time (F 9,144 = 64.09, P<0.0001) on the level of classification errors, as well as a significant interaction of Condition×Time×Group (F 18,288=3.71, P<0.0001). No other interactions were statistically significant. The main effect of Group indicates that PD subjects exhibited higher error levels than controls when the data are pooled across Condition and Time. The main effect of Time indicates that when the error data are pooled across the other factors classification errors decreased over time during the evolution of the reach. The main effect of Time is present in all analyses, therefore, it will not be discussed henceforth. The main effect of Condition shows that when pooled across Group and Time, there is an effect of the Visual Feedback manipulation. The three-way interaction of Condition×Time×Group indicates that differences exist between PD and control subjects that depend on each of the two other factors. Planned comparisons for each of the visual feedback conditions were conducted. Figure 5 shows the temporal patterns of classification errors for the CC-CX comparison on each of the three visual feedback conditions. Figure 5 shows that the classification errors fall rapidly in both groups in each condition. However, while those of the control group are below 5% by time point 35% in all conditions, the PD group error levels in the Full Vision and Object Vision conditions lag behind. Post hoc tests revealed that in the Full Vision condition, the handshape classification errors of PD subjects were significantly higher than those of controls at time points 35% through 85% (shaded area in Fig. 5a). These results are in accordance with our earlier studies regarding hand preshaping in normal controls and in PD subjects (Schettino et al. 2003a, b). In the Object Vision condition, PD subjects showed even greater deficits, having significantly elevated handshape classification errors from time points 35% through 95% of the movement (shaded area in Fig. 5b). Finally, in the No Vision condition, the PD subjects showed significantly increased handshape classification errors relative to controls at time points 25% and 35%. It is somewhat surprising that the PD subjects showed smaller deficits in the No Vision condition than in the other two conditions. This result may be due to a combination of longer MTs (resulting in the compression of early time points, see above) and the increase in hand aperture which is characteristic of reach-to-grasp tasks under reduced visual feedback conditions (Wing et al. 1986; Sivak and MacKenzie 1990; Berthier et al. 1996). This increase in finger extension would affect the abduction dimension as well, and PD subjects tend to use overly small abduction angles relative to controls (see below).
Patterns of classification errors for the control and PD groups in the CC-CX comparison at 10% epochs of time during a reach-to-grasp movement per Visual Feedback condition. a Full vision, b Object vision and, c No Vision condition. Shaded areas indicate regions where significant differences between the groups were found. Error bars are ± SEM
SQ-CC Comparison
A repeated measures ANOVA for the SQ-CC comparison revealed significant main effects of Group (F 1,16=11.46, P=0.0038) and Time (F 9,144=54.31, P<0.0001) on the level of handshape classification errors, as well as a significant interaction of Condition×Time×Group (F 18, 288=1.87, P=0.017). The main effect of Group indicates that overall, the PD subjects exhibited higher errors than the elderly controls. The significant three-way interaction indicates that differences exist between the experimental groups that depend on Condition and Time point. Figure 6 presents the temporal patterns of classification errors for the SQ-CC condition on each of the three visual feedback conditions. In contrast to those of the CC-CX comparison, and in accordance with our previous findings (Schettino et al. 2003a), the SQ-CC patterns show a slower rate of decline across time. Interestingly, the Object Vision condition is the only condition in which PD subjects show overall elevated error levels relative to age-matched controls; there is no overlap in the means or SEMs of the two groups. Repeated measures ANOVAs were conducted for each condition. In the Full Vision condition there were no significant main effects nor interactions except that of Time (F 9,144=24.12, P<0001) indicating that the experimental groups did not differ significantly in their error levels at any Time point or Condition. In the Object Vision condition, significant main effects of Group (F 1,16=12.8, P=0.002) and Time (F 9,144=22.6, P<0001) were revealed. There were no significant interactions. The main effect of Group indicates that PD subjects’ error levels were higher than those of elderly controls when pooled across Time. Finally, in the No Vision condition, a significant main effect of Time (F 9,144=17.47, P<0001) and a significant interaction of Group×Time (F 9,144 =2.14, P=0.029) were found. Subsequent post hoc tests were conducted on the significant Group×Time interaction. Significant differences between the experimental groups were found at time points 15%, 65% and 75%. The early difference between the groups reflects the fact that the control group had significantly higher classification error levels than the PD group at time 15%. This result is probably independent of hand preshaping processes, however, since the earlier evidence of preshaping does not normally occur before 35% of the movement (Schettino et al. 2003a). The PD subjects had significantly higher classification error rates than control subjects at times 65% and 75% of the movement (Fig. 6, shaded area in graph). The results for the reduced visual feedback conditions indicate that PD subjects are impaired in the modulation of hand configuration relative to normal controls. Surprisingly, in the Full Vision condition, for this object pair comparison and unlike for the more complex CC-CX comparison, PD subjects did not exhibit any impairment relative to normal elderly controls. A further point of interest is that compared to young controls (Schettino et al. 2003a), the age-matched controls preshaped their hands relatively quickly in normalized time. In our previous experiments, we found that for the SQ-CC comparison under full vision conditions, the error levels of young controls did not reach low levels until 75% of normalized time. The apparent discrepancy is resolved by noting the amount of time dedicated by each group to the deceleration section of the movement. While young controls dedicate 54% of the movement to this section, older controls dedicate more than 65%, suggesting that their use of visual feedback for the control of hand preshaping may start earlier than in young controls.
Patterns of classification error for the control and PD groups in the SQ-CC comparison at 10% epochs of time during a reach-to-grasp movement per Visual Feedback condition. a Full vision, b Object vision and, c No Vision condition. The asterisk in b indicates a main effect of group. The shaded area in c indicates a significant difference between the groups. Error bars are ± SEM
PD subjects did not abduct the fingers as much as control subjects in the Full Vision condition.
Abduction/adduction
We have previously shown that PD patients exhibit abnormal rates and modulation of finger abduction/adduction while grasping the CX object (Schettino et al. 2003b). Given that finger abduction is a major component of early preshaping, occurring as early as 35% of the movement, it can be considered the result of internally-guided processes. A repeated measures ANOVA was conducted on the mean abduction angles per subject during CX trials for joints ABD1 and ABD2 for each visual feedback condition. The within-subjects factors were Joint (ABD1, ABD2) and Time (5–95%) and the between-subjects factor was Group (Controls, PD). In the Full Vision condition, significant main effects of Group (F 1,16=5.14, P=0.03), Joint (F 1,1=20.12, P=0.0004) and Time (F 9,144=36.79, P<0.0001) were found. There were no significant interactions. The main effect for Joint simply indicates that ABD1 generally reached wider angles than ABD2. The main effect of Time reflects the increase in abduction angle throughout the movement. Finally, the significant effect of Group indicates that PD subjects did not reach the abduction levels observed in elderly controls. Figure 7 presents the abduction angles for ABD1 for both the control and the PD group in the Full Vision condition. Interestingly, PD subjects show a relative lack of modulation in the abduction angle at the end of the motion. Unlike controls, PD subjects show no decrease of the abduction angle at the end of the movement. Statistical comparisons for the Object Vision and No Vision conditions did not reach significance for the Group effect or for interactions including Group.
Discussion
Summary of results
We found several motor deficiencies in the PD subjects when in the Off state:
-
1.
Relative to controls, PD subjects were bradykinetic in the reach parameters of the reach-to-grasp movements, exhibiting lower peak velocities and longer movement times consistently across all visual feedback condition (Figs. 3, 4).
-
2.
In agreement with our earlier work, PD subjects were found to rely on visual feedback more than controls for the performance of this reach-to-grasp task (cf. Schettino et al. 2003b). In particular, while PD subjects’ levels of incorrect and failed grasps under Full Vision conditions were not different from those of elderly controls (suggesting that both subject groups were able to grasp the object relatively efficiently with the appropriate hand configuration), PD subjects showed significantly higher rates of incorrect and failed grasps in the Object Vision and No Vision conditions. It is possible that due to their lack of predictive control, PD subjects waited until their hand was visible during the last portion of the movement and only then attempted to position their fingers through online control, an extremely complex and error-prone process given the number of degrees of freedom involved (Jeannerod 1997).
-
3.
The PD subjects also exhibited specific deficits in the ability to use propioceptive information in order to execute grasping movements in a predictive fashion (cf. Schettino et al. 2003b). This is especially clear in the SQ-CC comparison where hand preshaping deficits are specifically evident in the reduced visual feedback conditions. While age-matched controls were able to switch to proprioceptive guidance in order to preshape their hands under reduced visual feedback conditions, PD subjects were not.
The PDPs’ deficits were evident even though normalized trajectories were considered, so that bradykinesia, if acting in a compensatory fashion, was still insufficient to allow normal coordination. It is also notable that the deficit in preshaping the hand was most pronounced in the Object Vision condition, for both CC-CX and SQ-CC comparisons, though present even in the Full Vision condition for the SQ-CC comparison.
In summary, there were two main types of deficits observed in the Parkinsonian patients. First, the PDPs manifested a generalized slowing of movement in this task, which we suggest stems from deficit of an intensive nature. Intensive deficits can be considered those in which the gain is maladaptively altered from normal so that output is either too great (fast, overshot) or too small (slow, undershot). Such deficits can be remedied by altering gain. While the actual circuitry involved is likely to be more complex, such deficits can be considered to depend on a rather straightforward input-output pathway. Second, the PDPs demonstrated an inability to use different information sources to generate appropriately patterned and coordinated motor output, which we have classified as being of coordinative nature. Coordinative deficits can include those involving the integration of different sensory modalities (e.g. proprioceptive with vision), the transformation of sensory input to motor output (e.g. target shape guiding hand preshaping), or the coordination of different motor components (e.g. reach and grasp). As a consequence of this inability, PDPs relied more upon visual feedback in which target information could be directly coded into motor output without intermediate transformations. They waited to shape their hands until both the target and effector (hand) were simultaneously visible within the workspace. Their deficits were most acute, relative to controls, in the Object Vision condition when they needed to integrate proprioceptive information about the arm position into motor commands in coordination with a visual target.
Sensorimotor processing in Parkinson’s disease
It has been frequently noted that PD subjects appear to rely excessively on sensory information in order to guide their movements (Flowers et al. 1976; Klockgether and Dichgans 1994; Beuter et al. 1990; Georgiou et al. 1993; Nixon and Passingham 1998; Poizner et al. 2000, Adamovich et al. 2001). Such a dependence could explain why, in the present study, hand preshaping was delayed until the hand was in the area of the target. We previoulsy found a similar failure of coordination in PDPs until an arm and trunk reach closed in upon its target (Poizner et al. 2000). Moreover, in that study, the PDPs not only had delayed preshaping, but had higher levels of incorrect and failed grasps despite the availability of visual feedback of the target object. This result suggests that PD subjects had difficulty with the transformation of visual input into the necessary motor commands for the coordination of hand preshaping (Klockgether et al. 1995; Demirci et al. 1997; Adamovich et al. 2001). Consistent with that idea, in the present study, PD subjects exhibited impairments in the earlier phase of the hand preshaping process (CC-CX comparison) regardless of visual feedback condition.
But PDPs also have an additional difficulty—the inability to integrate different sensory modalities to guide movements. We had previously suggested that PDPs’ reliance on vision can also arise from their difficulty in integrating proprioceptive signals with concurrent or remembered visual information in order to guide movements. (Adamovich et al. 2001). In accordance with our previous work (Schettino et al. 2003b), the present study also found that the need to integrate proprioceptive and visual information further impaired PDP performance relative to normals.
Loss of predictive control in PD
Closely related to the PDPs dependence on visual information is an inability to control movement predictively. Predictive control is that generation of movement which is based on a spatial and temporal pattern not specified by the movement target. To establish such control, subjects need to generate motor plans from limited sensory information (as available from a distant target rather than one adjacent to the hand in this task). In addition, they may need to coordinate one component of a movement (e.g. the grasp) with another (e.g. reach). Therefore, breakdown of the coordinative requirements for sensorimotor processing or for motor component coordination can impair the ability to choreograph predictive, open loop movement sequences. Learned motor behavior relies on assembled movement sequences that require considerable predictive control (Halsband and Freund 1993). It has been proposed that use of proprioceptive monitoring facilitiates rapid and predictive execution (Gentilucci et al. 1994), relegating the relatively slow visual feedback control mechanisms to the last phases of reaching movements which require higher degrees of accuracy (Desmurget and Grafton 2000). However, such motor sequences require the ability to coordinate diverse coordinate systems resident in a variety of different topographically and perhaps functionally organized brain regions. In the current study, we found that the PD subjects had difficulties with crucial elements of predictive control of grasping, such as early abduction during CX grasps. This result supports the notion that PD subjects exhibit impairments in the use of predictive control for the coordination of complex movement sequences (Benecke et al. 1987a; Schettino et al. 2003b).
Variation of bradykinesia with task requirements
Our current study also confirms observations that more complex motor tasks result in relatively greater slowing of movement in PDPs (Benecke et al. 1987a). In our previous studies involving a relatively simple pointing paradigm, PD subjects, although on average somewhat slower, were not significantly slower than elderly controls (Poizner et al. 1998, 2000; Adamovich et al. 2001). In contrast, in the current grasping paradigm, where precision requirements are increased due to the different target object shapes and the reach must be finely coordinated with the grasp, PD subjects show significantly longer MTs and lower peak velocities than controls (see also Schettino et al. 2003b). This supports the idea that bradykinesia may depend on the particular motor task as well as subject state (Majsak et al. 1998). Different motor deficits are interrelated such that deficiency in one deficit (coordinative deficits) may impact on another (intensive deficits). Movements may be slowed to allow the assembly of the correct motor coordination.
Experiment 2: Effects of Dopamine replacement therapy
Dopamine replacement therapy is the most common and effective therapy for the amelioration of several of the impairments stemming from Parkinson’s disease (PD) (Sage and Mark 1994; Hagan et al. 1997). While the effects of PD on motor control have been studied extensively, comparatively less has been written regarding the effects of dopamine (DA) replacement therapy on the same processes. DA therapy commonly results in a reduction in the four cardinal features of PD, namely, tremor, bradykinesia, rigidity and postural impairments (Sage and Mark 1994). In spite of the success of DA therapy in treating such impairments, it has become increasingly clear that a constellation of symptoms of PD may be refractory to the available therapeutic regimes (Blin et al. 1991; Horak et al. 1992; Johnson et al. 1994, 1996; Burleigh-Jacobs et al. 1997; Chong et al. 2000; Castiello et al. 2000; Kelly et al. 2002).
The most common finding in studies comparing the performance of PD patients on versus off DA therapy is an increase in movement speed and/or in the stability of performance of relatively simple tasks after levodopa (L-dopa) administration (Baroni et al. 1984; Benecke et al. 1987b; Johnson et al. 1994; Hocherman et al. 1998; Castiello et al. 2000; Kelly et al. 2002). Benecke et al. (1987b) reported an improvement in the performance of complex movements by PD subjects following L-dopa ingestion. In contrast, a number of studies have found little evidence of L-dopa derived amelioration in a variety of measurements (Blin et al. 1991; Caligiuri et al. 1992; Johnson et al. 1994, 1996; Horak et al. 1996; Chong et al. 2000).
Experiment 2 was designed to test whether currently available therapeutic approaches to PD are able to resolve the coordinative as well as the intensive deficits observed in PDPs.
Methods
In Experiment 2, PD subjects were tested ON medications and their performance compared to how they performed OFF medications in Experiment 1. Whereas, in Experiment 1 PD subjects were tested after being off of their medications for at least 12 hours (PD-OFF) (Langston et al. 1992), in Experiment 2 they were tested approximately two hours after taking their first medication of the day (PD-ON) when L-dopa blood concentrations are close to their peak (Duvoisin and Sage 2001). As previously mentioned, the order of the sessions was counter-balanced across subjects. The interval between the two testing sessions was approximately one week (8.22 days on average). The motor section of the Unified Parkinson’s Disease Rating Scale test (Stebbins and Goetz 1998) was evaluated for both sessions by a trained movement disorders specialist using a standardized protocol with videotaping of the testing for final scoring, and the relative amelioration of the clinical motor symptoms by DA therapy is presented in Table 1. All other methods and procedures were identical to those of Experiment 1 with the exception of medication state.
Statistical analysis
Repeated-measures ANOVAs were conducted on the kinematic measures in order to assess the effects of Meds (PD-ON, PD-OFF), Object shape (CC, CX, SQ) and Visual Feedback Condition (Full Vision, Object Vision, No Vision).
Hand preshaping classification errors
Although our second experiment focused on the performance of PD-ON versus PD-OFF subjects, in order to compare the hand preshaping classification error data of age-matched control subjects to those of the PD-ON group, we conducted a repeated measures ANOVA with Object Comparison (CC-CX, CX-SQ, SQ-CC), Condition (vision, object vision, no vision) and Time (5–95% at 10% intervals) as within factors and Group (Controls, PD-ON) as a between factor.
Repeated measures ANOVAs were conducted on the classification error data for the PD subjects in the present study with Meds (PD-ON, PD-OFF), Object Comparison, Condition and Time as factors. Post-hoc tests (Tukey HSD) were employed to test for significant differences between factor means in all significant main effects and second order interactions. Subsequent repeated measures ANOVAs were conducted on factors exhibiting statistically significant effects.
Results
PD –OFF and PD-ON subjects did not differ significantly in their number of incorrect and failed grasps.
Incorrect and failed grasps
Our first experiment found that PD-OFF subjects made significantly more incorrect and failed grasps than did the control subjects in the Object-Vision and No-Vision conditions. These incorrect or failed grasps occurred in a small percentage of the total number of trials (Average per group for the No Vision condition: PD-OFF 4.31%, PD-ON 4.68%, controls.73%). An ANOVA with Meds and Condition as within factors was conducted on the error totals for the PD subjects while OFF and ON their DA therapy. Only a significant main effect of condition (F=9.1, P=0.002) was found. No other significant main effects or interactions were found. Since there were no main effects or interactions with Meds, these results suggest that L-dopa did not improve successful prehension in PD subjects. Figure 8 presents these results.
Effects of DA therapy on kinematic parameters of the reach
DA therapy increased movement speed in PD subjects, having its greatest effect in the Object-Vision condition.
Peak velocity
An ANOVA on the PD subjects’ peak velocity (PV) with medication status and condition as within factors revealed a significant main effect of Meds (F 1,8=17.97, P=0.0028), and a significant interaction of Meds×Condition (F 2,16=4.33, P=0.031). All other main effects and interactions were non significant. Overall, PD-ON subjects were significantly faster than PD-OFF subjects. In our first experiment, PD-OFF subjects were significantly slower than control subjects independently of visual feedback condition. This result confirms earlier reports that L-dopa therapy in PD results in higher movement velocities for self-initiated tasks with high accuracy requirements (Kelly et al. 2002). A separate ANOVA conducted on the data for the PD-ON group revealed a significant main effect of Condition. Subsequent post hoc tests revealed that PD subjects achieved faster PV in the Object Vision condition relative to the Full Vision condition while on their DA therapy (Fig. 9). In contrast, a separate ANOVA on PV for the PD-OFF group showed no significant effect (see above). These results indicate that while medicated, PD subjects were able to significantly modulate their PV, but were unable to do so in the off medication state.
DA replacement therapy did not alleviate the hand preshaping deficits in any visual feedback condition.
Hand preshaping
A most critical analysis in the present study involved deficits in finger coordination during the reach (hand preshaping classification errors as revealed by the discriminative analysis). Our first experiment showed that PD-OFF subjects exhibited delayed specification of handshape during the reach across visual feedback conditions, but were particularly impaired in the Object-Vision condition. In order to contrast any hand preshaping deficits of PD patients on their DA replacement therapy to the normal evolution of handshape in elderly controls, a comparison of the handshape classification error data from the PD-ON subjects and elderly controls was performed through a repeated measures ANOVA including Object Comparison (CC-CX, CX-SQ, SQ-CC), Condition (vision, object vision, no vision) and Time (5–95%) as within factors and Group (Controls, PD-ON) as a between factor. The ANOVA revealed significant main effects of Group (F (1,16)=5.78, P=0.028), Comparison (F (2,32)=16.08, P<0.0001) and Time (F (9,144)=255.12, P< 0.0001). Significant interactions of Comparison×Condition×Group (F (4,64)=2.98, P=0.025), Comparison×Time (F (18, 288)=5.6, P< 0.0001) and Comparison×Condition×Time (F (36, 576)=2.44, P<0.0001) were also revealed. Significant effects of Time are expected, since handshape classification errors are naturally reduced as the movement has more and more time to unfold. The significant main effect of Group extends our earlier finding of hand preshaping deficits in PD-OFF subjects to include PD subjects while on their DA medication.
A four-way repeated measures ANOVA was conducted on the classification error data for all PD subjects with Meds (PD-ON, PD-OFF), Object Comparison, Condition and Time as factors. Statistical main effects of Comparison (F (2,16)=20.24, P<0.0001) and Time (F (9,72)=195.16, P<0.0001) were revealed. A significant interaction of Comparison×Time (F (18,144)=5.65, P<0.0001) was also revealed. There were no significant main effects nor interactions involving Meds as a factor. Furthermore, separate ANOVAs on the CC-CX and SQ-CC comparisons also failed to show significant effects of medication on reducing the classifications errors.
DA replacement therapy did not significantly affect finger abduction during the reach in any of the visual feedback conditions.
Abduction/adduction
Statistical comparisons between the OFF and ON data for finger abduction revealed no significant main effects nor interactions in any of the three visual feedback conditions except a main effect of Time. These results increase the evidence that a number of prehension parameters may not be sensitive to dopamine replacement therapy. However, it should be noted that despite the lack of improvement in the degree of abduction, the PD-ON group showed a pattern of abduction modulation more closely resembling that of the control group.
Discussion
Previous studies have suggested that the normal balance between the predictive and responsive control of behavior becomes disrupted in Parkinson’s disease (Briand et al. 1999; Poizner et al. 2000; Adamovich et al. 2001; Schettino et al. 2003b). Our first experiment probed the effects of visual input manipulation on hand preshaping in PD subjects and categorized their motor deficits as either intensive or coordinative. The results of our second experiment indicate that while intensive movement parameters are likely to be improved by dopamine therapy, coordinative control is more resistant to this type of intervention.
DA replacement therapy does not ameliorate hand preshaping deficits
Our results indicate that neither the hand preshaping nor the abduction deficits of PD subjects were ameliorated by DA replacement therapy. As mentioned before, hand preshaping requires the transformation of sensory information conveying the intrinsic characteristics of the target object (Jeannerod 1981) into a series of motor commands. Each one of our three visual feedback conditions requires a differential use of sensory information. While subjects may be able to process visual information in a closed-loop fashion in the Full Vision condition, the same is not true for the Object Vision and No Vision conditions. In the reduced visual feedback conditions information regarding arm position and hand configuration must be acquired kinesthetically, forcing subjects to employ predictive control (Schettino et al. 2003a). However, while in the Object Vision condition information regarding the extrinsic and intrinsic characteristics of the target object is available throughout the movement, in the No Vision condition the movement must be performed under mnemonic guidance. As shown in our first experiment, PD-OFF subjects show their largest differences in hand preshaping from elderly controls in the Object Vision condition. We found a similar result when PD subjects pointed to targets in a darkened room: the greatest deficit of the PD patients relative to control subjects was when they pointed, without vision of their arms, to a target that was visible throughout the movement (Adamovich et al. 2001). The inability of PD patients to visually track their moving hand is especially disabling, more than the need to use spatial memory to localize the target (see also Flash et al. 1992; Klockgether and Dichgans 1994). The result of the present experiment is interesting because it indicates that PD subjects are not able to use the visual information available during the Object Vision condition to implement the appropriate hand configuration changes, a condition during which they must extract critical proprioceptive information. Furthermore, the administration of DA replacement therapy did not remediate this deficit. This is evidence that somesthetic monitoring, sensorimotor integration and transformation, and, importantly, motor prediction may not be restored by dopamine replacement therapy.
Positive effects of DA replacement therapy on movement control
Consistent with several earlier reports, our results show that DA replacement therapy produced consistent and significant increases in the velocity of movement in the PD subjects (Benecke et al. 1987b; Castiello et al. 2000; Kelly et al. 2002). Our data show that while on their medications, PD subjects reached higher PV while executing grasping movements to all object shapes and under all visual feedback conditions. This suggests a generalized effect of dopamine replacement on movement speed. An interesting issue regarding increased movement speed is that there could be a speed/accuracy trade-off following L-dopa administration. In other words, given that PD subjects exhibit hand preshaping problems due in part to a reduction in predictive control and in sensorimotor integration, it may be expected that the higher PV would result in a decrease in the accuracy of grasping movements (Fitts 1954). Our results show that this was not the case: the number of incorrect and failed grasps was not higher in the PD-ON subjects relative to their PD-OFF state. The preshaping data of this study indicate that PD subjects, regardless of their medication state, were unable to preshape their hands in a predictive manner; therefore, hand configuration changes do not appear to account for the gain in movement speed.
General discussion
Neural bases of predictive and responsive control
Studies with imaging and animal models have suggested that grasping requires a substantial amount of inter-regional coordination in the brain. The reach-to-grasp movement has been reported to depend critically on the anterior intraparietal region (AIP) and the rostral portion of ventral premotor cortex (PMvr) (Rizzolatti et al. 1988; Luppino et al. 1999; Jeannerod et al. 1995). It has recently been suggested that interaction between these areas is responsible for the grip selection prior to the onset of movement and for the visuomotor control of the reach-to-grasp (Fagg and Arbib 1998).
Further evidence suggests that the BG may be needed to contribute to this coordination. It has been noted that these nuclei receive a variety of sensory inputs that are topographically organized. They receive profuse projections from parietal areas thought to be involved in propioceptive (Cavada and Goldman-Rakic 1991; Yeterian and Pandya 1993) and in visual analysis required for the control of movement (Saint-Cyr et al. 1990). Moreover, neuronal populations in the BG have been reported to respond to both tactile and visual inputs (Crutcher and DeLong 1984; Romo et al. 1995; Graziano and Gross 1995). In agreement with the notion of sensory integration in the BG, anatomical studies have described a high degree of convergence of cortical inputs onto single striatal neurons, indicating their probable function as integrators (Percheron et al. 1990). Indeed, the coordination of complex motor sequences has been suggested to depend critically on the intact striatum (Aldridge and Berridge 1998).
The involvement of the BG in such complex sequences is also reflected in their functional importance in the predictive control of movement. In Goldberg’s (1985) model, they are a critical component of the ‘medial’ circuit involving the basal ganglia (BG) and the supplementary motor area (SMA). While the lateral pathway (cortico-cortical projections between parietal and premotor regions) is involved in motor behaviors dependent on an explicit external input, a relatively straightforward matching of input and output, the medial circuit operates in a ‘projectional’ or predictive fashion. The results of the present study and of our previous work (Schettino et al. 2003b) suggest that PD subjects exhibit deficits in predictive control and in the coordination of movement consistent with this proposed role for the basal ganglia. Furthermore, PD subjects’ increased reliance on sensory input is consistent with the idea that following BG dysfunction and the consequent loss of predictive control, PD subjects must rely preferentially on their responsive lateral pathways for the control of movement.
Dopamine replacement therapy and the Parkinsonian brain
Recent work conducted at the cellular level indicates that loss of dopamine terminals results in reorganization within the striatum (Cho et al. 2002). One of the outcomes of such a process could be a disruption of the response patterns established during previous motor learning (Jog et al. 1999), observable behaviorally as a disruption in the execution of motor routines. While DA replacement through L-dopa intake may partially reinstate striatal connectivity (Calabresi et al. 2000), thereby relieving the motor system from the maladaptive braking influence exerted by tonic inhibitory projections from the GPi on the motor thalamus (Albin et al. 1989), our results suggest that the coordinated control of behavior remains disrupted.
At the systems level, the loss of balance between predictive and responsive control in PD has been documented by numerous brain mapping studies (Playford et al. 1992; Jahanshahi et al. 1995; Samuel et al. 1997a, b; Owen et al. 1998; Catalan et al. 1999; Sabatini et al. 2000; Haslinger et al. 2001; Fukuda et al. 2001; Rowe et al. 2002, Mattay et al. 2002). Converging evidence from this brain imaging work has indicated that during the execution of complex movements there is, in PD, a hypoactivation of rostral SMA, which has been proposed to underlie akinesia (Samuel et al. 1997a; Sabatini et al. 2000; Haslinger et al. 2001; see also Rowe et al. 2002) and abnormal nonspecific hyperactivity of motor cortex, which has been proposed to underlie bradykinesia (Haslinger et al. 2001). Dopamine replacement therapy results in a partial reversal of the abnormal activation patterns concomitant with the amelioration of clinical parameters of bradykinesia, akinesia and rigidity (Haslinger et al. 2001; Mattay et al. 2002). Interestingly, studies comparing surgical procedures geared to improve those precise parameters have reached similar conclusions (Samuel et al. 1997b; Fukuda et al. 2001). Some of these studies have also uncovered an increase in activation in parietal and premotor cortical fields (Samuel et al. 1997a; Catalan et al. 1999; Sabatini et al. 2000; Haslinger et al. 2001), which has been suggested to represent a reorganization of motor circuits from striato-mesial loops to cortico-cortical networks (Samuel et al. 1997a; Sabatini et al. 2000). The overall effect of this reorganization, based on anatomical and physiological evidence (Luppino et al. 1993), is thought to favor an externally-guided pattern of motor control as an alternative to the dysfunctional internally-guided loops. The behavioral correlates of this reorganization would be an increased reliance on external guidance for movement control, consistent with the experimental data from the present study and others (Flowers et al. 1976; Klockgether and Dichgans 1994; Beuter et al. 1990; Georgiou et al. 1993; Nixon and Passingham 1998; Poizner et al. 2000, Adamovich et al. 2001).
Two kinds of Parkinsonian deficits
We propose that in Parkinson’s disease it is possible to differentiate two distinct types of motor deficits. The first, a simple loss of motor power or gain, is likely to be a more generalized decrease in facilitation of motor output due to decreased BG contributions that normally operate in an intensive manner. Clinical signs of bradykinesia and rigidity may be consequences of this loss of facilitation. The second is due to the loss of a more precise, differentiated BG function that is topographically organized and facilitates the integration of different brain regions needed for coordinated motor output. A neurophysiological sign of this loss may be the decrease in synchronized cortical activity previously noted in Parkinson’s disease (Wang et al. 1999). It is our suggestion that the first kind of deficit will be more amenable to the repletion of dopaminergic drive through PD treatment while the second kind of deficit may remain impervious to treatment. In so far as the Parkinsonian basal ganglia either fail to facilitate normal movement or act as a brake upon it, simply restoring an appropriate level of neuronal activity through repletion of dopamine should reduce bradykinesia. However, the degenerated BG will be unable to reconstitute the pattern of inhibitory and excitatory activities required to support coordinative sensorimotor processing (Cho et al. 2002).
References
Adamovich SV, Berkinblit MB et al (2001) The interaction of visual and proprioceptive inputs in pointing to actual and remembered targets in Parkinson’s disease. Neuroscience 104:1027–1041
Agostino R, Berardelli A et al (1998) Clinical impairment of sequential finger movements in Parkinson’s disease. Mov Disord 13(3):418–421
Alberts JL, Saling M et al (2000) Disruptions in the reach-to-grasp actions of Parkinson’s patients. Exp Brain Res 134:353–362
Alberts JL, Tresilian JR et al (1998) The co-ordination and phasing of a bilateral prehension task. The influence of Parkinson’s disease. Brain 121(Pt 4):725–742
Albin RL, Young AB et al (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–375
Aldridge JW, Berridge KC (1998) Coding of serial order by neostriatal neurons: a natural action approach to movement sequence. J Neurosci 18(7):2777–2787
Baroni A, Benvenuti F et al (1984) Human ballistic arm abduction movements: effects of L-dopa treatment in Parkinson’s disease. Neurology 34:868–874
Benecke R, Rothwell JC et al (1987a) Disturbance of sequential movements in patients with Parkinson’s disease. Brain 110(Pt 2):361–379
Benecke R, Rothwell JC et al (1987b) Simple and complex movements off and on treatment in patients with Parkinson’s disease. J Neurol Neurosurg Psychiat 50:296–303
Berardelli A, Rothwell JC et al (2001) Pathophysiology of bradykinesia in Parkinson’s disease. Brain 124:2131–2146
Berthier NE,Clifton RK et al (1996) Visual information and object size in the control of reaching. J Motor Behav 28:187–197
Beuter A, Milton JG et al (1990) Delayed visual feedback and movement control in Parkinson’s disease. Exp Neurol 110(2):228–235
Blin O, Ferrandez AM et al (1991) Dopa-sensitive and dopa-resistant gait parameters in Parkinson’s disease. J neurolog sci 103:51–54
Bonfiglioli C, De Berti G et al (1998) Kinematic analysis of the reach to grasp movement in Parkinson’s and Huntington’s disease subjects. Neuropsychologia 36(11):1203–1208
Briand KA, Strallow D et al (1999) Control of voluntary and reflexive saccades in Parkinson’s disease. Exp Brain Res 129(1):38–48
Brown P, Marsden CD (1999) Bradykinesia and impairment of EEG desynchronization in Parkinson’s disease. Mov Disord 14(3):423–429
Burleigh-Jacobs A, Horak FB et al (1997) Step initiation in Parkinson’s disease: influence of levodopa and external sensory triggers. Mov Disord 12(2):206–215
Calabresi P, Centonze D et al (2000) Synaptic transmission in the striatum: from plasticity to neurodegeneration [In Process Citation]. Prog Neurobiol 61(3):231–265
Caligiuri MP, Galasko DR (1992) Quantifying drug-induced changes in parkinsonian rigidity using an instrumental measure of activated stiffness. Clin Neuropharmacol 15(1):1–12
Castiello U, Bennett MB et al (1994) The Reach to grasp movement of Parkinson’s disease subjects. In: Bennett MB, Castiello U (eds) Insights into the Reach to Grasp Movement. Elsevier Science, pp 215–237
Castiello U, Bennett MB (1997) The bilateral reach-to-grasp movement of Parkinson’s disease subjects. Brain 120:593–604
Castiello U, Bennett K et al (1999) The reach-to-grasp movement in Parkinson’s disease: response to a simultaneous perturbation of object position and object size. Exp Brain Res 125(4):453–462
Castiello U, Bennett M et al (2000) The reach-to-grasp movement in Parkinson’s disease before and after dopaminergic medication. Neuropsychologia 38:46–59
Catalan MJ, Ishii K et al (1999) A PET study of sequential finger movements of varying length in patients with Parkinson’s disease. Brain 122:483–495
Cavada C, Goldman-Rakic P (1991) Topographic segregation of corticostriatal projections from posterior parietal subdivisions in the macaque monkey. Neuroscience 42:683–696
Chieffi S, Gentilucci M (1993) Coordination between the transport and the grasp components during prehension movements. Exp Brain Res 94(3):471–477
Cho J, Duke D et al (2002) Dopamine depletion causes fragmented clustering of neurons in the sensorimotor striatum: evidence of lasting reorganization of corticostriatal input. J Comp Neurol 452:24–37
Chong RKY, Horak FB et al (2000) Parkinson’s disease impairs the ability to change set quickly. J Neurolog Sci 175:57–70
Churchill A, Hopkins B et al (2000) Vision of the hand and environmental context in human prehension. Exp Brain Res 134:81–89
Connoly JD, Goodale MA (1999) The role of visual feedback of hand position in the control of manual prehension. Exp Brain Res 125:281–386
Crutcher MD, DeLong MR (1984) Single cell studies of the primate putamen. II. Relations to direction of movement and pattern of muscular activity. Exp Brain Res 53(2):244–258
Demirci M, Grill S et al (1997) A mismatch between kinesthetic and visual perception in Parkinson’s disease. Ann Neurol 41(6):781–788
Desmurget M, Grafton S (2000) Forward Modeling allows Feedback Control for Fast Reaching Movements. Trend Cogn Sci 4(11):423–431
Duvoisin RC, Sage J (2001) Parkinson’s disease. A guide for patient and family. Williams & Wilkins, Philadelphia, Lippincot
Fagg AH, Arbib MA (1998) Modeling parietal-premotor interactions in primate control of grasping. Neural Networks 11:1277–1303
Feigin A, Ghilardi MF et al (2002) Effects of levodopa infusion on motor activation responses in Parkinson’s disease. Neurology 59:220–226
Fitts P (1954) The information capacity of the human motor system in controlling the amplitude of movement. J Exp Psychol 47:381–391
Flash T, Inzelberg R et al (1992) Kinematic analysis of upper limb trajectories in Parkinson’s disease. Exp Neurol 118(2):215–226
Flowers KA (1976) Visual closed-loop and open-loop characteristics of voluntary movement in patients with parkinsonism and intention tremor. Brain 99:269–310
Fukuda M, Mentis MJ et al (2001) Networks mediating the clinical effects of pallidal brain stimulation for Parkinson’s disease. A PET study of resting-state glucose metabolism. Brain 124:1601–1609
Gentilucci M, Jeannerod M et al (1994) Dissociating visual and kinesthetic coordinates during pointing movements. Exp Brain Res 102(2):359–366
Gentilucci M, Negrotti A (1999a) Planning and executing an action in Parkinson’s disease. Mov Disord 14(1):69–79
Gentilucci M, Negrotti A (1999b) The control of an action in Parkinson’s disease. Exp Brain Res 129(2):269–277
Georgiou N, Iansek R et al (1993) An evaluation of the role of internal cues in the pathogenesis of parkinsonian hypokinesia. Brain 116(Pt 6):1575–1587
Godaux E, Koulischer D et al (1992) Parkinsonian bradykinesia is due to depression in the rate of rise of muscle activity. Ann Neurol 31:93–100
Goldberg G (1985) Supplementary motor area structure and function: review and hypotheses. Behav Brain Sci 8:567–616
Graziano M, Gross C (1995) The representation of extrapersonal space: a possible role for bimodal visual-tactile neurons. In: Gazzaniga M (ed) The Cognitive Neurosciences. MIT Press, Cambridge, pp 1021–1034
Hagan JJ, Middlemiss DN et al (1997) Parkinson’s disease: prospects for improved drug therapy. Trends Pharmacol Sci 18:156–163
Hallett M, Khoshbin S (1980) A physiological mechanism of bradykinesia. Brain 103:301–314
Halsband U, Freund HJ (1993) Motor learning. Curr Opin Neurobiol 3(6):940–949
Haslinger B, Erhard P et al (2001) Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain 124:558–570
Hocherman S, Giladi N (1998) Visuomotor control abnormalities in patients with unilateral parkinsonism. Neurology 50(6):1648–1654
Horak FB, Frank J et al (1996) Effects of dopamine on postural control in parkinsonian subjects: scaling, set and tone. J Neurophysiol 2380–2396
Horak FB, Nutt JG et al (1992) Postural inflexibility in parkinsonian subjects. J Neurol Sci 111(1):46–58
Ingvarsson PE, Gordon AM et al (1997) Coordination of manipulative forces in Parkinson’s disease. Exp Neurol 145(2 Pt 1):489–501
Jackson SR, Jackson GM et al (1995) The internal control of action and Parkinson’s Disease: a kinematic analysis of visually-guided and memory-guided prehension movements. Exp Brain Res 105:147–162
Jahanshahi M, Jenkins IH et al (1995) Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 118(Pt 4):913–933
Jakobson LS, Goodale MA (1991) Factors affecting higher-order movement planning: a kinematic analysis of human prehension. Exp Brain Res 86(1):199–208
Jeannerod M (1981) Intersegmental coordination during reaching at natural visual objects. In: Long J, Baddeley A (eds) Attention and performance XI. Hillsdale, Erlbaum
Jeannerod M (1984) The timing of a natural prehension movement. J Motor Behav 26:235–254
Jeannerod M (1997) The cognitive neuroscience of action. Blackwell, Cambridge
Jeannerod M, Arbib M et al (1995) Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci 18:314–320
Jog MS, Kubota Y et al (1999) Building neural representations of habits. Science 286:1745–1749
Johnson MT, Kipnis AN et al (1996) Effects of levodopa and viscosity on the velocity and accuracy of visually guided tracking in Parkinson’s disease. Brain 119:801–813
Johnson M, Mendez A et al (1994) Acute effects of levodopa on wrist movement in Parkinson’s disease. Kinematics, volitional EMG modulation and reflex amplitude modulation. Brain 117:1409–1422
Kelly V, Hyngstrom A et al (2002) Interaction of levodopa and cues on voluntary reaching in Parkinson’s disease. Mov Disord 17:38–44
Klockgether T, Borutta M et al (1995) A defect of kinesthesia in Parkinson’s disease. Mov Disord 10(4):460–465
Klockgether T, Dichgans J (1994) Visual control of arm movement in Parkinson’s disease [see comments]. Mov Disord 9(1):48–56
Kothari A, Poizner H et al (1992) Interactive three-dimensional graphic analysis for studies of neural disorders of movement. SPIE Visual Data Interpret 1668:82–92
Langston JW, Widner H et al (1992) Core assessment program for intracerebral transplantations (CAPIT). Mov Disord 7(1):2–13
Luppino G, Matelli M et al (1993) Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comparat Neurol 338:114–140
Luppino G, Murata A et al (1999) Largely segregated parietofrontal connections linking rostral intraparietal cortex (areas AIP and VIP) and the ventral premotor cortex (areas F5 and F4). Exp Brain Res 128:181–187
Majsak MJ, Kaminski T et al (1998) The reaching movements of patients with Parkinson’s disease under self- determined maximal speed and visually cued conditions. Brain 121(Pt 4):755–766
Mattay VS, Tessitore A et al (2002) Dopaminergic modulation of cortical function in patients with Parkinson’s disease. Ann Neurol 51:156–164
Nixon PD, Passingham RE (1998) The striatum and self-paced movements. Behav Neurosci 112(3):719–724
O’Suilleabhain P, Bullard J et al (2001) Proprioception in Parkinson’s disease is acutely depressed by dopaminergic medications. J Neurol Neurosurg Psychiatry 71:607–610
Owen AM, Doyon J et al (1998) Abnormal basal ganglia outflow in Parkinson’s disease identified with PET. Implications for higher cortical functions. Brain 121(Pt 5):949–965
Percheron G, Parent A et al (1990) Substrat Anatomo-physiologique de L’akinesie Chez Le Primate. Rev Neurolog 146(10):575–584
Pine ZM, Krakauer JW et al (1996) Learning of scaling factors and reference axes for reaching movements. Neuroreport 7(14):2357–2361
Playford ED, Jenkins IH et al (1992) Impaired mesial frontal and putamen activation in Parinson’s disease: a positron emission tomography study. Ann Neurol 32:151–161
Poizner H, Feldman AG et al (2000) The timing of arm-trunk coordination is deficient and vision-dependent in Parkinson’s patients during reaching movements. Exp Brain Res 133:279–292
Poizner H, Fookson OI et al (1998) Pointing to remembered targets in 3-D space in Parkinson’s disease. Motor Control 2(3):251–277
Poizner H, Mack L et al (1990) Three-dimensional computergraphic analysis of apraxia. Brain 113:85–101
Poizner H, Wooten E et al (1986) Computergraphic modeling and analysis: a portable system for tracking arm movements in three-dimensional space. Behav Res Methods Instr Comput 18(5):427–433
Rizzolatti G, Camarda R et al (1988) Functional organization of inferior area 6 in the macaque monkey. II Area F5 and the control of distal movements. Exp Brain Res 71:491–507
Romo R, Merchant H et al (1995) Neuronal acitvity of primate putamen during categorical perception of somaesthetic stimuli. NeuroReport 6:1013–1017
Rowe J, Stephan KE et al (2002) Attention to action in Parkinson’s disease. Impaired effective connectivity among frontal cortical regions. Brain 125:276–289
Sabatini U, Boulanouar K et al (2000) Cortical motor reorganization in akinetic patients with Parkinson’s disease. Brain 123:394–403
Sage JI, Mark MH (1994) Basic mechanisms of motor fluctuations. Neurology 44:S10–S14
Saint-Cyr J, Ungerleider L et al (1990) Organization of visual cortical inputs to the striatum and subsequent outputs to the pallido-nigral complex in the monkey. J Comp Neurol 298:129–156
Samuel M, Ceballos-Baumann AO et al (1997a) Evidence for lateral premotor and parietal overactivity in Parkinson’s disease during sequential and bimanual movements. A PET study. Brain 120(1):963–976
Samuel M, Ceballos-Baumann AO et al (1997b) Pallidotomy in Parkinson’s Disease increases supplementary motor area and prefrontal activation during performance of volitional movements. An H 152 O PET study. Brain 120:1201–1213
Santello M, Soechting JF (1998) Gradual molding of the hand to object contours. J Neurophys 79:1307–1320
Schettino LF, Adamovich SV et al (2003a) Effects of object shape and visual feedback on hand configuration during grasping. Exp Brain Res 151:158–166
Schettino LF, Rajaraman V et al (2003b) Deficits in the evolution of hand preshaping in Parkinson’s disease. Neuropsychologia 42:82–94
Sivak B, MacKenzie C (1990) Integration of visual information and motor output in reaching and grasping: the contributions of peripheral and central vision. Neuropsychologia 28:1095–1106
Stebbins GT, Goetz CG (1998) Factor structure of the Unified Parkinson’s disease rating scale: motor examination section. Mov Disord 13(4):633–636
Teulings HL, Contreras-Vidal JL et al (2002) Adaptation of handwriting size under distorted visual feedback in patients with Parkinson’s disease and elderly and young controls. J Neurol Neurosurg Psychyatry 72:315–324
Tresilian JR, Stelmach GE et al (1997) Stability of reach-to-grasp movement patterns in Parkinson’s disease. Brain 120(Pt 11):2093–2111
Wang HC, Lees AJ et al (1999) Impairment of EEG desynchronisation before and during movement and its relation to bradykinesia in Parkinson’s disease. J Neurol Neurosurg Psychiatry 66(4):442–446
Wing A, Turton A et al (1986) Grasp size and accuracy of approach in reaching. J Motor Behavior 18:245–260
Yeterian E, Pandya D (1993) Striatal connections of the parietal association cortices in rhesus monkeys. J Comp Neurol 332:175–197
Acknowledgements
This research was supported in part by research grant 7 R01 NS36449 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health to UCSD and by a center grant from the American Parkinson Disease Association to Robert Wood Johnson Medical School.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schettino, L.F., Adamovich, S.V., Hening, W. et al. Hand preshaping in Parkinson’s disease: effects of visual feedback and medication state. Exp Brain Res 168, 186–202 (2006). https://doi.org/10.1007/s00221-005-0080-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-005-0080-4