Abstract
We describe in detail the frequency response of the human three-dimensional angular vestibulo-ocular response (3-D aVOR) over a frequency range of 0.05–1 Hz. Gain and phase of the human aVOR were determined for passive head rotations in the dark, with the rotation axis either aligned with or perpendicular to the direction of gravity (earth-vertical or earth-horizontal). In the latter case, the oscillations dynamically stimulated both the otolith organs and the semi-circular canals. We conducted experiments in pitch and yaw, and compared the results with previously-published roll data. Regardless of the axis of rotation and the orientation of the subject, the gain in aVOR increased with frequency to about 0.3 Hz, and was approximately constant from 0.3 to 1 Hz. The aVOR gain during pitch and yaw rotations was larger than during roll rotations. Otolith and canal cues combined differently depending upon the axis of rotation: for torsional and pitch rotations, aVOR gain was higher with otolith input; for yaw rotations the aVOR was not affected by otolith stimulation. There was a phase lead in all three dimensions for frequencies below 0.3 Hz when only the canals were stimulated. For roll and pitch rotations this phase lead vanished with dynamic otolith stimulation. In contrast, the horizontal phase showed no improvement with additional otolith input during yaw rotations. The lack of a significant otolith contribution to the yaw aVOR was observed when subjects were supine, prone or lying on their sides. Our results confirm studies with less-natural stimuli (off-vertical axis rotation) that the otoliths contribute a head-rotation signal to the aVOR. However, the magnitude of the contribution depends on the axis of rotation, with the gain in otolith-canal cross-coupling being smallest for yaw axis rotations. This could be because, in humans, typical yaw head movements will stimulate the otoliths to a much lesser extent then typical pitch and roll head movements.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eye velocity during passive head movements in the dark is a measure of the vestibular response to head motion. Vestibular information is augmented with additional information about head motion, such as visual slip, efferent copy of body movements, or muscle proprioception, to improve the human angular vestibular ocular reflex (aVOR) and so stabilize the retinal image and improve vision (Demer et al. 1993; Baloh and Demer 1991; Kasteel-van Linge and Maas 1990).
The peripheral vestibular system is comprised of two types of motion sensors: the semi-circular canals, which respond to angular acceleration; and the otoliths, which sense linear acceleration caused by translation and gravity. Since a change in head orientation relative to gravity is a rotation, otolith afferents also carry implicit information about head rotation. We expect that the nervous system would use all available information to compensate for any inherent limitations in sensory systems (Zupan et al. 2002; Angelaki et al. 1999; Bockisch et al. 2003), and to adapt to changes that occur due to development, aging, disease, and injury. One limitation of the semi-circular canals is their high-pass frequency characteristics, caused primarily by the elastic restoring force of the cupula (Fernandez and Goldberg 1971). Since the otolith organs respond well to low frequency stimulation, we would expect them to contribute to the low frequency aVOR when possible. Evidence for otolith contribution to the aVOR in non-humans exists [cat: (Rude and Baker 1988; Blanks et al. 1978; Tomko et al. 1988); rabbit: (Barmack and Pettorossi 1988; Pettorossi et al. 1991); monkey: (Angelaki and Hess 1996; Angelaki et al. 2002)]. In humans, similar evidence for a contribution of dynamic otolith signals to the aVOR mainly comes from experiments with stimuli that do not occur in everyday life, for example, long-duration off-vertical axis rotation (Haslwanter et al. 2000; Harris and Barnes 1987; Furman et al. 1992; Darlot et al. 1988), per- or post-rotatory tilt (Bockisch et al. 2003; Fetter et al. 1996; Zupan et al. 2000), or eccentric rotation about the earth-vertical axis (Merfeld et al. 2001; Lansberg et al. 1965). These stimuli include strong, unusual conflicts between sensory cues.
Numerous studies of the frequency response of the human aVOR have been published (Mathog 1972; Peterka et al. 1990; Demer et al. 1993; Schmid-Priscoveanu et al. 2000; Groen et al. 1999; Peterka 1992; Clarke et al. 2000; Kasteel-van Linge and Maas 1990; Hyden and Larsby 1991). However, no study has investigated the horizontal, vertical, and torsional responses under identical conditions in humans across a range of frequencies. Tweed et al. (1994) studied the passive human 3-D aVOR, including body orientations that elicited dynamic otolith stimulation, but their study was restricted to a single frequency (0.3 Hz).
The purpose of the present study was to describe the frequency response of the human three-dimensional aVOR to canal-alone and to canal-plus-otolith stimulation, using more natural stimuli than the experiments with cue-conflicts mentioned above. We used the same stimuli and equipment as Schmid-Priscoveanu et al. (2000) in their study of the torsional aVOR, and combined our measurements during pitch and yaw rotations with their roll data to provide a full description of the frequency response of the human 3-D aVOR.
Materials and methods
Subjects
The 20 volunteer subjects, which included the authors, were free of any known vestibular or ocular pathologies. The experimental protocols were approved by a local ethics committee at Zürich University Hospital, and adhered to the Declaration of Helsinki for research involving human subjects.
Apparatus
The three-axis rotational stimulator was driven by three servo-controlled motorized axes (Acutronic, Switzerland), controlled with Acutrol software and hardware, and interfaced with LabVIEW software. Subjects were comfortably seated in a chair and secured with safety belts and evacuation pillows molded to the upper body and legs. The center of the head was positioned at the center of rotations. Individually adjusted masks (Sinmed BV, Reeuwijk, The Netherlands), made of a thermoplastic material (Posicast), were molded to the contour of the head after warming, with openings in the mask made for the eyes and mouth. The mask was attached to the back of the chair, and restricted head movements very effectively without causing discomfort.
The 3-D position of one eye (usually the left) was measured with search coils manufactured by Skalar (Delft, The Netherlands). A chair-fixed coil frame (side length 0.5 m) surrounded the head and produced three orthogonal magnetic fields with frequencies of 80, 96 and 120 kHz. The signals were amplified and multiplexed before passing through the turntable slip rings. A high performance digital signal processor computed a Fast Fourier Transform in real time on the digitized search coil signal to determine the voltage induced in the coil by each magnetic field (system manufactured by Primelec, Regensdorf, Switzerland). Eye position signals were digitized with 12-bit accuracy, and chair position signals with 16-bit. All data were sampled at 1 kHz, and analyzed offline with MatLab software (The MathWorks, Boston, MA).
To calibrate the coils, we first zeroed voltage offsets while placing the search coils in a metal tube to shield them from the magnetic fields. We measured the relative gains of the three magnetic fields with the search coils on a gimbal placed in the magnetic field at the same location as the measured eye.
Procedure
Subjects were oscillated with different frequencies in pitch (about the inter-aural axis) or yaw (rostro-caudal axis) in separate sessions. The corresponding roll data presented here were taken from the study of Schmid-Priscoveanu et al. (2000). We instructed subjects to fixate an imagined, distant, and space-fixed target in the “straight ahead” position at the beginning of each trial. We monitored eye position during each trial, and spoke to the subject if their attention appeared to wander.
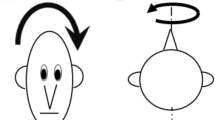
Subjects were rotated about the earth-vertical axis (spinning on an office chair, for example), so that the forces on the otoliths were constant (gravity-neutral; Fig. 1, top row), and about an earth-horizontal axis (such as a “barbeque spit” rotation), which dynamically stimulated the otolith organs (gravity-assisted; Fig. 1, bottom row). For yaw rotations subjects were rotated while upright (gravity-neutral) or supine (gravity-assisted); for pitch rotations while on the side (gravity-neutral) or upright (gravity-assisted). To facilitate comparisons with previous studies, we used the same frequencies and amplitudes as Schmid-Priscoveanu et al. (2000) in their study of the torsional aVOR. The frequencies were 0.05, 0.1, 0.3, 0.5, 0.7, and 1 Hz. Due to the torque limitations of our turntable, the amplitude changed with frequency and yielded peak velocities of 12.6, 25.1, 37.7, 25.1, 17.6, and 12.6 deg/s. Subjects were oscillated for either five complete cycles (for the lowest frequencies) or for 30 s, whichever was longer. Before each oscillation, the subject was returned to the upright orientation and fixated a 1.5 m distant target in the mid-sagittal plane prior to the start of the next trial. Breaks were taken periodically, about every 5–7 minutes, with the room lights turned on.
The types of rotation used in these experiments. The gray rod represents the axis of rotation, which passes through the center of the subject’s head. The top row shows the gravity-neutral rotations, or rotations about the earth-vertical axis, which do not dynamically stimulate the otolith organs. All other rotations were about the earth-horizontal axis, or gravity-assisted, because the head changes orientation relative to gravity and so stimulates the otolith organs. The roll experiments were conducted by Schmid-Priscoveanu et al. (2000)
The results of the first set of experiments led us to perform additional low frequency yaw oscillation experiments when subjects were upright, left side down (LSD, Fig. 1 bottom right), and prone. The procedure was the same as described above, except we only tested with frequencies of 0.05, 0.1, and 0.3 Hz.
Data analysis
We represent eye positions as 3-D rotation vectors in a head-fixed coordinate system. Positive eye position values indicate clockwise, up, and right from the subject’s perspectiveFootnote 1. We call rotation around the naso-occipital axis “torsion”, rotations about the interaural axis “vertical” eye movements, and rotations about the axis pointing through the top of the head “horizontal” eye movements. The reference position [0°, 0°, 0°] was always determined by the eye position when the subject was upright and fixating a 1.5 m distant target in the mid-sagittal plane prior to the start of each trial.
Rotation vectors were smoothed, and angular eye velocity was computed as described previously (Hepp 1990; Tweed et al. 1990). Slow phase eye velocity was found with an interactive computer program that first automatically detected saccades based on velocity and noise criteria, and then allowed the user to adjust the automatically-marked saccades and to remove blink artifacts.
Periods of unusually low eye velocity, which clearly deviated from surrounding data and indicated inattentiveness by the subject, were identified and removed from further analysis (on average, less than 6% of each trial was omitted). We fit sinusoidal curves to the desaccaded eye velocity, to calculate the amplitude, phase and offset of the aVOR. The frequency was fixed to the frequency of the head rotation. Gain was defined as the eye velocity amplitude divided by the peak head velocity. We reversed the sign of the head velocity so that a gain of +1 and a phase of 0° indicate a perfect compensatory eye movement, and a positive phase lead, with peak eye velocity preceding peak head velocity.
In these experiments, subjects were instructed to fixate an imagined spatially-fixed target that was “straight ahead” prior to the start of rotation. As a result, eye position did not systematically vary orthogonal to the axis of rotation, so the effect of eye position on the axis of rotation (like tilts of Listing’s Plane) was not addressed.
The average phase was calculated using statistics for circular data. The mean phase is given by the geometric mean of the corresponding points on a unit circle. The circular standard deviation was determined assuming a wrapped normal distribution, using the corresponding gain value to weigh each phase contribution (Mardia 1972).
For statistical comparison of gains we computed repeated-measures analyses of variance (ANOVAs) with body orientation and frequency as factors, using the statistical software program MINITAB. Separate ANOVAs were computed for each axis of eye velocity (torsional, vertical, and horizontal) and each experiment. When the eye-velocity axis was not the same as the axis of head rotation, measured gains were low and their distributions positively skewed, which violates the normality assumptions necessary for the ANOVAs. So, we normalized these gain distributions by performing a log transformation, and then we performed ANOVAs on the transformed gain (Kleinbaum et al. 1988). For circular data (phase response), no equivalent for a t-test exists. Also, while two sets of “reasonably well” oriented data can be compared with the Williams-Watson test, no test that would be equivalent to ANOVAs could be found. However, when the axis of head velocity is the same as the axis of eye velocity the variation in phase values is small and the “wrapping” problem is also avoided, so we treat the phases like normal numbers and compute repeated-measures ANOVAs. When the eye velocity response is small, however, we report the phase values without any formal statistical analysis.
To ensure that the same analysis procedures were used on all data, we reanalyzed the roll oscillation data of Schmid-Priscoveanu et al. (2000). The conclusions and interpretations we obtained were the same as the ones in the original analysis.
Results
Figure 2 shows an example of vertical eye-position and velocity for a subject rotated at 0.1 Hz while upright (A, B) and lying on their left side (C, D). In both orientations, pitch oscillation stimulates primarily the posterior and anterior semi-circular canals. In addition, head pitch movements in the upright condition also stimulate the otolith organs (both the utricle and the saccule are stimulated). It is readily apparent that the eye position and velocity traces are more regular when the subject is upright (gravity-assisted: A, B). Also noticeable in Fig. 2 is the difference in phase between the head and eye velocity when the subject is in the gravity-neutral orientation (bottom rows: C, D), with eye velocity showing a phase lead compared to head velocity. Our subjects typically reported that during gravity-assisted rotations they had a more certain feeling of their body movement in space as compared to the gravity-neutral conditions.
Example data from a pitch oscillation experiment. A Vertical eye-in-head (solid lines) and inverted head (dotted lines) pitch position, when a subject was upright and oscillated at 0.1 Hz. B Corresponding eye and head velocity. C Vertical eye-in-head and inverted head pitch position, when a subject was oriented on his side and oscillated at 0.1 Hz. D Corresponding eye and head velocity
Gain
Figure 3 summarizes the human angular vestibular ocular response to low and medium frequency stimulation. We first describe the main components of the aVOR (the cases when the axis of eye velocity aligned with the axis of head velocity), shaded in Fig. 3. We discuss the off-axis components separately.
The frequency response of the human 3-D aVOR in roll (top row), pitch (middle), and yaw (bottom). The roll oscillation data were taken from Schmid-Priscoveanu et al. (2000). Solid lines indicate data collected when the head orientation changed with respect to gravity (see legend on left). Dotted lines show data collected when the head orientation did not change with respect to gravity. Points are the means of ten subjects, and the error bars are ±1 standard error. Cases where the axis of eye velocity was aligned with the axis of head velocity are shaded
In all three dimensions, the aVOR gain, averaged across subjects, increased sharply from 0.05 Hz to 0.3 Hz, and was then steady up to 1 Hz. The average gain of the torsional aVOR (data from Schmid-Priscoveanu et al. 2000), which reaches a maximum of about 0.35, is about half the average gain of the pitch and yaw aVOR, which both peak between 0.6–0.7. For roll and pitch aVOR, gravity-assisted rotations caused a significant increase in aVOR gain compared to the gravity-neutral conditions. Torsional aVOR gain was about 0.1 higher in the gravity-assisted condition than in the gravity-neutral condition. For the pitch aVOR, the gravity-assisted gain was, on average, 0.054 higher than the gravity-neutral condition. For the yaw aVOR, gain in the gravity-assisted and gravity-neutral conditions appear similar. Repeated-measures ANOVAs conducted separately for each experiment with body orientation and oscillation frequency as factors showed that the effect of body orientation was significant for roll (ANOVA main effect of orientation, F(1,97)=71.78, p<0.001) and for pitch (F(1,99)=7.45, p<0.01). For yaw there was no significant effect of body orientation (F(1,99)=0.74, p<0.4).
In all three experiments, the main effect of oscillation frequency on gain was significant (roll: F(5,97)=12.14, p<0.001; pitch: F(5,99)=12.72, p<0.001; yaw: F(5,99)=10.97, p<0.001). The interaction of orientation and frequency was never significant (roll: F(5,97)=0.15; pitch: F(5,99)=0.34, yaw: F(5,99)=0.28; all values of p>0.8).
Phase
When the axis of eye velocity was aligned with the axis of head velocity, the phase of the low frequency aVOR depended upon head orientation relative to gravity for roll and pitch, but not for yaw. For low frequency roll and pitch oscillations (0.05–0.1 Hz), the gravity-neutral aVOR showed an average phase lead of 25–30°, which approached zero for frequencies above 0.5 Hz (Fig. 3, left top, and middle center). This phase lead was eliminated in the gravity-assisted paradigms.
For the yaw aVOR the average phase for both upright and supine orientations show a phase lead of 10–20° below 0.3 Hz, which changes to a small phase lag above 0.5 Hz (Fig. 3, right bottom).
We conducted repeated-measures ANOVAs on the phases in each experiment, and an identical pattern of results to the analysis of gain was found: body orientation affects the roll and pitch aVOR, but not the yaw aVOR. For the roll data of Schmid-Priscoveanu et al. (2000), the main effects of orientation (F(1,97)=4.09, p<0.05), and frequency (F(5,97)=2.84, p<0.02) were both significant, as well as their interaction (F(5,97)=4.16, p<0.01). A similar pattern was found for the pitch experiment, where the main effects of orientation (F(1,99)=32.9, p<0.001) and frequency (F(5,97)=22.5, p<0.001) were statistically significant, as well as their interaction (F(5,99)=15.01, p<0.001).
The ANOVA for the yaw experiment was different, however. While the main effect of frequency was statistically significant (F(1,99)=46.96, p<0.001), the effect of orientation was not (F(1,99)=1.01, p>0.3), nor was their interaction significant (F(5,99)=1.05, p>0.3).
Alignment of eye and head axes of rotation
The axis of eye rotation was generally well aligned with the axis of head rotation. The off-diagonal, non-shaded plots in Fig. 3 show gain (eye velocity/peak head velocity) and phase of the off-axis eye movement components.
There were a few cases when the axis of eye velocity did not perfectly align with the head rotation axis. Horizontal eye velocity was observed during upright and supine roll stimulation, and torsional velocity was seen during both pitch and yaw oscillations. In all cases the gain was quite low.
For the roll data collected by Schmid-Priscoveanu et al. (2000), ANOVAs revealed that neither horizontal nor vertical gain varied significantly with body orientation or frequency. In the pitch experiment, there was a main effect of frequency on log torsional gain (F(5,99)=3.25, p<0.01), reflecting the rise in gain with frequency seen Fig. 3. All other effects failed to reach statistical significance at a level of 0.05.
In the yaw experiment, the ANOVA of the log torsional gain revealed a main effect of orientation (F(5,99)=35.26, p<0.001), and an interaction of frequency and orientation (F(5,99) =4.67; p<0.001). The ANOVA on log vertical eye gain found no significant effect of frequency or body orientation.
Yaw oscillation when side down and prone
The results presented above show that otolith-canal interactions during yaw aVOR are different than during roll and pitch aVOR: simultaneous otolith stimulation does not improve yaw aVOR gain and does not reduce the phase lead at very low frequencies. To be certain that this was not due to the particular orientation (supine) that we tested, we conducted a further study with yaw rotations where subjects were prone, lying with their left side down (LSD), or upright. We concentrated on the lowest frequencies (0.05, 0.1, and 0.3 Hz), where differences due to the direction of gravity were most apparent during roll and pitch oscillation.
Figure 4 shows an example trial in one subject when they were oscillated at 0.05 Hz in the LSD orientation. Torsional, vertical, and horizontal eye velocity is shown (Fig. 4, top to bottom). Peak horizontal eye velocity (bottom panel) is about half the peak head velocity (dotted line, and inverted in the figure to facilitate comparison), and eye velocity is in-phase with inverted head velocity. Vertical eye velocity, of lower amplitude, is apparent in the middle panel.
The average horizontal gain at 0.05 Hz was about 0.5, increased to 0.6–0.7 at 0.3 Hz, and was similar for upright, LSD, and prone orientations (Fig. 5, right). Horizontal phase was also similar in all three orientations: a phase lead of about 15°, on average, was observed at 0.05 Hz, which declined to about 2° at 0.3 Hz (Fig. 5, far right). These results are very similar to those we found when subjects were oscillated in the upright and supine orientations (Fig. 3, bottom panels).
In contrast to the upright and supine orientation, we found consistent vertical eye velocity in the LSD orientation (Fig. 4, middle; and Fig. 5, middle left). While the magnitude was small (at 0.05 Hz the mean amplitude was 2 deg/s), this finding was also reflected in the perceived orientation of the rotation: all subjects reported that the yaw oscillations in the LSD orientation felt very different to the other orientations, but that the differences were difficult to put in words. They described the rotation more like a combined yaw-roll movement. This perception decreased at higher frequencies.
Repeated-measures ANOVA on the horizontal gain found a significant main effect of frequency (F(2,81)=39.6; p<0.01). The main effect of orientation was not statistically significant (F(2,81)=1.5; p>0.2), but the interaction of orientation and frequency was significant (F(4,81)=5.5, p<0.005). For the horizontal phase, an ANOVA found significant main effects of body orientation (F(2,81)=4.96, p<0.01), and oscillation frequency (F(2,81)=65.7, p<0.001), as well as their interaction (F(4,81)=3.27, p<0.05). Multiple comparison tests found that the phase when upright differed from the phase when prone (mean phase difference was 3.5°) (t=3.13, p<0.01), but no other comparison reached statistical significance. This suggests a modest effect of body orientation on the phase of the yaw aVOR, yet it appears considerably reduced compared to those found for pitch and roll (Fig. 3).
Repeated-measures ANOVA on the log torsional gain found significant main effects of frequency (F(2,81)=7.2; p<0.001) and body orientation (F(2,81)=6.9; p<0.002). The interaction of frequency and orientation was not significant. Multiple comparisons found that the torsional gain in the gravity-neutral (upright) condition was significantly different from both of the gravity-assisted orientations (LSD: t=2.9, p<0.05; prone: t=3.37, p<0.01), but the gravity-assisted orientations were not significantly different.
For log vertical gain, the ANOVA showed a significant main effect of body orientation (F(2,81)=45.1; p<0.001). Multiple comparisons found that the vertical gain was different in all body orientations (LSD-prone t=6.2, p<0.001; LSD-upright t=9.3, p<0.001; prone-upright t=2.9, p<0.02). The main effect of frequency did not quite reach statistical significance (F(2,81)=3.0; p<0.06). The interaction of frequency and body orientation was not significant.
Discussion
For rotations about the head fixed roll, pitch, and yaw axes, the gain of the human aVOR increased with stimulus frequency from 0.05 to 0.3 Hz, and then was about constant to 1 Hz. Roll gain, which was determined by Schmid-Priscoveanu et al. (2000) under identical conditions, was roughly half of the pitch and yaw gain. We unexpectedly found that the otolith contribution to the aVOR depended strongly on the axis of rotation: stimulating the otoliths improved the pitch and roll aVOR, but not the yaw aVOR. For pitch and roll, stimulating the otoliths produced a constant increase in aVOR (0.05 for pitch, Fig. 3, middle-row; and 0.1 for roll, Fig. 3, top row, courtesy of Schmid-Priscoveanu et al. 2000), and for both pitch and roll the phase misalignment seen at low frequencies for canal-only stimulation was improved with otolith stimulation. For yaw, however, we found no improvement of the gain, and only a slight effect on phase in the second experiment, where the phase in the prone position was slightly improved (Fig. 3, bottom-row, and Fig. 5).
The relatively low maximum gains we found contrast with the aVOR gains of near unity observed with very short duration, high acceleration movements (Aw et al. 1996; Crane et al. 2000). The decreased gain in our paradigm is probably not due to the attentional demands of the task (where each trial is at least 30 s), because the torsional gain is also low, and torsional eye movements are generally not thought to be under conscious control. Crane et al. (1997) measured the yaw aVOR at a higher frequency (1.2 Hz), and found gains similar to ours at 1 Hz. With the higher accelerations attained with rapid head impulses, non-linear VOR pathways may be recruited (Minor et al. 1999), leading to higher gains (Aw et al. 1996). Rapid head impulses are also non-periodic, and lead to a predominantly unilateral canal stimulation. The lower gain we observed for the oscillations may therefore arise due to the process of combining signals from the labyrinth on each side.
Alignment of eye and head axes of rotation
We did not expect to find large, consistent misalignments of the axis of eye and head velocity when only the canals were stimulated, and in general we found the axes were closely aligned, confirming the results of Tweed et al. (1994) who tested only at 0.3 Hz. There are, however, several reasons to expect off-axis velocity when the otolith organs are stimulated, particularly with low frequency oscillations. When the otolith organs are stimulated during head tilt, it is possible the stimulation could be interpreted as a head translation, thereby invoking the translational VOR (tVOR). The nervous system could distinguish tilt from translation through frequency segregation (Paige and Tomko 1991; Mayne 1974), or by combining otolith signals with other sensory signals such as canal signals (Merfeld and Young 1995; Merfeld et al. 1993; Zupan et al. 2002; Angelaki et al. 1999). Our data cannot distinguish between these proposals, but they do indicate how well the tilt/translation ambiguity is resolved.
Misalignments of the axis of eye velocity can also be introduced by erroneous estimates of the direction of gravity. Such a misestimation can, for example, be caused by a bias of the perceived vertical towards the body’s longitudinal axis, the “idiotropic vector” introduced by Mittelstaedt (1983) and observed more recently (Dai et al. 1989; Mittelstaedt and Glasauer 1993; Glasauer and Mittelstaedt 1998). Recent models of otolith-canal interaction (Haslwanter et al. 2000; Zupan et al. 2002) suggest that the resulting mismatch between the expected direction of linear accelerations (including the idiotropic vector) and the actually sensed one is interpreted as rotation. These models can correctly predict the vertical eye movements that we found during the yaw oscillations in the ear-down orientation (Figs. 4 and 5). However, the predicted torsional components should be larger than the vertical components, which we did not observe. In our second experiment, the torsional eye velocity components during yaw were slightly higher in the gravity-assisted conditions, which could be a tVOR response. Torsional velocity was also seen in upright yaw, and could be related to the canal-elicited static torsion during yaw velocity steps found by Smith et al. (1995).
In the pitch experiment there was a small, statistically significant increase in torsional eye movements with frequency. Torsional velocity during head pitch would be expected if subjects fixed a near target, due to the tilt of Listing’s Plane with vergence (Mok et al. 1992). While we asked subjects to fix on an imagined, distant target, it is possible that they maintained a smaller vergence angle. Since that component did not depend on body orientation, it is probably due to canal stimulation.
Our analysis of the off-axis data during roll stimulation conducted by Schmidt-Priscoveanu et al. revealed horizontal eye velocity during both upright and supine stimulation, with a frequency-independent gain. Tweed et al. (1994) made similar observations, even though they only tested at 0.3 Hz. Again, the frequency- and orientation-independence indicates that the horizontal eye movements may arise from canal stimulation. Further, if the brain interpreted linear acceleration during roll as arising from a translational movement, we would expect horizontal components to be higher in the upright condition. This was not observed.
Multisensory contribution to the aVOR
Previous experiments have demonstrated that dynamic otolith signals contribute to the estimate of head angular velocity in the VOR. Angelaki and Hess (1996) collected data similar to ours in the monkey but over a wider frequency range (0.01–1.0 Hz). For frequencies above 0.1 Hz, the aVOR gain in monkeys was close to unity, even for gravity-neutral oscillations. The drop in gain at low frequencies, which in humans begins at 0.3 Hz, appears in monkeys only below 0.05 Hz. As a result of the high gain in these conditions with canal-only stimulation, it is difficult to identify an otolith contribution to the aVOR. At the very lowest frequency tested, 0.01 Hz, the difference between yaw and pitch/roll did appear in monkeys: dynamic otolith input improved the pitch and roll aVOR, but not the yaw (Angelaki and Hess 1996, Fig. 10). The relatively low aVOR gain in humans compared to monkeys may suggest that humans typically rely more on visual signals to drive compensatory eye movements at low and medium frequencies.
In humans, otolith-canal interactions have been studied in other paradigms such as long duration off-vertical axis rotations, where compensatory eye velocity remains after canal signals have decayed (Haslwanter et al. 2000; Harris and Barnes 1987; Furman et al. 1992; Darlot et al. 1988). While some studies suggest caution in interpreting results from paradigms with strong cue-conflicts (Bockisch et al. 2003), it is interesting that they produce comparable results: during 100 deg/s rotation about an earth-horizontal axis with subjects oriented parallel to the rotation axis, a step in velocity produces horizontal nystagmus that decays to a very small offset value of perhaps 5% of the rotation velocity (Haslwanter et al. 2000). Therefore, that study suggests that dynamic otolith stimulation alone at a frequency of 0.28 Hz (100 deg/s/360°) produces a yaw aVOR gain of less than 0.05.
The quality of the aVOR for low frequency head movements (<1 Hz) can also, of course, be improved with visual pursuit mechanisms (Baloh and Demer 1991; Demer et al. 1993). This does not obviate the need for accurate head rotation signals at low frequencies, since retinal slip could be misinterpreted as arising from object motion rather than self-motion. The improvement we found of the aVOR at low frequencies when the head changes orientation relative to gravity suggests that the nervous system does use vestibular mechanisms for improving the estimates of the head movement at these very low frequencies, and does not rely solely on extra-vestibular information.
Why is yaw different?
The relative lack of otolith contribution to the yaw VOR is surprising. The approximately orthogonal arrangement of utricle and saccule, together with the curved shape of the otolith surfaces, guarantees that in every orientation the otoliths provide some information about our spatial orientation (Takagi and Sando 1988; Sato et al. 1992; Jaeger et al. 2002). So a yaw rotation signal from the otoliths is available to the nervous system, yet it is apparently not used for oculo-motor control, even though the eye movements are not nearly compensatory to the head movement.
Consistent with our results, however, is the finding in cats of less convergence of horizontal canal signals onto otolith-sensitive neurons in the vestibular nucleus compared to anterior and posterior canal-otolith convergence (Zhang et al. 2001, 2002). Eye movements after post-rotary tilts also indicate that in rhesus monkeys otolith signals transform horizontal canals signals differently than vertical canal signals, rotating the former while projecting the latter onto gravity (Angelaki and Hess 1995). During re-orientation, only those components of the vertical canal signals that are parallel to gravity are retained, while the magnitude of horizontal canal signals remains almost unchanged. The resulting signals are such that an eye velocity elicited by an earth-vertical axis rotation tends to align with gravity after reorientation.
Differences in the interactions of otolith and canal signals may in part originate in the biomechanics of head-movements: in the common upright orientation, roll and pitch movements of the head typically induce strong otolith stimulation. In contrast, yaw head movements stimulate the otoliths to a much smaller degree. These differences might result in different processing patterns for the different sensory inputs.
Notes
We adopt the sign conventions for horizontal and vertical eye movements that are traditionally used in studies of 2-D eye movements. By deviating from the “right hand rule”, we are not forced, for example, to represent a downward eye movement as an upward deflection on a graph.
References
Angelaki DE, Hess BJ (1995) Inertial representation of angular motion in the vestibular system of rhesus monkeys. II. Otolith-controlled transformation that depends on an intact cerebellar nodulus. J Neurophysiol 73:1729–1751
Angelaki DE, Hess BJ (1996) Three-dimensional organization of otolith-ocular reflexes in rhesus monkeys. II. Inertial detection of angular velocity. J Neurophysiol 75:2425–2440
Angelaki DE, McHenry MQ, Dickman JD, Newlands SD, Hess BJM (1999) Computation of inertial motion: neural strategies to resolve ambiguous otolith information. J Neurosci 19:316–327
Angelaki DE, Newlands SD, Dickman JD (2002) Inactivation of semicircular canals causes adaptive increases in otolith-driven tilt responses. J Neurophysiol 87:1635–1640
Aw ST, Haslwanter T, Halmagyi GM, Curthoys IS, Yavor RA, Todd MJ (1996) Three-dimensional vector analysis of the human vestibuloocular reflex in response to high-acceleration head rotations I. Responses in normal subjects. J Neurophysiol 76:4009–4020
Baloh RW, Demer J (1991) Gravity and the vertical vestibulo-ocular reflex. Exp Brain Res 83:427–433
Barmack NH, Pettorossi VE (1988) The otolithic origin of the vertical vestibuloocular reflex following bilateral blockage of the vertical semicircular canals in the rabbit. J Neurosci 8:2827–2835
Blanks RH, Anderson JH, Precht W (1978) Response characteristics of semicircular canal and otolith systems in cat. II. Responses of trochlear motoneurons. Exp Brain Res 32:509–528
Bockisch CJ, Straumann D, Haslwanter T (2003) Eye movements during multiaxis whole-body rotations. J Neurophysiol 89:355–366
Clarke AH, Grigull J, Mueller R, Scherer H (2000) The three-dimensional vestibulo-ocular reflex during prolonged microgravity. Exp Brain Res 134:322–334
Crane BT, Viirre ES, Demer JL (1997) The human horizontal vestibulo-ocular reflex during combined linear and angular acceleration. Exp Brain Res 114:304–320
Crane BT, Tian JR, Demer JL (2000) Initial vestibulo-ocular reflex during transient angular and linear acceleration in human cerebellar dysfunction. Exp Brain Res 130:486–496
Dai MJ, Curthoys IS, Halmagyi GM (1989) A model of otolith stimulation. Biol Cybern 60:185–194
Darlot C, Denise P, Droulez J, Cohen B, Berthoz A (1988) Eye movements induced by off-vertical axis rotation (OVAR) at small angles of tilt. Exp Brain Res 73:91–105
Demer JL, Oas JG, Baloh RW (1993) Visual-vestibular interaction in humans during active and passive, vertical head movement. J Vestibul Res 3:101–114
Fernandez C, Goldberg JM (1971) Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol 34:661–675
Fetter M, Heimberger J, Black RA, Hermann W, Sievering F, Dichgans J (1996) Otolith-semicircular canal interaction during postrotatory nystagmus in humans. Exp Brain Res 108:463–472
Furman JM, Schor RH, Schumann TL (1992) Off-vertical axis rotation: a test of the otolith-ocular reflex. Ann Oto Rhinol Laryn 101:643–650
Glasauer S, Mittelstaedt H (1998) Perception of spatial orientation in microgravity. Brain Res Brain Res Rev 28:185–193
Groen E, Bos JE, De Graaf B (1999) Contribution of the otoliths to the human torsional vestibulo-ocular reflex. J Vestibul Res 9:27–36
Harris LR, Barnes GR (1987) Orientation of vestibular nystagmus is modified by head tilt. In: Graham MD, Kemink JL (eds) The vestibular system: Neurophysiologic and clinical research. Raven, New York, pp 539–548
Haslwanter T, Jaeger R, Mayr S, Fetter M (2000) Three-dimensional eye-movement responses to off-vertical axis rotations in humans. Exp Brain Res 134:96–106
Hepp K (1990) On Listing’s law. Commun Math Phys 132:285–292
Hyden D, Larsby B (1991) Velocity dependence of the vestibulo-ocular reflex over a broad frequency range. Acta Otolaryngol Suppl 481:293–294
Jaeger R, Takagi A, Haslwanter T (2002) Modeling the relation between head orientations and otolith responses in humans. Hearing Res 173:29–42
Kasteel-van Linge A, Maas AJ (1990) Quantification of visuo-vestibular interaction up to 5.0 Hz in normal subjects. Acta Otolaryngol 110:18–24
Kleinbaum DG, Kupper LL, Muller KE (1988) Applied regression analysis and other multivariable methods. PWS-Kent, Boston, MA
Lansberg MP, Guedry J, Graybiel A (1965) Effect of changing resultant linear acceleration relative to the subject on nystagmus generated by angular acceleration. Aerosp Med 456–460
Mardia KV (1972) Statistics of directional data. Academic, New York
Mathog RH (1972) Testing of the vestibular system by sinusoidal angular acceleration. Acta Otolaryngol 74:96–103
Mayne R (1974) A systems concept of the vestibular organs. In: Kornhuber HH (ed) Handbook of sensory physiology. Springer, Berlin Heidelberg New York, pp 493–580
Merfeld DM, Young LR (1995) The vestibulo-ocular reflex of the squirrel monkey during eccentric rotation and roll tilt. Exp Brain Res 106:111–122
Merfeld D, Young LR, Oman CM, Shelhamer M (1993) A multidimensional model of the effect of gravity on the spatial orientation of the monkey. J Vestibul Res 3:141–161
Merfeld DM, Zupan LH, Gifford CA (2001) Neural processing of gravito-inertial cues in humans. II. Influence of the semicircular canals during eccentric rotation. J Neurophysiol 85:1648–1660
Minor LB, Lasker DM, Backous DD, Hullar TE (1999) Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey. I. Normal responses. J Neurophysiol 82:1254–1270
Mittelstaedt H (1983) A new solution to the problem of the subjective vertical. Naturwissenschaften 70:272–281
Mittelstaedt H, Glasauer S (1993) Crucial effects of weightlessness on human orientation. J Vestibul Res 3:307–314
Mok D, Ro A, Cadera W, Crawford DJ, Vilis T (1992) Rotation of Listing’s Plane during vergence. Vision Res 32:2055–2064
Paige GD, Tomko DL (1991) Eye movement responses to linear head motion in the squirrel monkey. I. Basic characteristics. J Neurophysiol 65:1170–1182
Peterka RJ (1992) Response characteristics of the human torsional vestibulo-ocular reflex. Ann NY Acad Sci 656:877–879
Peterka RJ, Black FO, Schoenhoff MB (1990) Age-related changes in human vestibulo-ocular reflexes: Sinusoidal rotation and caloric tests. J Vestibul Res 1:49–59
Pettorossi VE, Errico P, Santarelli RM (1991) Contribution of the maculo-ocular reflex to gaze stability in the rabbit. Exp Brain Res 83:366–374
Rude SA, Baker JF (1988) Dynamic otolith stimulation improves the low frequency horizontal vestibulo-ocular reflex. Exp Brain Res 73:357–363
Sato H, Sando I, Takahashi H (1992) Computer-aided three-dimensional measurement of the human vestibular apparatus. Otolaryng Head Neck 107:405–409
Schmid-Priscoveanu A, Straumann D, Kori A (2000) Torsional vestibulo-ocular reflex during whole-body oscillation in the upright and the supine position: I. Responses in healthy human subjects. Exp Brain Res 134:212–219
Smith ST, Curthoys IS, Moore ST (1995) The human ocular torsion position response during yaw angular acceleration. Vision Res 35:2045–2055
Takagi A, Sando I (1988) Computer-aided three-dimensional reconstruction and measurement of the vestibular end-organs. Otolaryng Head Neck 98:195–202
Tomko DL, Wall C III, Robinson FR, Staab JP (1988) Influence of gravity on cat vertical vestibulo-ocular reflex. Exp Brain Res 69:307–314
Tweed D, Cadera W, Vilis T (1990) Computing three-dimensional eye position quaternions and eye velocity from search coil signals. Vision Res 30:97–110
Tweed D, Sievering D, Misslisch H, Fetter M, Zee D, Koenig E (1994) Rotational kinematics of the human vestibuloocular reflex I. Gain matrices. J Neurophysiol 72:2467–2479
Zhang X, Zakir M, Meng H, Sato H, Uchino Y (2001) Convergence of the horizontal semicircular canal and otolith afferents on cat single vestibular neurons. Exp Brain Res 140:1–11
Zhang X, Sasaki M, Sato H, Meng H, Bai RS, Imagawa M, Uchino Y (2002) Convergence of the anterior semicircular canal and otolith afferents on cat single vestibular neurons. Exp Brain Res 147:407–417
Zupan LH, Peterka RJ, Merfeld DM (2000) Neural processing of gravito-inertial cues in humans. I. Influence of the semicircular canals following post-rotatory tilt. J Neurophysiol 84:2001–2015
Zupan LH, Merfeld DM, Darlot C (2002) Using sensory weighting to model the influence of canal, otolith and visual cues on spatial orientation and eye movements. Biol Cybern 86:209–230
Acknowledgements
We thank B.J.M. Hess for valuable discussions about the data; A. Schmid-Priscoveanu and A.A. Kori for the use of their data; A. Züger for technical assistance, and T. Schmückle and K. Weber for assistance when conducting the experiments. Supported by the Swiss National Science Foundation [3100–063669 (T.H.); 32–51938.97 SCORE A (D.S.) / 31–63465.00 (D.S.)]; Olga-Mayenfisch Foundation, Hartmann-Mueller Foundation, and the Betty and David Koetser Foundation for Brain Research, Zürich, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bockisch, C.J., Straumann, D. & Haslwanter, T. Human 3-D aVOR with and without otolith stimulation. Exp Brain Res 161, 358–367 (2005). https://doi.org/10.1007/s00221-004-2080-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-2080-1