Abstract
Saccade characteristics in response to moving and stationary targets were studied in three monkeys (Macaca mulatta) that had been trained to look at a target, which after an initial jump either remained in place or moved forward or backward with constant velocity (10°/s). Eye movements were recorded using a search coil. The contribution of smooth pursuit to the saccade amplitude was small (<0.25°). Saccades having the same amplitude (5.67–6.83° for different monkeys) to forward and backward moving targets were compared. Peak velocity was higher (37–42°/s on average for different monkeys) and saccade duration was shorter (8–10 ms on average) for backward saccades than for forward saccades These differences were highly significant (t-test: P<0.001). Thus, forward and backward saccades are not on the same main sequence. This suggests that saccade dynamics are affected not only by the retinal position error but also by target motion. Further analysis revealed that saccade peak velocity mainly depends on the retinal position error, but saccade amplitude also depends on a stimulus-related velocity factor, which affects the saccade mainly during deceleration. This velocity factor could be retinal slip or target velocity, which was the same under our conditions. Our results experimentally support recent models that propose that the saccade acceleration in response to moving targets might be controlled by the superior colliculus, whereas the deceleration changes are fine-tuned by the cerebellum. This prediction must still be tested on a neuronal level.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many current models of saccade generation use the retinal position error as the main driving stimulus (see e.g., Becker 1989). It is thought to be sampled shortly before the saccade starts. Stepping the target to another location during a “dead” interval, about 80 ms before saccade onset, does not affect the movement (Becker and Jürgens 1979). However, if the amplitude of a saccade to a moving target (“catch-up saccade”) were also determined by the retinal error sampled 80 ms before saccade onset, the endpoint of such a saccade would systematically miss the target. Therefore, additional information about target velocity seems to be necessary to improve saccade accuracy. Contrary to earlier studies (Heywood and Churcher 1981; Ron et al. 1989), later investigations showed significant effects of target velocity on saccades in monkeys (Keller and Johnsen 1990), humans (Gellman and Carl 1991), and in the cat (de Brouwer et al. 2001). These studies found that with identical retinal position error 100 ms before movement onset, saccades have larger amplitude when the target moves toward higher eccentricities compared to stationary targets or targets moving in the opposite direction. This effect of the target velocity on the saccade amplitude is not just a by-product of superimposed smooth pursuit components, since it was still observed after subtracting the pursuit-related intrasaccadic eye displacement from the saccade amplitude (Keller and Johnsen 1990; de Brouwer et al. 2002). Not all types of saccades show such predictive abilities, e.g., saccades elicited during sustained smooth pursuit do depend exclusively on the retinal error immediately after the target step (Smeets and Bekkering 2000).
It is not exactly known which parts of the saccadic system are involved in this velocity-induced change of the saccade amplitude. Keller et al. (1996) found that the activity of cells in the superior colliculus was not altered by changes of the saccade amplitude induced by target velocity. This finding is compatible with the idea that “...one path is concerned with correction of a presaccadic retinal position error (a path that includes the colliculus) and another path is concerned with position extrapolations based on the velocity of the moving target (a path that does not include the colliculus)” (Keller et al. 1996). This idea was developed further by a model of Optican and Quaia (2002) which assumed that both the superior colliculus (SC) and the cerebellum, especially lobuli VI and VII (the so-called oculomotor vermis) (Yamada and Noda 1987) and the underlying fastigial oculomotor region (FOR) (Fuchs et al. 1993; Helmchen et al. 1994), interact and fine-tune the activity of the brainstem saccade generator, making saccade amplitude and direction accurate (Dean 1995; Robinson and Fuchs 2001). The cerebellum (oculomotor vermis and FOR) may be the structure that provides the additional neuronal input necessary for the velocity extrapolation. If this hypothesis were true, not only the amplitude of the saccades but also their dynamic properties would be affected by target motion, because the FOR signals reflect much of the dynamic properties of the saccade (Robinson et al. 1993; Kleine et al. 2002).
Some previous studies investigated not only the effect of target motion on the saccade amplitude but also on saccade dynamics. In a study on superior colliculus (SC) activity, Keller et al. (1996) observed that saccades to forward moving targets had prolonged deceleration phases. de Brouwer et al. (2002) showed in humans that the dynamics of catch-up saccades during sustained pursuit did not differ from the dynamics of saccades to stationary targets, suggesting that the dynamics of saccades to moving targets can be explained by the superposition of main sequence saccades and pursuit (superposition hypothesis). However, during sustained or anticipatory smooth pursuit, a great deal of the intrasaccadic target displacement can be accounted for by the pursuit system. Under these conditions the requirements for altering the amplitude and possibly the dynamics of the saccade are relatively small.
The aim of this study was to compare the dynamics of saccades to stationary targets with that to moving targets under conditions with no (saccade onset) or little (saccade end) smooth pursuit contribution. This was done by training monkeys to make saccades to stationary targets as well as to targets that, after an initial target step, moved forward (forward saccades) or backward (backward saccades). Step size and target velocity were chosen in such a way that saccades of the same amplitude were performed. The results show differences between saccades made in these two different paradigms. They also reveal that forward saccades have less deceleration and backward saccades more deceleration than saccades to stationary targets. These differences cannot be explained by the superposition hypothesis alone. Parts of our results have been published as an extended poster abstract (Guan et al. 2003).
Materials and methods
Experiments were performed with three adult monkeys (Macaca mulatta). Prior to the experiments, the monkeys were familiarized with the laboratory environment and trained to sit erect in a primate chair. Under general anesthesia and aseptic conditions, they were prepared for chronic, monocular, two-dimensional eye position recordings. A head holder was attached to the skull to maintain a stable head position throughout the experiment. Details of the surgical procedure have been described previously (Boyle et al. 1985). Eye movements were recorded with the scleral search coil method (Judge et al. 1980) using a 40×40×40 cm three-field system (Remmel Labs, Ashland, Mass., USA). The calibration of the coil system was based on 70 fixations at known target positions between ±15° horizontal and ±10° vertical eccentricity. The three-dimensional Cartesian vector of the gaze direction c was computed from the three measured voltages v, which were induced in the coil by the three magnetic fields, using the following equation:
in which the three-dimensional vector o denotes the offset voltages induced in the feedline of the coil and the electronically generated offsets. The rows of the transposed 3×3 matrix HT contain the magnetic field vectors of each of the three fields (\( \underline{h} ^{T}_{i} \)), multiplied by the inductivity (l) of the coil and the amplifier gain of each field (g i ):
All nine elements of H and the three offset voltages o were fitted by minimizing the squared distance between the gaze direction c and the target position vector, summed over all 70 fixations.
Training and stimuli
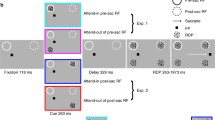
Monkeys were trained to fixate a light spot with a diameter of 0.4° (visual angle) as visual target on a video monitor at a viewing distance of 76 cm. There were two main stimulus conditions: step and step-ramp (Fig. 1). Each step-ramp trial started with the light spot in the center of the screen, after which a step-ramp stimulus was presented as follows (Fig. 1). For monkeys A and C, targets stepped to the initial position of 4.8° or 9.2° left or right from the center, and then immediately started to move with a velocity of 10°/s. For the small initial step size (4.8°), movement was in the direction of the step (forward), and for the large initial step size (9.2°) it was in the opposite direction (backward) (Fig. 1a). The steps were chosen in such a way that the moving targets were always at the same eccentricity (at 7°) when a saccade started, assuming an average saccade latency of 200 ms. Target motion stopped after 500 ms. The target disappeared 1 s later, and 1 s thereafter a new central fixation target was presented. In addition to the step-ramp stimuli, trials were performed with different steps that lacked subsequent target motion but had amplitudes of ±4.8°, ±7.0°, and ±9.2° (Fig. 1b). A correct saccade was recorded when the monkey’s eye entered a 3° window area around the target location not later than 400 ms after the step. Such a correct saccade was contingently rewarded with fruit juice.
Target position (dashed) and eye position (solid) for the two step-ramp stimuli (a) and the three different stationary target steps (b). For these stimuli the primary target steps were chosen to converge after 200 ms at the same position (7°) as the medium-sized stationary target step. Eye position shows average data for monkey A. Saccades to the left side were mirrored before averaging. The two saccade amplitudes are similar for forward and backward moving targets (a). For these trials, saccades are followed by SPEM. Note that there is no indication that pursuit occurred before the saccades.
To reduce the monkey’s ability to guess the new target position, the sequences of step-ramp and pure-step target movements were chosen at random. The directions of the initial target steps (left or right) were also randomly varied.
The step amplitudes were slightly modified for monkey B in order to elicit similar saccade amplitudes for stationary and moving targets as for monkeys A and C. Stationary targets were presented with amplitudes of 5.3°, 7.0°, and 8.7°, forward moving targets with a step amplitude of 5.3°, and backward moving targets with a step amplitude of 8.7°. The target velocity of moving targets was 10°/s.
To prevent the monkey from predicting that small steps would lead to forward movements and large steps to backward ones (Fig. 1), monkey B was required in another set of trials to make saccades to stationary targets with step sizes of 3° or 12°, and to moving targets with initial step sizes of 9.2° or 5.8° in both forward and backward directions, with a moving target velocity of 10°/s. In this way, forward saccades after an initial step of 5.8° and backward saccades after an initial step of 9.2° had an identical amplitude of ca. 7°.
Data collection and analysis
The stimuli and the collection, presentation, and storage of data were controlled by a PC using a real-time software package (REX; Hays et al. 1982). Eye position and target position signals were sampled and stored on hard disk at a sampling frequency of 1 kHz. To parameterize the saccades, the calibrated eye position was marked on the basis of velocity criteria. Eye velocity was computed using a symmetrical two-point differentiator after low-pass filtering with a Gaussian filter with a cutoff frequency of 33 Hz (transmission gain of 0.1 at 85 Hz). Fast eye movements occurring between 50 ms and 300 ms after the initial target step were marked as a saccade if the peak velocity was more than 100°/s, the duration shorter than 100 ms, or the amplitude more than 2.0°. Start and end of the saccade were defined as the points at which eye velocity rose above or dropped below 10% of peak velocity, respectively. The latency of a saccade was computed as the time interval between the initial target step and saccade onset, and saccade amplitude as the eye position difference between start and end of a saccade.
In the step-ramp paradigm, the target is likely to evoke a smooth pursuit eye movement (SPEM) after the initial step-induced saccade (Fig. 1). There is evidence that a SPEM component is added to the saccadic component during a saccade (de Brouwer et al. 2002; Blohm et al. 2003). To separate saccade parameters from such SPEM components, the SPEM was estimated for each saccade during an interval starting 50 ms before and ending 300 ms after saccade onset, in the following way (Fig. 2). First, the saccades were removed from the eye velocity data. The eye velocity during the saccade was replaced by constantly increasing or decreasing velocity starting at the velocity immediately before the saccade and ending at the velocity immediately after the saccade. This “saccade-free” velocity trace was then submitted to low-pass filtering (symmetrical Gaussian low-pass filter with 5 Hz frequency limit). This procedure yielded the postsaccadic SPEM velocity (solid line in Fig. 2c). Finally, the complete SPEM velocity (during and after the saccade) as a function of time (t) was approximated by an exponential function, starting from the average eye velocity during 50 ms before the start of a saccade (\( \overline{{SPV}} _{0} \)), as follows:
Decomposition of the eye position and eye velocity in saccade and pursuit-related components. The plots show data from a single saccade of monkey A. The eye position signal (a, solid) is differentiated to compute the eye velocity (b, solid). The saccade is then cut out from the velocity trace, and the remaining postsaccadic pursuit velocity is low-pass filtered with a cutoff frequency of 5 Hz (c, solid). The time course of SPEM velocity during the saccade is fitted by an exponential function (c, dashed), which is then subtracted from the eye velocity. The result (b, dashed) is the saccadic component of eye velocity. Integration of this trace gives the eye position trace, compensated for SPEM (a, dashed).
with
- A :
-
=sustained pursuit velocity
- SPV :
-
=estimated SPEM velocity
- t 0 :
-
=start of saccade
- τ :
-
=time constant
The two unknown parameters A and τ were determined by minimizing the mean squared difference between the estimated SPV given in Eq. 1 and the postsaccadic SPEM velocity. Finally, the SPV (dashed line in Fig. 2c) was subtracted from the original velocity data, resulting in the saccadic velocity profile without the SPEM component (dashed line in Fig. 2b). Numerical integration of this velocity profile over time led to the respective position data (dashed line in Fig. 2a).
These eye position data, corrected for SPEM components, were used for the subsequent re-evaluation of the saccade parameters. To detect the onset and the end of the corrected saccade, the same criteria were used as before, after execution of the described SPEM correction algorithm.
Results
The results are based on the analyses of 1953 (monkey A), 1843 (monkey B), and 1453 (monkey C) saccades, recorded in eight to ten sessions. The results were basically the same for all monkeys.
Smooth pursuit contribution
In trials with moving targets, a possible interaction with SPEM components was considered. They were not evident immediately prior to the saccades (Fig. 2). Table 1 shows the mean eye velocity during a 50-ms period before saccade onset (mean presaccadic velocity) and during a time interval from 200 to 300 ms after the end of the saccade. When the initial target step and the target motion were in opposite directions, the eye velocity immediately before saccade onset was very small and even in the direction of the saccade (see column 3 of Table 1). When saccade and target moved in the same direction, the mean presaccadic velocity ranged between 0.99°/s (monkey A) and 0.48°/s (monkey B). These data show that there was no presaccadic smooth pursuit under our experimental conditions. The saccade was generally followed by a SPEM that accelerated during the postsaccadic period. The average eye velocities during an interval from 200 to 300 ms after the end of the saccade ranged between 2.0°/s in monkey C and 5.1°/s in monkey A (see column 4 of Table 1, Fig. 2c). The contribution of a SPEM component was subtracted from the primary saccade data. SPEM compensation reduced saccade velocities of forward saccades by not more than 3°/s. Backward saccades were 0.9°/s faster with than without SPEM compensation. Even when these effects were small (see Table 1), we will consider only corrected saccade data in the following. Uncorrected saccades are only considered under “the velocity profile”.
Latency
Latencies varied between 150 and 300 ms and were similar for stationary and moving targets (Table 2, Table 3, Table 4). Latency increase led to larger saccades for forward saccades, whereas the amplitude decreased for backward saccades (Fig. 3). From the regression lines in Fig. 3a, c (monkey A), it follows that per 100 ms latency, saccades to forward moving targets increased in amplitude by 1.5° (monkeys A, B: 1.6°; C: 0.7°), while backward saccades decreased by 1.0° (monkeys A, B: 0.8°; C: 0.7°). These slopes (solid lines in Fig. 3a, c) indicate that the increase or decrease in saccade amplitude with increasing latency corresponds fairly well with the position of the target, which increased or decreased by 1.0°/100 ms (dashed lines in Fig. 3a, c). In contrast, saccade amplitude for stationary targets increased only slightly with latency (0.5°/100 ms; B: 0.4°/100 ms; C: 0.1°/100 ms: Fig. 3b). The effect of latency on amplitude can be easily explained on the basis of the retinal position error sampled at a fixed time before the saccade since the velocity of its increase during the latency is identical to the velocity of the target. The dependence of the amplitude on the latency of the saccade is not related to an effect of target velocity on the saccade.
Saccade amplitude in relation to saccade latency for forward (a), stationary (b), and backward (c) saccades (monkey A). The slope of the regression lines (solid) shows that saccade amplitude increases with latency for forward saccades (15°/s) and decreases for backward saccades (10°/s). Dashed lines show the retinal position error 100 ms before saccade onset. The average values of the initial fixation error were close to zero (Table 2, Table 3, Table 4). The slopes of the dashed lines correspond to the target velocity of +10, 0, and −10°/s in A, B, and C, respectively. The dependencies of the saccade amplitude and retinal position error on latency are similar, but the difference between saccade amplitude and retinal position error differs between conditions.
However, the effect of target velocity on saccade amplitude becomes evident, when the relation between retinal position error and saccade amplitude is considered in more detail. The dashed lines in Fig. 3 indicate the retinal error 100 ms before saccade onset. While the saccade amplitude was larger than the retinal position errors for forward moving targets (Fig. 3a), the amplitude was smaller than the retinal position error for stationary targets (Fig. 3b) and smaller still for backward moving targets (Fig. 3c). The saccade errors, defined by the difference between eye position and target position at the end of the movement (Table 2, Table 3, Table 4), show that for moving targets the saccades are more accurate than expected on the basis of the presaccadic retinal error. Thus, saccade amplitude did not exclusively rely on the retinal position error sampled about 100 ms before saccade onset, but were partly compensated for the target displacement occurring during the “dead interval” and during the saccade.
Effect of target velocity on saccade duration and peak velocity
To determine whether saccade dynamics were also affected by target motion, we compared peak velocity and saccade duration between saccades to moving and to stationary targets. This analysis was performed on the data after correcting for smooth pursuit. By choosing the appropriate target step amplitude, mean amplitudes of the saccades to forward and backward moving targets were adjusted to be as similar as possible (monkey A forward: 6.53°, backward: 6.55°, Table 5). However, even so, any difference between the amplitude histograms of forward and backward saccades would prevent a fair comparison of saccade peak velocities and durations. To avoid this, we randomly drew a subsample from each saccade population according to the following two criteria: (1) the amplitude histograms of the two subsamples had to be proportional to each other, and (2) the total number of saccades in the two subsamples had to be as large as possible. Statistical analyses of differences were carried out only on the subsamples. With nearly identical amplitude histograms (Fig. 4 a1, b1), the average peak velocity of backward saccades (244.3°/s; Fig. 4b2) is significantly higher (t-test: P<0.001) than that of forward saccades (207.4°/s; Fig. 4a2). According to the main sequence (Bahill et al. 1975), saccades of lower velocity should have a shorter duration. This, however, was not the case in our study. On the contrary, the duration of saccades to forward moving targets was significantly longer (57.4 ms) than to reverse moving target (49.9 ms) (t-test: P<0.001; Fig. 4a3, b3). Thus, neither forward nor reverse saccades are on the main sequence.
Histograms of amplitude, peak velocity, and duration of saccades to forward (a1–3) and backward (b1–3) moving targets for monkey A. Data were first corrected for SPEM components (Fig. 2). A selection is shown from both forward and backward trials which matches the frequency distribution of the amplitudes as closely as possible (a1, b1). For backward saccades, peak velocity is higher and duration is shorter than that of saccades to forward moving targets of identical size.
These results are consistent with the outcome of the one-way analysis of variance (ANOVA) of the overall effects of target motion including saccades to stationary, forward, and backward moving targets (Table 5, Table 6, Table 7).
Regression analysis
The results presented so far show that the saccade dynamics are affected by the target velocity. To investigate in more detail how this effect concerns different parts of the saccade, we quantified the relative contribution of the retinal position error and the target velocity for the saccade amplitude and for the peak velocity. Two multiple regression analyses were performed for each monkey across all stationary and moving trials.
In the first regression, saccade amplitude (corrected for SPEM) was fitted by a weighted sum of the retinal position error, evaluated 100 ms before the start of each saccade (RetErr 100 ), and the target velocity (V):
Both partial correlations of the dependent variable saccade amplitude with the retinal position error (R AE ) and with target velocity (R AV ) were highly significant for all three monkeys (Table 8, left column). The gain factor a2 for the target velocity ranged between 0.067 s and 0.095 s. Thus, the target velocity of 10°/s affected the saccade amplitudes by about 0.95° (monkey A), 0.85° (monkey B), and 0.67° (monkey C).
If saccade amplitude and peak velocity of saccades to stationary and moving targets belonged to the same main sequence, i.e., if peak velocity and saccade amplitude determined each other, then the ratio between the partial correlations of the retinal position error and the target velocity should be similar, regardless of whether the saccade amplitude (R AE , R AV ) or the peak velocity (R PE , R PV ) is used as a dependent variable. To test this hypothesis, the second regression analysis used saccade peak velocity (corrected for SPEM) as the dependent variable:
The partial correlations of the saccade peak velocity with the retinal position error (R PE ) and with target velocity (R PV ) were also highly significant (Table 8, right column). Comparison with the left column shows the following: (1) For all monkeys all partial correlations with saccade peak velocity (R PE , R PV ) are smaller than those with the saccade amplitude (R AE , R AV ), indicating a higher noise level of peak velocity than of saccade amplitude. (2) More important for our consideration is the finding that the ratio between the partial correlations of the retinal position and the target velocity was larger for the dependent variable peak velocity (R PE /R PV =8.4±4.2) than for the dependent variable saccade amplitude (R AE /R AV =1.5±0.1), indicating that the target velocity had a stronger influence on the saccade amplitude than on the peak velocity. This was particularly the case for monkeys A and B and, to a lesser extent, also for monkey C.
The velocity profile
The computations in the last paragraphs were based on saccade profiles that compensated for possible pursuit components. This compensation was based on the assumption of an exponential increase of the pursuit velocity (see Eq. 1). However, the time course of postsaccadic eye velocity did not always show an exponential profile. For example, the exponential fit shown in Fig. 2 (Fig. 2c, dashed) matches the actual eye velocity in the early postsaccadic period, but is too small in the later postsaccadic period. To test whether the effects of target velocity on the saccadic velocity profile may result from incomplete pursuit compensation, we compared the velocity profiles of saccades to moving and to stationary targets. Figure 5a, b shows the original individual and averaged eye velocities (not compensated for pursuit). The average amplitudes of the saccades to moving and stationary targets were identical. Even though the trial by trial variability was quite large, saccades to forward moving targets clearly showed a longer duration and a slower peak velocity. This became obvious when the average time courses were superimposed (Fig. 5c, dotted, solid traces).
Comparison of eye velocity traces of saccades to stationary and forward moving targets (identical means of saccade amplitude). Graphs show data of monkey A. Individual eye velocity traces (gray lines) for stationary targets (a) and moving targets (b). Superimposed lines show the time course of the mean (solid) ±standard deviations (dashed). These data show raw recordings (not compensated for SPEM). c Solid line: mean velocity of saccades to moving targets (identical to solid line in b). Dotted line: mean velocity of saccades to stationary targets (identical to solid line in a). Dashed line: three saccades to stationary targets with smaller, identical, and larger initial acceleration than the saccades to moving targets. d Differences between the velocity of the saccade to moving targets and the three dashed velocity traces in c. The superposition hypothesis predicts that one of these differences should approximate the intrasaccadic SPEM velocity.
According to the superposition hypothesis, these differences must be explained by the assumption that the saccade velocity to the forward moving target is the sum of a main sequence velocity profile of a smaller saccade and a forward pursuit velocity. To test this hypothesis, we subtracted the velocity profiles of variously sized saccades to stationary targets (dashed in Fig. 5c) from the velocity of the saccade to moving targets (solid in Fig. 5c). The resulting differences are shown in Fig. 5d. Even if we know neither the respective contribution of saccade and pursuit to the complete movement nor the exact time course of intrasaccadic pursuit velocity, according to the superposition hypothesis we can expect to find a main sequence saccade for which the difference results in the presumed monotonic increase of the pursuit velocity. Figure 5d shows that there is no main sequence saccade that complies with this expectation. The velocity of small main sequence saccades, when subtracted from the velocity of saccades to forward moving targets, led to a peak of the remaining velocity component far above 10°/s (Fig. 5d, top trace). Subtracting the velocity of large main sequence saccades resulted in “pursuit” components that begin even in the negative direction (Fig. 5d, lower trace). Even the minimum overshoot of the difference that does not involve negative “pursuit” velocities for forward saccades is about 60–70°/s (Fig. 5d, central trace), which is much higher than the pursuit velocity of 5°/s at the end of the saccade. For backward saccades the overshoot was smaller and ranged from 20 to 40°/s for the different monkeys (e.g., Fig. 6). Thus, these differences are not compatible with the superposition hypothesis and suggest that the velocity during the deceleration phase of saccades to forward (backward) moving targets is higher (lower) than that of main sequence saccades.
Comparison of eye velocity traces of saccades to stationary and backward moving targets (identical means of saccade amplitude). Graphs show data of monkey B. This monkey showed very little pursuit as indicated by the small final value of the difference trace in d. For description of subplots see Fig. 5.
Forward and backward saccades after the same initial step size
In our experiment forward target motion always occurred after small target steps and backward target motion always after large target steps. It was thus possible that the monkey could have used the detection of the target step as a signal to prolong or to shorten the deceleration phase of the saccade. In this case target velocity would not have been used. In order to exclude this possibility, monkey B was presented with a second setup that required him to make saccades to two groups of stationary targets, and saccades to a target that moved in different directions (forward and backward) but with the same step size (see “Methods”).With this the monkey made forward and backward saccades after a small step (5.8°) and forward and backward saccades after a big step (9.2°). The forward saccades after the small step and backward saccades after the big step led to saccades of similar amplitude which were considered further. By applying the same “subsampling” approach, we compared the backward (n=678) and forward (n=646) saccades with the same amplitude (7.22° and 7.18°, respectively). Also under these conditions backward saccades had a higher peak velocity than forward saccades (backward: 225.9°/s, forward: 192.2°/s; t-test: P<0.01) and they were shorter than forward ones (backward: 66.1 ms, forward: 72.5 ms; t-test: P<0.01), which fully confirms the results obtained with the first setup. Thus, the same results were obtained when the monkey did not know if a target step was followed by a forward or backward moving target.
Discussion
Saccade duration and peak velocity and their relation to SPEM
We have demonstrated that saccades to moving targets can be as accurate as saccades to stationary targets (see Fig. 3, Table 2, Table 3, Table 4) and that these saccades are on different main sequences than are saccades to stationary targets. Catch-up saccades made during sustained SPEM have also been shown to be on a different main sequence (de Brouwer et al. 2002) than saccades to stationary targets (Bahill et al. 1975). This difference in saccadic profile has been assumed to be caused by a SPEM component that is added to or subtracted from the saccadic component. However, we show for the first time that even after correcting for the SPEM component, catch-up and stationary saccades still occur on different main sequences. Backward saccades are faster and shorter than forward saccades. How can these different findings be explained?
In agreement with de Brouwer et al. (2002) and Blohm et al. (2003), we found that retinal slip velocity had an effect on saccade amplitude. The effects in the monkey (regression coefficients a2 between 0.067 and 0.095; see Table 8) were in between the coefficients that de Brouwer et al. (2002) found for the cat (0.162) and Blohm et al. (2003) found in humans (0.059). Although all our findings for the saccade amplitude are in the range of previous results, they cannot be explained by a superposition of main sequence saccades with pursuit, since our correlation analysis revealed that saccade amplitude is more affected by the target velocity than is the peak velocity. This result is not compatible with the superposition hypothesis and seems to be in conflict with the results of Blohm et al. (2003) who, after correcting for SPEM, found that these saccades were indistinguishable from saccades to stationary targets. One important difference between their experiments and ours is that the SPEM component in our experiment was very small. Before saccade onset the eye velocity stayed below 1°/s. In contrast, the major interest of the study of Blohm et al. (2003) was focused on predictive SPEM, and subjects were particularly trained to produce anticipatory SPEM. Therefore, in their study the eye velocity exceeded 10°/s before saccade onset. In our experiment, the contribution of the SPEM to the saccade amplitude (between 0.02° and 0.25°; see Table 1) was smaller than the effect of target velocity (between 0.67° and 0.95°; see Table 8).
It is quite possible that the absence of effects of target velocity on the main sequence in previous experiments is due to the activity of the pursuit system before the saccade. In primates, who have a gain of SPEM near one, the activity of the pursuit system is normally involved with the target motion during the latency period of the saccade and during its duration. Therefore, saccades usually do not have to change much with target velocity, if the pursuit system is already active. It is conceivable that the dependence of the time course of saccadic eye velocity on target velocity can only be observed if, as in our experiment, there is no pursuit activity before the saccade. Our results also clearly revealed that the accuracy of saccades to moving targets is not the result of preprogrammed learning to execute velocity profiles specific for certain target steps. This is shown by the fact that randomization of the target step and of the direction of the following target motion does not affect this accuracy.
Latency
In case of a stationary target, the latency between the target start and the onset of a saccade is generally 150–250 ms (Becker 1989; Krauzlis and Miles 1996). This is in line with our findings of latencies that varied between 150 and 300 ms. There are some reports that larger saccades after larger target steps have longer latencies (Becker 1989). However, latency has basically no effect on the amplitude of saccades to a stationary target of a given size (Fig. 3). On average, we hardly observed any latency difference between saccades to moving and to stationary targets (Table 2, Table 3, Table 4). During the latent period, the distance is calculated which the eye has to move to fixate the target. The difference between the initial and intended eye positions is translated into a motor command that activates the extraocular muscles to move the eye over the correct distance in the appropriate direction. For a saccade made to a moving target, the situation is clearly more complicated due to the continuous change of the retinal error during the latent period.
We show that the latency of saccades to moving targets is linearly related to the amplitude of the saccade (Fig. 3). This relation is expected since in our experiment, the saccade latency was linearly related to the retinal position error, sampled at a fixed time before saccade onset, and because the retinal position error is the main determining factor for the saccade amplitude. Thus, this finding is a further confirmation of the generally accepted view that the position error is sampled at a fixed time before saccade onset.
Retinal error, stimulus-related velocity factor, and saccade velocity profile
In this context, the retinal position error is defined as the distance between target and eye position 100 ms before saccade onset. Saccade amplitudes are smaller than the retinal position error for backward moving targets and larger for forward moving targets (Fig. 3). This dissociation of saccade amplitude and retinal error shows that the retinal position error cannot be the only factor involved.
This result is in line with previous studies showing that an additional velocity factor affects saccade amplitude (Keller and Johnsen 1990; Gellman and Carl 1991; de Brouwer et al. 2001). This factor could be retinal slip or target velocity, which was the same under our conditions. de Brouwer et al. (2002) and Blohm et al. (2003) recently demonstrated that retinal slip is the relevant factor, which we will call stimulus-related velocity factor in the following. We hypothesize that saccade amplitude depends on both the retinal position error and a stimulus-related velocity factor, which affects the saccade mainly after the peak velocity, i.e., during the deceleration phase of the saccade. This hypothesis explains why the partial correlations of target velocity with saccade amplitude are much larger than that of target velocity with eye peak velocity (Table 8). This interpretation is fully compatible with the finding that saccades with the same amplitude have different peak velocities, depending on the direction of target motion. The reason why saccades to backward moving targets have higher peak velocities than saccades to forward moving targets is not that peak velocity depends on target motion. In our experiment, the retinal error sampled 100 ms before saccade onset was larger for backward saccades than for forward ones. Our experiments show that forward saccades are initially planned with a smaller amplitude and smaller peak velocity (because of the smaller primary target step) and are then decelerated more slowly than saccades to stationary targets, in order to increase the amplitude. The opposite procedure is followed in the case of backward moving targets.
This can be seen in Fig. 5 and Fig. 6, in which the velocity profiles of saccades to moving and stationary targets are compared. When comparing the size of the velocity overshoots of the velocity differences shown in Figure 5d and Fig. 6d with the much smaller size of the SPEM components (see Table 1), it also becomes obvious that the differences in the main sequences of saccades to forward and backward moving targets which we report here cannot be explained as simply an artifact of the imperfections of our algorithm to compensate for SPEM components. The method we applied may not be appropriate to estimate the exact time course of intrasaccadic pursuit because of the effects of saccades on the pursuit gain known as “postsaccadic enhancement” (Lisberger 1998; Churchland and Lisberger 2002). However, even postsaccadic enhancement does not predict that the pursuit velocity during the saccade is larger than after the saccade. Therefore, the large differences during the deceleration phase of the eye velocities between saccades to stationary and to moving targets cannot be attributed to pursuit: They indicate differences in the dynamics of the saccadic component.
Quantitatively the regression analysis (Table 8) showed that the relative predictive power of the target velocity (with respect to that of the retinal error) was 8.9, 5.7, and 2.9 times larger for saccade amplitude than for peak velocity as indicated by the ratio [R AV /R AE ]/[R PV /R PE ] for monkeys A, B, and C, respectively. This is a further confirmation of our interpretation that saccade amplitude is more affected by target velocity than is peak velocity.
The acceleration phases of saccades to stationary and moving targets appear to be similar, but forward saccades have a higher velocity in the deceleration phase and last longer than backward saccades. Therefore, we assume that target velocity is used by the oculomotor system to shorten or to prolong the deceleration phase, which was originally preprogrammed on the basis of the retinal position error in order to improve the accuracy of saccades to moving targets.
Relation to models and neuronal mechanisms
In many models of saccade generation the signal driving excitatory burst neurons (EBN) in the brainstem is controlled by a local feedback loop (Robinson 1975; Jürgens 1981; Scudder 1988; for a review see Scudder et al. 2002). All neural structures in this feedback loop are candidates that potentially affect the kinematics and dynamics of saccades. For example, Erkelens and Sloot (1995) used feedback control to explain that the variability of effective saccade direction is smaller than that of initial direction. Structures of the feedback loop which are also involved in the processing of presaccadic target velocity could cause the effects observed in the present study. One recent model of the interaction of the SC and cerebellum favors the cerebellum to explain such effects (Quaia et al. 1999; Optican and Quaia 2002). This model does not consider the SPEM component and thus is similar to our approach. The saccade simulations presented in Fig. 4 in Optican and Quaia (2002) also show some differences in the saccade profile, when saccades to stationary and to moving targets are compared. These differences are similar to the differences we observed.
Briefly, their model predicts that the SC generates a saccadic signal based on the retinal position error, an assumption supported by experimental studies (Keller et al. 1996; Klier et al. 2001). The source of the neuronal activity for the stimulus-related velocity component is not known. According to the model of Optican and Quaia (2002), the cerebellum with its relevant output structure (the FOR) might be a candidate. Saccade-related burst neurons have been encountered in the FOR (Fuchs et al. 1994; Helmchen et al. 1994; Robinson and Fuchs 2001). The stimulus-related velocity component seems to play only a minor role during the acceleration phase of the saccade, but it becomes manifest mainly during the deceleration phase, resulting in larger amplitudes of forward saccades and smaller amplitudes of backward saccades.
Conclusions
Our results confirm that saccades to moving targets are highly accurate. The saccadic system achieves this accuracy by taking not only the retinal position error into account but also by generating a component related to stimulus velocity that is independent of any SPEM contribution. This component leads to an altered saccade velocity profile, which becomes manifest mainly during the deceleration phase. Future studies will have to determine how different neuronal structures such as the SC and the cerebellum contribute to this saccade control process.
References
Bahill AT, Clark MR, Stark L (1975) Τhe main sequence, a tool for studying human eye movements. Math Biosci 24:191–204
Becker W, Jürgens R (1979) An analysis of the saccadic system by means of double step stimuli. Vision Res 19:967–983
Becker W (1989) The neurobiology of saccadic eye movements. Metrics. Rev Oculomot Res 3:13–67
Blohm G, Missal M, Lefevre P (2003) Interaction between smooth anticipation and saccades during ocular orientation in darkness. J Neurophysiol 89:1423–1433
Boyle R, Buttner U, Markert G (1985) Vestibular nuclei activity and eye movements in the alert monkey during sinusoidal optokinetic stimulation. Exp Brain Res 57:362–369
de Brouwer S, Missal M, Lefevre P (2001) Role of retinal slip in the prediction of target motion during smooth and saccadic pursuit. J Neurophysiol 86:550–558
de Brouwer S, Missal M, Barnes G, Lefevre P (2002) Quantitative analysis of catch-up saccades during sustained pursuit. J Neurophysiol 87:1772–1780
Churchland AK, Lisberger SG (2002) Gain control in human smooth-pursuit eye movements. J Neurophysiol 87:2936–2945
Dean P (1995) Modelling the role of the cerebellar fastigial nuclei in producing accurate saccades: the importance of burst timing. Neuroscience 68:1059–1077
Erkelens AJ, Sloot OB (1995) Initial directions and landing positions of binocular saccades. Vision Res 35:3297–3303
Fuchs AF, Robinson FR, Straube A (1993) Role of the caudal fastigial nucleus in saccade generation. I. Neuronal discharge pattern. J Neurophysiol 70:1723–1740
Fuchs AF, Robinson FR, Straube A (1994) Participation of the caudal fastigial nucleus in smooth-pursuit eye movements. I. Neuronal activity. J Neurophysiol 72:2714–2728
Gellman RS, Carl JR (1991) Motion processing for saccadic eye movements in humans. Exp Brain Res 84:660–667
Guan YF, Eggert T, Büttner U (2003) Analysis of saccades to stationary and moving targets in the monkey. Ann N Y Acad Sci 1004:385–388
Hays AV, Richmont BJ, Optican LM (1982) A Unix-based multiple process system for real-time data acquisition and control. Wescon Conf Proc 2:100–105
Helmchen C, Straube A, Buttner U (1994) Saccade-related activity in the fastigial oculomotor region of the macaque monkey during spontaneous eye movements in light and darkness. Exp Brain Res 98:474–482
Heywood S, Churcher J (1981) Saccades to step-ramp stimuli. Vision Res 21:479–490
Judge SJ, Richmond BJ, Chu FC (1980) Implantation of magnetic search coils for measurement of eye position: an improved method. Vision Res 20:535–538
Jürgens R, Becker W, Kornhuber HH (1981) Natural and drug-induced variations of velocity and duration of human saccadic eye movements: evidence for a control of the neural pulse generator by local feedback. Biol Cybern 39:87–96
Keller E, Johnsen SD (1990) Velocity prediction in corrective saccades during smooth-pursuit eye movements in monkey. Exp Brain Res 80:525–531
Keller EL, Gandhi NJ, Weir PT (1996) Discharge of superior collicular neurons during saccades made to moving targets. J Neurophysiol 76:3573–3577
Kleine JF, Guan YF, Langer S, Tchelidze T, Büttner U (2002) Discharge properties of saccade-related neurons in the oculomotor region of primate fastigial nucleus. Ann N Y Acad Sci 978:526–528
Klier EM, Wang H, Crawford JD (2001) The superior colliculus encodes gaze commands in retinal coordinates. Nat Neurosci 4:627–632
Krauzlis RJ, Miles FA (1996) Decreases in the latency of smooth pursuit and saccadic eye movements produced by the “gap paradigm” in the monkey. Vision Res 36:1973–1985
Lisberger SG (1998) Postsaccadic enhancement of initiation of smooth pursuit eye movements in monkeys. J Neurophysiol 79:1918–1930
Optican LM, Quaia C (2002) Distributed model of collicular and cerebellar function during saccades. Ann N Y Acad Sci 956:164–177
Quaia C, Lefevre P, Optican LM (1999) Model of the control of saccades by superior colliculus and cerebellum. J Neurophysiol 82:999–1018
Robinson DA (1975) Oculomotor control signals. In: Lennerstrand G, Bach-y-Rita P (eds) Basic mechanisms of ocular motility and their clinical implications. Pergamon, Oxford, pp 337–374
Robinson FR, Fuchs AF (2001) The role of the cerebellum in voluntary eye movements. Annu Rev Neurosci 24:981–1004
Robinson FR, Straube A, Fuchs AF (1993) Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J Neurophysiol 70:1741–1758
Ron S, Vieville T, Droulez J (1989) Target velocity based prediction in saccadic vector programming. Vision Res 29:1103–1114
Scudder CA (1988) A new local feedback model of the saccadic burst generator. J Neurophysiol 59:1455–1475
Scudder CA, Kaneko CS, Fuchs AF (2002) The brainstem burst generator for saccadic eye movements: a modern synthesis. Exp Brain Res 142:439–462
Smeets J, Bekkering H (2000) Prediction of saccadic amplitude during smooth pursuit eye movements. Hum Movement Sci 19:275–295
Yamada J, Noda H (1987) Afferent and efferent connections of the oculomotor cerebellar vermis in the macaque monkey. J Comp Neurol 265:224–241
Acknowledgements
The authors are grateful to Ms. S. Langer for technical assistance, Dr. E.W. Roubos for his valuable advice, Ms. B. Pfreundner and Ms. I. Wendl for preparing the manuscript, and Ms. J. Benson for copyediting the English text. This work was supported by the Deutsche Forschungsgemeinschaft (SFB 462).
Author information
Authors and Affiliations
Corresponding author
Additional information
Both Yanfang Guan and Thomas Eggert contributed equally
Rights and permissions
About this article
Cite this article
Guan, Y., Eggert, T., Bayer, O. et al. Saccades to stationary and moving targets differ in the monkey. Exp Brain Res 161, 220–232 (2005). https://doi.org/10.1007/s00221-004-2070-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-2070-3